Abstract
Upper limits for the supplementation of micronutrients are still undecided in many European countries. These limits are of particular interest for selenium because of the narrow range of acceptable intake. Standard analytical protocols to check for compliance must be consistent for a variety of dietary supplements. Emerging products in the evolving market for dietary supplements and over-the-counter (OTC)-medications must be factored in as well. The ICP-MS determination of selenium is well established for scientific research, but a systematic comparison of the operation modes for a realistic set of samples is still missing. In this manuscript, five common cell-techniques for interference removal were approved by a certified reference material (CRM) and applied to a variety of 28 different dietary supplements after microwave digestion. The comparison reveals these operation modes are less consistent for some supplements than for the CRM, whereas 78Se in collision mode was identified as being the most robust. The results for the supplements varied within tolerable limits from the labeled dosage. Extraction with TRIS-buffer was employed as a minimally invasive method to determine the impact in comparison to the total selenium quantification, showing variation among the labeled species (selenomethionine (∼100%), methyl-seleno-cysteine (94%), selenite and selenate (60–100%), selenized yeast (11–28%), spirulina algae (70%)).
Introduction
Selenium is among the most-supplemented micronutrients (Willers et al. Citation2015), providing several health benefits but poses severe risks when administered in higher doses (Kieliszek and Błażejak Citation2016; Kieliszek Citation2019). The safety margin between the dietary reference values of 70 µg d−1 (European Food Safety Authority (EFSA) 2014; Kipp et al. 2015; European Food Safety Authority Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products 2018) and the upper limit of 300 µg d−1 (European Food Safety Authority (EFSA) et al. Citation2006) has been recommended for the European population.
Recently, several European nations have proposed different values for upper dosage limits in dietary supplements, e.g., Germany 45 µg d−1 (Weißenborn et al. Citation2018). Deviations from the labeled dosage up to +45% and as low as −20% are considered to be legally tolerable due to variabilities in natural or technical production and composition changes during storage (European Commission 2012). The future implementation of legal limits for maximum acceptable selenium intake is calling for the development of new standard analytical protocols for selenium supplements.
Inductively coupled plasma – mass spectrometry (ICP-MS) is an internationally established method for quantitative elemental analysis. The analysis and detection of different isotopes of selenium are severely hindered by interferences from argon dimers. An appropriate determination of selenium with ICP-MS is only possible after the removal of these interferences by the use of a dynamic reaction cell (DRC) or kinetic energy discrimination (KED). This requirement is achieved by introducing methane (CH4) or helium (He) after ionization.
Whereas methane chemically reacts with the most abundant interference 40Ar40Ar+ in the DRC mode, the introduction of helium allows the removal of polyatomic species based on kinetic energy discrimination (KED), because of their average diameters. These techniques have been applied in numerous publications since 1998 and are therefore considered to be the most important tools for selenium ICP-MS-analytics in the research environment (May and Wiedmeyer Citation1998; Sloth and Larsen Citation2000; Darrouzès et al. Citation2005; Paucot Citation2006; D’Ilio et al. Citation2011; Thomas Citation2013; Constantinescu-Aruxandei et al. Citation2018; Wilschefski and Baxter Citation2019).
Despite of the breadth of research on ICP-MS for selenium detection, the effects of the different methods on the determination of selenium in a broad range of actual dietary supplements have never been examined to any appreciable extend. The results of this study emphasize that a check of those methods with one reference material for selenium does not fulfill the requirements to obtain valid and robust results for a whole set of real-life samples.
To fulfill the demands of the upcoming legal guidelines, ICP-MS must deliver explicit results across a multitude of matrices. Instead of analyzing the effects on the ICP-MS-operation modes or sample-preparation-optimization for one matrix, the approach of this study is to design a robust and versatile method for the analysis of real samples. This will support governmental and industrial analytical chemists reaching out for a manageable application of ICP-MS in order to prove the compliance with these legal limits.
High-value selenium compounds from biomass
The effect of dietary supplements on human health is influenced by many factors including the nutrient concentration, the chemical species in which the nutrient is being delivered and the bioavailability of the nutrient within the dosage form.
Typical selenium-providing ingredients in dietary supplements are salts of selenite (SeO3 2−) or selenate (SeO4 2−), amino acids such as selenomethionine (SeMet), and biofortified biological material such as selenized yeast or algae. While selenium salts are rather inexpensive inorganic compounds, organic selenium compounds (most commonly seleno-amino-acids or selenized yeast) are considered to be more valuable. When yeast or algae are grown in a selenium-enriched medium, selenium can be converted from inorganic selenium salts to organic selenium compounds (Schrauzer Citation2006; Schiavon et al. Citation2017; Wells et al. Citation2017), which have a greater bioaccessibility and a higher economic value.
SeMet and selenocysteine (SeCys) are the two most common natural seleno-amino-acids (Połatajko, Jakubowski, and Szpunar Citation2006; Constantinescu-Aruxandei et al. Citation2018). Whereas SeMet can be metabolized nonspecifically like methionine and is bound in different places in proteins, SeCys appears like a dimer with a diselenide-bond or with a Se-S-bond, which influences the functionality of enzymes. SeCys shows high reactivity and as a result is primarily present in derivatives such as the dimerized form selenocystine (SeCys2) or the methylated form methyl-seleno-cysteine (MeSeCys), which is one of the selenium-ingredients declared on the label of dietary supplements. MeSeCys is not registered in the positive lists of mineral substances which may be used in the production of food supplements in the EC-Directive on food supplements (European Commission 2002). According to a footnote in this directive, organic compounds not mentioned explicitly may be present in dietary supplements in the case that these are derived from selenized yeast.
Extraction techniques for selenium speciation
Despite its importance to economic value and health issues, up to now there is no officially approved method for the determination of selenium species. The first step toward speciation analysis is sample preparation. Hence, the extraction of the hydrophilic selenium compounds especially from selenized yeast has been examined extensively. Thus far, mild to partially harsh conditions for the extraction of selenium species from supplements or yeast samples have been carried out, with most focusing upon the decomposition of polymeric compounds in selenized yeast.
On the other hand, for total selenium analysis, digestion is often assisted by HNO3 (Ducros et al. Citation1994; Dernovics and Lobinski Citation2008; Kokarnig et al. Citation2015) or mixtures of HNO3 with H2O2 (Reyes et al. Citation2006; Casal et al. Citation2010) or H2O2 (Kokarnig et al. Citation2015). The other objective of the present work is to describe an extraction method suitable for the preservation of monomeric selenium species from a broad spectrum of dietary supplements. Hence, the analysis must be nondestructive to be capable of preserving different selenium species (B’Hymer and Caruso Citation2000; Bendahl and Gammelgaard Citation2004; Yang et al. Citation2004; B’Hymer and Caruso Citation2006; Połatajko, Jakubowski, and Szpunar Citation2006; Tastet et al. Citation2008).
In order to accomplish this goal, an ultrasonication-assisted extraction was executed at room temperature. TRIS-buffer as a solvent without a digestion step may not extract a high percentage of protein-bound selenium but provides a mild extraction procedure, preserving the labile compounds. (Kannamkumarath, Wrobel and Wuilloud Citation2005; Lipiec et al. Citation2010)
Materials and methods
Standards and reagents
Ultrapure water (resistivity of 18.2 MΩ·cm−1) was used at all times in this work and was produced with a Millipore Direct-q 3 UV-system from Merck Millipore (Darmstadt, Germany). Aristar nitric acid (HNO3) 69% for trace analysis (SigmaAldrich, Germany) and hydrogen peroxide (H2O2) were used for microwave digestion. Calibrations for total selenium analysis in 2% w/w HNO3 and in TRIS buffer were executed by Fluka Trace Cert selenium-standard for AAS 1000 mg L−1 ± 4 mg L−1 in 2% HNO3 and Ge PerkinElmer Pure Plus atomic spectroscopy standard 10 mg L−1.
Microwave digestion of the standard reference material SELM-1 (NRC – National Research Council Canada) was used throughout the analyses to verify the accuracy of total selenium content.
The extraction was performed with TRIS buffer (tris(hydroxymethyl-)aminomethane) gr for analysis (Merck, Germany) dissolved in water with approximately 200 mg L−1 of sodium azide (purity > 99.0%, SigmaAldrich, Germany) added.
The dilution and extraction of the dietary supplements were performed in single use falcon tubes.
Dietary supplement samples
28 dietary supplement samples examined in this work represent the German market and were purchased in the customary way via different distribution channels (online, pharmacies (Bonn, Germany), supermarkets (Bonn, Germany) and drug stores (Bonn, Germany). These are therefore subject to German/European regulations including the planned upper limits, the limitation of selenium sources allowed for dietary supplements and more general food regulations such as for novel food. The samples are different dosage forms and contain 10 to 200 µg selenium/portion from the various selenium sources.
Microwave digestion and sample preparation for total selenium analysis
Sample preparation for total selenium measurements was achieved by microwave-digestion similar to DIN EN 13805:2014-1212 Citation2014.
Tablets and solid materials were ground by mortar and pestle; soft capsules were cut with a single use sterile scalpel (Braun, Aesculap AG, Germany). Capsules with free-flowing content that could be taken out were opened and the content was homogenized. 0.5 g of the homogenized material was weighed in PTFE vessels. Soft capsules were prepared all at once as a whole capsule.
6 mL HNO3 and 2 mL H2O2 30% were added for closed-vessel microwave extraction. The microwave parameters are provided in .
Table 1. Microwave digestion parameters.
Extraction
An aliquot of 0.3 g of the homogenized supplement was shaken with 10 mL of TRIS buffer solution. The suspension was sonicated for one hour at room temperature. After filling to 15 mL with the same solution, the suspension was centrifuged at 5000 rpm at a temperature of 20 °C for 15 minutes. 8 mL of the solution were decanted from the pellet and the remaining solution was homogenized by vortexing before dilution with additional TRIS buffer. During the dilution step, 10 µg L−1 of Ge was added.
Instrumentation
Quantification was done with an ICP-MS Instrument (Nexion 350 D, PerkinElmer, USA), equipped with an autosampler (SC-2-DXS-Fast, Elemental Scientific, USA). The system contained a Meinhard concentric nebulizer and a baffled cyclonic quartz spray chamber, nickel sampler and skimmer cones and an aluminum hyperskimmer cone. The autosampler probe and tube were rinsed with 1% HNO3 after each analysis.
The parameters optimized daily for maximal sensitivity and minimal oxidation (CeO+) were torch position and nebulizer gas flow and the voltages for the quadrupole ion deflector. The performance characteristics were tested with a standard solution (Nexion solution, PerkinElmer (USA)) daily after optimization. The validation parameters are provided in the supplementary material.
Calibration
The analyte signal was corrected by 10 µg L−1 Ge as an internal standard in each sample and a blank was subtracted after the internal standard correction. Calibration was performed by diluting the above mentioned standards to 5, 10, 25, 50 and 100 µg L−1, respectively, in 2% HNO3 for the analysis of microwave digests. These were diluted to a content of 2% HNO3 and approximately 50 µg L−1 Se calculated from the labeled selenium amount of the supplements. Quality control was performed by analyzing SELM-1 throughout the supplement analyses.
For the determination of selenium in the extracts, the same calibration procedure was carried out in TRIS buffer solution. SELM-1 does not serve as a reference for extraction because the extraction process does not include any enzymolysis. It must be assumed that high amounts of selenium in selenized yeast are insoluble in water.
Cell gas parameters
The aim of the optimization was to accomplish the most reliable interference-removal-method. In standard mode (Std) the collision/reaction cell (CRC) was evacuated and the analysis was performed without any gas for interference removal. Dynamic reaction cell mode (DRC) used methane (CH4). In the kinetic energy discrimination mode (KED), the cell was filled with unreactive helium (He). shows the employed conditions.
Table 2. ICP-conditions.
Results
Trueness evaluation of the total selenium determination after microwave digestion and ICP-MS
The certified selenium content of the reference material is 2.059 ± 0.064 µg kg−1 (Mester et al. Citation2006). shows the selenium content determined in SELM-1 after microwave-assisted digestion.
Figure 1. Selenium content determined in SELM-1 for different ICP-conditions. The 80Se and 82Se isotopes are measured in DRC (dynamic reaction cell mode), KED (kinetic energy discrimination mode, Std (standard mode). The error bars = standard deviation; certified concentration for SELM-1; red lines: confidence limits for SELM-1.
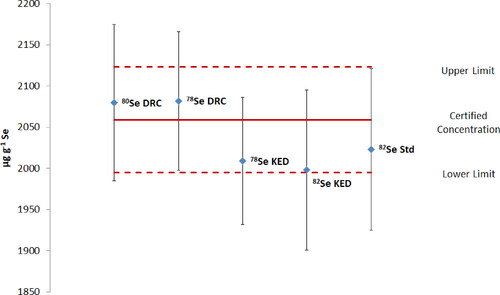
The concentrations determined with the different sets of conditions are all in accordance with the certified uncertainties for the reference. lists the bias and precision measurements calculated from the concentrations determined for the reference material:
Table 3. Selenium-content determined in SELM-1 for different ICP conditions: isotopes 80Se, 78Se, 82Se are measured in DRC (dynamic reaction cell mode), KED (kinetic energy discrimination mode), Std (standard mode), s = standard deviation; s[%] = relative standard deviation.
The smallest bias was analyzed with 82Se Std, but the values found with all of the tested methods are smaller than the standard deviation. Furthermore, bias values up to ± 15% are recognized to be acceptable (European Commission 2002; Peters et al. Citation2009). Therefore the trueness is tolerable for every set analyzed in this work. The precision data at the different settings are comparable within the acceptable limits.
The differences are smaller than the precision of the certified selenium concentration. Furthermore, SELM-1 served as a reference for one matrix only. Therefore, the method of choice is based upon the comparison of the deviations in the various dietary supplements.
Comparison of the ICP-conditions upon dietary supplements
All five sets of conditions were applied for the determination of total selenium content in 28 dietary supplements. These were mineralized as described in the Materials and Methods section and analyzed after calibration.
shows the results from the total content analysis of the dietary supplements compared to the labeled contents.
Figure 2. Selenium content in microwave digests of dietary supplements determined with different ICP conditions (DRC, KED and Std) and different istotopes (78Se 80Se and 82Se) (circles, squares and triangles); declaration = declared selenium content [μg Se g−1] (bars); small bars: standard deviation of the whole procedure; numbers listed in supplementary material.
![Figure 2. Selenium content in microwave digests of dietary supplements determined with different ICP conditions (DRC, KED and Std) and different istotopes (78Se 80Se and 82Se) (circles, squares and triangles); declaration = declared selenium content [μg Se g−1] (bars); small bars: standard deviation of the whole procedure; numbers listed in supplementary material.](/cms/asset/0128da26-60c1-4c2c-aa12-e312b34feb7d/lanl_a_1746328_f0002_c.jpg)
Outliers were eliminated with the Dixon test (Dixon Citation1951). These are not consequences of one or more explicable fact. Instead of elucidating the origin in every single case, the interferences were identified referring to the other settings:
The overall results of the different conditions did not vary significantly according to Kruskall-Wallis-H. When measuring standards in nitric acid, the highest number of unexplained errors was found for 80Se in the DRC mode. These data were eliminated because they were identified to be outliers or were measured after incorrect results for the reference material. The differences between the results of the settings clearly show that these settings are not equivalent, although all were demonstrated to be correct by applying the procedure to the certified reference material. It becomes clear that the reliability of the data must be verified for the various ICP-MS-settings within the same run.
When analyzing the dietary supplement digests the highest number of acceptable results, which do not have to be eliminated due to similar reasons, were found for 78Se in the KED mode. The concentrations determined in the DRC mode are often not in accordance with the concentrations obtained in the other modes. These values obtained in the KED or Std-mode are generally in accordance with the selenium content which was declared for the dietary supplements.
Standard deviations shown as error bars in include the whole procedure, beginning with homogenization through the result. In most cases, the standard deviations were not higher than 15% of the mean.
The two samples with the highest deviations were obtained in the DRC mode and were medium concentrated supplements. Accordingly, they are represented in the 153 to 394 µg g−1 chart in . However, no clear deduction can be made from these measurements, because the concentrations were medium level and both the 80Se and 78Se isotopes were affected.
In addition to the selenium concentration in the supplements, the list of inorganic ingredients was analyzed. lists the ingredients declared to be in these supplements. The order is the same as in , based on increasing concentration.
Table 4. List of ingredients of the supplements containing 153 to 394 µg g−1 declared selenium-concentration [µg g−1Se], the declared source of selenium, additional inorganic ingredients, which could be expected in the microwave digests.
The similarity between the supplements with severe effects on 80Se DRC is the ingredient zinc. Nevertheless, a whole list of inorganic ingredients may be found in Table S6 in supplementary material. Table S6 shows zinc as an inorganic ingredient in many of the dietary supplements that contain 5 to 152 µg g−1 selenium, but the measured concentrations differ from the declared concentration to an acceptable extent.
Quantification of the extracted selenium
The TRIS-extraction was incomplete for most samples, resulting in the observation of a solid residue found in most of the extraction tubes. The extraction efficiency is shown in , where the relationships between the extracted content and the total content are shown for each supplement sample.
Figure 3. Extraction efficiency where recovery [%] = selenium in extract (78Se KED)/total selenium; tall bars = recovery of one supplement grouped by declared selenium sources; MeSeCys = methylselenocysteine; SeMet = selenomethionine; small bars = standard deviation for the procedure.
![Figure 3. Extraction efficiency where recovery [%] = selenium in extract (78Se KED)/total selenium; tall bars = recovery of one supplement grouped by declared selenium sources; MeSeCys = methylselenocysteine; SeMet = selenomethionine; small bars = standard deviation for the procedure.](/cms/asset/0575cb63-3dc6-4865-90cb-85de262952e4/lanl_a_1746328_f0003_c.jpg)
The overall pattern of the extraction efficiency is inhomogeneous. shows the results organized in groups of declared selenium sources, because these are to be expected to be the primary selenium delivering compounds. The extracted content differs between the declared selenium sources and within the groups of samples containing the same source of selenium.
The highest and most repeatable selenium extraction yield was found for the supplements containing SeMet and MeSeCys. The extraction yields for these samples were nearly 100%. For selenized yeast, the overall extraction efficiency is the lowest (11 to 28%) with one exception, when 91% selenium was determined in the extract. The extraction efficiency of inorganic selenium salts is higher than for the selenium yeast overall but varies from sample to sample.
Discussion
Comparison of ICP-MS conditions
In order to discuss the differences between the operational modes of ICP-MS, the trueness, precision and applicability of real life samples were examined. The Se-concentration in the dietary supplement samples is unknown, so the trueness of the application of different operation modes of ICP-MS was evaluated on the basis of the results obtained for the analysis of a microwave digest of certified reference material SELM-1. The quantities of selenium content and the standard deviations are within the acceptance criteria derived from the certification limits of the reference material and from the maximum acceptable bias (European Commission 2002; Peters et al. Citation2009). Nevertheless, the different operation modes can be accepted but do not deliver the same values.
These results are especially visible in : in some cases, the measurement of one ICP-MS mode results in an underestimated selenium dosage, whereas the results of the other settings reveal the unmatching setting interfered. Returning to the CRM, the differences between the ICP-MS-operation modes are lower than the variance of the Se-content in SELM-1. The trueness of the settings must therefore be considered to be equivalent and therefore do not serve as an evaluation criterion for the optimal method.
Nevertheless, the question arises, which characteristics are the most important? The methods were examined as a comparison of the settings in application on a broad spectrum of dietary supplements with selenium. The applicability of the ICP-MS methods may be enormously influenced by the samples because of the high inorganic content. Additionally, the values measured for dietary supplements and standards had to be checked for outliers, even when no discrepancies are obvious.
shows the differences between the DRC mode and the other ICP-MS conditions. It may be derived that the inorganic sample matrix affects DRC mode the most. This is in accordance with the literature: DRC-mode-optimization is quite specific. That is, on the one hand, the background is low due to the efficient elimination of the highly-abundant Ar-Ar-interference, but on the other hand, the DRC-mode is prone to side reactions in the reaction cell. These are designed to be reduced by dynamic bandpass scanning in DRC (Thomas Citation2013), but the measurements obtained for the other methods are more robust.
When an optimization of DRC for a more robust method is desired, the matrix compounds must be evaluated. Instead of performing a total component analysis, these must be examined based on the selenium concentration and labeled ingredients. Three samples with obvious deviations from the concentrations delivered by DRC contained medium selenium concentrations. It was therefore assumed that the method is sensitive for selenium detection, but is at the same time, is robust to varying concentrations of selenium in the samples by the selenium concentration.
The determination of the other inorganic ingredients on the label revealed that zinc is the only similarity amongst the supplements with the most interference. The other samples containing 153 to 394 µg g−1 selenium were unaffected. There were other samples containing zinc and selenium content in the range from 5 to 152 µg g−1 that did not exhibit significant differences between the cell gas modes. As a result, none of the ingredients may be identified as the key influence affecting the results obtained in DRC mode.
None of the available measurements can be identified as repeatable that may be eliminated via method development. Consequently, this study focuses upon the overall applicability for dietary supplements with different ingredient lists. This approach is more feasible with regard to rapidly changing supplement compositions and the plethora of possible ingredient combinations in dietary supplements.
The basis of decision may be derived from the above mentioned inconsistencies. The conclusions should be rated based on criteria including the highest precision values and the lowest number of outliers. Among the compared conditions, 78Se in the KED-mode was the method fulfilling these criteria to the highest degree. Nevertheless, the discussed interferences are still not fully understood. Therefore the results from 78Se in KED-mode are proposed to be checked with other operation modes of ICP-MS as reference methods in one run.
Extraction efficiency
Aqueous extractions do not require much equipment and are therefore applicable in most laboratories in order to present an initial step toward speciation. TRIS buffer is designed to provide a soft extraction without any changes in the molecular structure. The selenium species that are bound to water-insoluble molecules may therefore not be extracted.
The aqueous extraction efficiency of selenium from dietary supplements is inhomogeneous. Several trends may be observed from the results obtained here. Among the different categories of supplements, the lowest extraction efficiency was observed for selenized yeast, whereas there were variations between the supplements with selenized yeast.
The high extraction efficiency of one supplement compared to the other selenized yeast preparations may be connected to a high content of a water-soluble selenium species, which were added to the yeast instead of cultivation in a Se-enriched medium. In case of the other yeast samples, the loss of extraction efficiency was accepted in favor of a low-invasive extraction of selenium species, especially selenium-containing proteins and proteins with seleno amino acids (selenoproteins). The fact that the extraction efficiency of monomeric selenium species from dietary supplements is significantly higher is an indication that the samples with lower extraction efficiency contain poorly water-soluble selenium species such as oligomeric or polymeric compounds.
Aqueous drug solubility has an enormous impact upon the bioaccessibility and is therefore one of the most important challenges in pharmaceutical formulation development (Savjani, Gajjar, and Savjani Citation2012). The active ingredient (selenium) in the analyzed supplements can only be utilized when it is available for digestion. Selenized yeast is considered to be an excellent option for selenium supplementation because of the high amount of organic selenium species found therein. However, in comparison to lower-price inorganic selenium, its digestibility is reduced by the low water solubility. The absorption of selenium from selenized yeast may take place only after enzymolysis.
Additionally, the formulation itself has to be taken into consideration. The differences in the water solubility of total selenium from the analyzed dietary supplements within one group of selenium species show the impact of the composition of the supplements. In particular, the extraction efficiency of the soft capsule samples is limited. In such compositions, the selenium sources are administered in a viscose lipophilic mixture, which cannot be extracted hydrophilically without any digestion step. Some of the supplement samples analyzed here are purported to provide a “depot-effect”. A prolonged release of selenium from the supplements is advertised to accomplish a continuous dosage over the whole digestion process. It is possible that the extraction efficiency changes when the extraction time varies.
The water-solubility may at first approximation be attributed to the bioaccessibility of selenium within the supplement composition. The near-100% extraction efficiency of SeMet may be due to the fact that it is highly bioaccessible, whereas selenite and selenate are less soluble. The bioaccessibility of selenized yeast is even lower. However, it must be kept in mind that pre-intestinal digestion would likely enhance the extractability.
Evaluation of selenium concentrations in the supplements
The results of the different methods confirm the selenium contents declared on the supplement labels. It must be pointed out that none of the supplements reaches the UL of 300 µg d−1 that was suggested by the EC Scientific Committee of Food (European Food Safety Authority (EFSA) et al. Citation2006). The daily dosages calculated from the contents are shown in the supplementary material. The differences between declared and determined total selenium content (78Se, KED mode) are acceptable according to the EU-Guidance document on tolerances for every sample (European Commission 2012). Still, most of the declared selenium contents do not conform to the proposed German dosage limit of 45 µg d−1 (Weißenborn et al. Citation2018).
Conclusion
A feasible approach for selenium determination in dietary supplements was established. The total selenium content determination in dietary supplements was achieved. Five different combinations of measured isotope and cell modes of the second quadrupole (CRC) were compared: standard mode without a gas cell (82Se), KED mode using helium for kinetic discrimination of the argon-dimer (78Se, 82Se) and DRC mode with methane (80Se, 78Se).
The results for the SELM-1 yeast reference material in each of the sets of conditions were in agreement with the certified value. In contrast, when analyzing mineralized dietary supplements, the results of the five sets of conditions did not agree with each other. It is obvious that method development cannot be verified only with the certified reference material, but must be controlled by different ICP-MS-operational-modes in case of every single digest. The determination of 78Se in the KED mode was the most robust. The selenium contents of the dietary supplements analyzed here vary within the tolerable deviation limits from the labeled dose and conform to the actual legal regulations.
Additionally, an initial step for selenium-speciation sample preparation was performed by extracting selenium with TRIS-buffer. It was based on ultrasonication without further treatment with enzymes or other additives. This sample preparation step is low-invasive and leads to an extraction efficiency of 60% or higher in case of monomeric selenium species. However, the extraction efficiency was from 10 to 30% in most cases of selenized yeast with the exception of one supplement.
The extraction results reflect the bioaccessibility of the different selenium species. It must be assumed that selenized amino acids are highly accessible from dietary supplement preparations, whereas, for selenized yeast, a digestion step is required. The solubility of inorganic species as selenite and selenate differs the most between the different supplement samples.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable.
Supplemental Material
Download MS Word (53.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bendahl, L. , and B. Gammelgaard . 2004. Separation of selenium compounds by CE-ICP-MS in dynamically coated capillaries applied to selenized yeast samples. Journal of Analytical Atomic Spectrometry 19 (1):143–8. doi:https://doi.org/10.1039/b307474a.
- B’Hymer, C. , and J. A. Caruso . 2000. Evaluation of yeast-based selenium food supplements using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry 15 (12):1531–9. doi:https://doi.org/10.1039/b006437h.
- B’Hymer, C. , and J. A. Caruso . 2006. Selenium speciation analysis using inductively coupled plasma-mass spectrometry. Journal of Chromatography A 1114 (1):1–20. doi:https://doi.org/10.1016/j.chroma.2006.02.063.
- Casal, S. G. , J. Far , K. Bierla , L. Ouerdane , and J. Szpunar . 2010. Study of the Se-containing metabolomes in Se-rich yeast by size-exclusion-cation-exchange HPLC with the parallel ICP MS and electrospray orbital ion trap detection. Metallomics : Integrated Biometal Science 2 (8):535–48. doi:https://doi.org/10.1039/c0mt00002g.
- Constantinescu-Aruxandei, D. , R. M. Frîncu , L. Capră , and F. Oancea . 2018. Selenium analysis and speciation in dietary supplements based on next-generation selenium ingredients. Nutrients 10 (10):1466. doi:https://doi.org/10.3390/nu10101466.
- Darrouzès, J. , M. Bueno , G. Lespès , and M. Potin-Gautier . 2005. Operational optimisation of ICP—octopole collision/reaction cell—MS for applications to ultratrace selenium total and speciation determination. Journal of Analytical Atomic Spectrometry 20 (2):88–94. doi:https://doi.org/10.1039/B410142A.
- Dernovics, M. , and R. Lobinski . 2008. Characterization of the selenocysteine-containing metabolome in selenium-rich yeast: Part 1. Identification of new species by multi-dimensional liquid chromatography with parallel ICP-MS and electrospray Q-TOFMS/MS detection. Journal of Analytical Atomic Spectrometry 23 (1):72–83. doi:https://doi.org/10.1039/B708294K.
- D’Ilio, S. , N. Violante , C. Majorani , and F. Petrucci . 2011. Dynamic reaction cell ICP-MS for determination of total As, Cr, Se and V in complex matrices: Still a challenge? A review. Analytica Chimica Acta 698 (1–2):6–13. doi:https://doi.org/10.1016/j.aca.2011.04.052.
- DIN EN 13805:2014-12 . 2014. Foodstuffs - Determination of trace elements - Pressure digestion, EN_13805 . Berlin: Beuth Verlag GmbH.
- Dixon, W. J. 1951. Ratios involving extreme values. The Annals of Mathematical Statistics 22 (1):68–78. doi:https://doi.org/10.1214/aoms/1177729693.
- Ducros, V. , D. Ruffieux , N. Belin , and A. Favier . 1994. Comparison of two digestion methods for the determination of selenium in biological samples. The Analyst 119 (8):1715–7. doi:https://doi.org/10.1039/an9941901715.
- European Food Safety Authority (EFSA), Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies . 2006. Tolerable upper intake levels for vitamins and minerals: Opinion of the Scientific Committee on food on tolerable upper intake level of selenium (expressed on 19 October 2000). Accessed March 3, 2020. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf.
- European Food Safety Authority (EFSA) . 2014. Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on Dietary Reference Values for selenium. EFSA Journal 12:3846. doi:https://doi.org/10.2903/j.efsa.2014.3846.
- European Food Safety Authority, Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products . 2018. Nutrition and Allergies (NDA). (EFSA), Overview on Tolerable Upper Intake Levels (September 2018). Accessed March 3, 2020. www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf
- European Commission. 2002. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements (Text with EEA relevance): 2002/46/EC.
- European Commission . 2012. Draft guidance document for competent authorities for the control of compliance with EU legislation on : Regulation ( EU ) No 1169 / 2011 and Council Directive 90 / 496 / EEC and Directive 2002 / 46 / EC : European Commision - Health and Consumers Directorate - General ( Ed .).
- Kannamkumarath, S. S. , K. Wrobel , and R. G. Wuilloud . 2005. Studying the distribution pattern of selenium in nut proteins with information obtained from SEC-UV-ICP-MS and CE-ICP-MS. Talanta 66 (1):153–9. doi:https://doi.org/10.1016/j.talanta.2004.10.010.
- Kieliszek, M. 2019. Selenium-fascinating microelement, properties and sources in food. Molecules 24 (7):1298. doi:https://doi.org/10.3390/molecules2407.
- Kieliszek, M. , and S. Błażejak . 2016. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 21 (5):609. doi:https://doi.org/10.3390/molecules21050.
- Kipp, A. P. , D. Strohm , R. Brigelius-Flohé , L. Schomburg , A. Bechthold , E. Leschik-Bonnet , and H. Heseker , German Nutrition Society (DGE) . 2015. Revised reference values for selenium intake. Journal of Trace Elements in Medicine and Biology: Biology 32:195–9. doi:https://doi.org/10.1016/j.jtemb.2015.07.005.
- Kokarnig, S. , A. Tsirigotaki , T. Wiesenhofer , V. Lackner , K. A. Francesconi , S. A. Pergantis , and D. Kuehnelt . 2015. Concurrent quantitative HPLC-mass spectrometry profiling of small selenium species in human serum and urine after ingestion of selenium supplements. Journal of Trace Elements in Medicine and Biology: Biology 29:83–90. doi:https://doi.org/10.1016/j.jtemb.2014.06.012.
- Lipiec, E. , G. Siara , K. Bierla , L. Ouerdane , and J. Szpunar . 2010. Determination of selenomethionine, selenocysteine, and inorganic selenium in eggs by HPLC-inductively coupled plasma mass spectrometry. Analytical and Bioanalytical Chemistry 397 (2):731–41. doi:https://doi.org/10.1007/s00216-010-3544-8.
- May, T. W. , and R. H. Wiedmeyer . 1998. A table of polyatomic interferences in ICP-MS. Atomic Spectroscopy 19:150–5.
- Mester, Z. , S. Willie , L. Yang , R. Sturgeon , J. A. Caruso , M. L. Fernández , P. Fodor , R. J. Goldschmidt , H. Goenaga-Infante , R. Lobinski , et al. 2006. Certification of a new selenized yeast reference material (SELM-1) for methionine, selenomethinone and total selenium content and its use in an intercomparison exercise for quantifying these analytes. Analytical and Bioanalytical Chemistry 385 (1):168–80. doi:https://doi.org/10.1007/s00216-006-0338-0.
- Paucot, H. 2006. Les dispositifs de collision/réaction en ICP-MS: Revue descriptive et modes de fonctionnement. Spectra Analyse 252:23–7.
- Peters, F. T. , M. Hartung , M. Herbold , U. G. Schmitt , and T. D. F. Musshoff . 2009. Appendix B to the GTFCh guidelines for quality insurance. Toxichem. Krimtech 76:185–208.
- Połatajko, A. , N. Jakubowski , and J. Szpunar . 2006. State of the art report of selenium speciation in biological samples. Journal of Analytical Atomic Spectrometry 21 (7):639–54. doi:https://doi.org/10.1039/B605654G.
- Reyes, L. H. , J. R. Encinar , J. M. Marchante-Gayón , J. I. G. Alonso , and A. Sanz-Medel . 2006. Selenium bioaccessibility assessment in selenized yeast after “in vitro” gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. Journal of Chromatography. A 1110 (1–2):108–16. doi:https://doi.org/10.1016/j.chroma.2006.01.088.
- Savjani, K. T. , A. K. Gajjar , and J. K. Savjani . 2012. Drug solubility: Importance and enhancement techniques. ISRN Pharmaceutics 2012:195727 doi:https://doi.org/10.5402/2012/195727.
- Schiavon, M. , A. Ertani , S. Parrasia , and F. D. Vecchia . 2017. Selenium accumulation and metabolism in algae. Aquatic Toxicology (Amsterdam, Netherlands) 189:1–8. doi:https://doi.org/10.1016/j.aquatox.2017.05.011.
- Schrauzer, G. N. 2006. Selenium yeast: Composition, quality, analysis, and safety. Pure and Applied Chemistry 78 (1):105–9. doi:https://doi.org/10.1351/pac200678010105.
- Sloth, J. J. , and E. H. Larsen . 2000. The application of inductively coupled plasma dynamic reaction cell mass spectrometry for measurement of selenium isotopes, isotope ratios and chromatographic detection of selenoamino acids. Journal of Analytical Atomic Spectrometry 15 (6):669–72. doi:https://doi.org/10.1039/b001798l.
- Tastet, L. , D. Schaumlöffel , B. Bouyssiere , and R. Lobinski . 2008. Identification of selenium-containing proteins in selenium-rich yeast aqueous extract by 2D gel electrophoresis, nanoHPLC-ICP MS and nanoHPLC-ESI MS/MS. Talanta 75 (4):1140–5. doi:https://doi.org/10.1016/j.talanta.2008.01.003.
- Thomas, R. 2013. Practical guide to ICP-MS: A tutorial for beginners . 3rd ed. Boca Raton, FL: CRC Press; 418 p. (Practical Spectrosc., vol. 38). ISBN: 9781466555433.
- Weißenborn, A. , N. Bakhiya , I. Demuth , A. Ehlers , M. Ewald , B. Niemann , K. Richter , I. Trefflich , R. Ziegenhagen , K. I. Hirsch-Ernst , et al. 2018. Höchstmengen für Vitamine und Mineralstoffe in Nahrungsergänzungsmitteln. Journal of Consumer Protection and Food Safety 13 (1):25–39. doi:https://doi.org/10.1007/s00003-017-1140-y.
- Wells, M. L. , P. Potin , J. S. Craigie , J. A. Raven , S. S. Merchant , K. E. Helliwell , A. G. Smith , M. E. Camire , and S. H. Brawley . 2017. Algae as nutritional and functional food sources: Revisiting our understanding. Journal of Applied Phycology 29 (2):949–82. doi:https://doi.org/10.1007/s10811-016-0974-5.
- Willers, J. , M. Heinemann , N. Bitterlich , and A. Hahn . 2015. Intake of minerals from food supplements in a German population—A nationwide survey. Food and Nutrition Sciences 6 (2):205–15. doi:https://doi.org/10.4236/fns.2015.62021.
- Wilschefski, S. C. , and M. R. Baxter . 2019. Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. The Clinical Biochemist. Reviews 40 (3):115–33. doi:https://doi.org/10.33176/AACB-19-00024.
- Yang, L. , R. E. Sturgeon , S. McSheehy , and Z. Mester . 2004. Comparison of extraction methods for quantitation of methionine and selenomethionine in yeast by species specific isotope dilution gas chromatography-mass spectrometry. Journal of Chromatography A 1055 (1–2):177–84. doi:https://doi.org/10.1016/j.chroma.2004.09.018.
