?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Santalum macgregorii (sandalwood), which is endemic to the southern part of Papua New Guinea (PNG), has been heavily exploited for its fragrant heartwood and is classified as threatened across its natural range. Domestication and smallholder agroforestry offer the means to preserve remaining diversity. This study was undertaken to understand the extent of remaining natural variation to support the species’s conservation and domestication. We evaluated morphological, heartwood and essential oil characters in 126 S. macgregorii trees in five populations (districts) in PNG’s Central, Gulf and Western provinces. The heartwood oil of this species is characterised by extreme tree-to-tree variation in key fragrant compounds. Proportions of individual compounds range from negligible to high for (Z)-α-santalol (0.5–51%), (Z)-β-santalol (0–24.2%), (Z)-nuciferol (0–40.5%) and (Z)-lanceol (0–72%). Despite the wide variation found within populations, an ordination of seven oil constituents revealed broad provenance-based variation in which trees from the eastern provinces (i.e. Central and Gulf) were more influenced by (Z)-nuciferol content and the trees from the Western Province site were more strongly influenced by (Z)-lanceol. The driver of this variation was the different associations between oil constituents, with (Z)-α- and (Z)-β-santalol both negatively correlated with (Z)-nuciferol for sites in the eastern provinces and (Z)-lanceol in Western Province. No evidence of distinct chemotypes was found, with continuous variation demonstrated across all major oil constituents. Of the trees surveyed with a basal diameter of >10 cm, 79% had heartwood. Mean heartwood percentage was 15.8% of basal area, with no significant differences between sites. Significant tree-to-tree variation in heartwood percentage (0–61%) was found. A modest positive correlation was found between stem and heartwood diameter (r = 0.39). Heartwood percentage and heartwood oil quality varied independently and, therefore, independent selection of these traits may be required for their simultaneous improvement. The population in Western Province is non-contiguous with those in the eastern part of the species distribution. It also has a distinct phenotype based on oil composition, leaf shape, flower colour and potential reproductive failure. It is possible that sandalwood in Western Province is more closely related to the proximal populations of S. lanceolatum in Cape York Peninsula, Queensland, than the more distant populations of S. macgregorii in PNG. While these phenotypic features do not necessarily discriminate a new species, molecular genetic research is required to determine the potential existence of a cryptic species of sandalwood. The implications of the variation found in S. macgregorii are discussed with respect to its domestication and conservation.
Introduction
Trees in the hemiparasitic Santalum genus (Santalaceae) are distributed across South and Southeast Asia, Oceania and Australia (Applegate et al. Citation1990; Harbaugh & Baldwin Citation2007). Sandalwood is valued for its fragrant heartwood, and existing strong international demand is projected to continue for the next 30 years (Thomson Citation2020). Santalum macgregorii F. Muell. is endemic to the southern coast of mainland Papua New Guinea (PNG) in dry savannah woodland up to 750 m above sea level, with a mean annual rainfall of around 1000 mm. The species, also known as PNG sandalwood, is commercially important; its valuable oil-rich fragrant heartwood was exploited from the early 1890s to the late 1930s and then again from the mid-1960s. In the early 2000s, harvesting increased to unsustainable levels, which contributed to a significant decline in mature stands (Rome et al. Citation2020) and prompted the International Union for Conservation of Nature to list the species as threatened (Eddowes Citation1998). Gunn et al. (Citation2002) developed a strategy for conserving and managing the wild resources of PNG sandalwood, although few of the recommendations have been implemented (Bosimbi & Bewang Citation2007).
To improve the prospects for the conservation and development of S. macgregorii in PNG, Bosimbi (Citation2006) strongly recommended establishing in situ and ex situ conservation stands as well as building the capacity of local farmers to establish plantings. It was evident by 2012 that little of this recommended work had been conducted, and Kiapranis (Citation2012) called for action to establish a breeding program to reinvigorate the significantly depleted resource. Although a formal program is still lacking, landowners have shown considerable interest in planting S. macgregorii for future income (Rome et al. Citation2020). Across most areas, planting has been undertaken informally by landowners, in which they have transplanted suckers and seedlings from mature harvested trees to village areas, but this approach remains largely opportunistic and not targeted towards developing specific traits. More formal and managed plantings can potentially lead to the community-based conservation of S. macgregorii. Dawson et al. (Citation2014) suggested that genetic selection and improved cultivation of culturally and commercially important trees can support the conservation of their wild sources. Strong evidence exists for the livelihood benefits of domestication and smallholder production of previously wild harvested trees (Leakey et al. Citation2012; Leakey Citation2019). The process of tree domestication depends on genetic variation for commercially important traits (Simons & Leakey Citation2004; Miller & Gross Citation2011).
The genetic diversity available in natural stands of S. macgregorii can help underpin conservation and domestication efforts for PNG sandalwood. This has been effective in the initial domestication of Santalum austrocaledonicum Vieill. in Vanuatu (Page et al. Citation2020). Understanding the natural phenotypic and genetic variation of S. macgregorii is the first step in formally domesticating PNG sandalwood. Oil quality is a key trait, which is influenced by the relative level of α- and β-santalol contained within. Variation in mean levels of α- and β-santalol is found in natural populations of Santalum album L. (30–60% and 10–25%, respectively; Subasinghe et al. Citation2017), S. austrocaledonicum (9–37% and 11–17%; Page et al. Citation2010), Santalum yasi Seem. (16–57% and 10–34%; Doran et al. Citation2005), and Santalum lanceolatum R.Br. (5–25% and 5–10%; Page et al. Citation2007). Despite the scientific knowledge of natural variation in oil-quality traits within and between species, there is a lack of published studies that determine the genetic influence on and heritability of oil traits in any sandalwood species.
Brophy et al. (Citation2009) examined the variation in oil chemistry and yield in 14 heartwood samples of S. macgregorii from five areas in PNG's Central, Gulf and Western provinces and found significant phenotypic variation in oil composition. They identified three extremes of composition, with high relative levels of (1) (Z)-α- and β-santalol; (2) (Z)-lanceol; and (3) the combined four constituents (E,E)-farnesol, (Z)-γ-curcumen-12-ol, (Z)-b-curcumen-12-ol and (Z)-nuciferol. No other studies have been published on the natural phenotypic and oil chemistry variation of S. macgregorii. This paper describes the tree-to-tree morphological variation and heartwood characteristics, including oil composition, of 126 trees sampled from the known natural distribution of S. macgregorii in PNG and the implications for conservation and commercial purposes.
Materials and methods
Study area
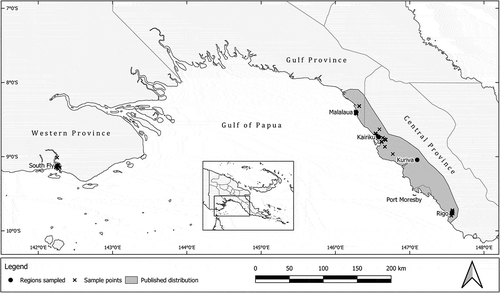
shows the natural range of S. macgregorii in PNG. Sampling was undertaken in: Rigo (9°46’S 147°34ʹE 30–150 m asl); Kairuku (8°44’S 146°35ʹE 10–130 m asl)—both districts within Central Province; Malalaua (8°24’S 146°17ʹE 05–85 m asl) District in Gulf Province; and a small area of the South Fly District to the east of the Mai Kussa River around the villages of Buzi, Berr and Sibidiri (9°07’S 142°15ʹE 10–30 m asl) in Western Province (). The sampled areas have a dry tropical monsoonal climate. The mean annual temperature ranges from 25.9°C to 26.8°C and the mean monthly temperatures range from 20.1–21.0°C to 31.0–32.2°C. The annual rainfall ranges from 1664 mm to 2122 mm, with the wettest month typically receiving 243–273 mm and the driest month 35–102 mm (Fick & Hijmans Citation2017). The sampled trees were located in cultivated areas close to or within village areas, in protected situations in savannah woodlands, and in or adjacent to vine thickets along water courses/drainage lines that run through savannah woodlands. In Western Province, trees were found in savannah woodlands only.
Table 1. The number and proportion of trees established via planting and assisted natural regeneration (ANR) for five populations of Santalum macgregorii in southern Papua New Guinea
Tree selection
Reconnaissance in Central and Gulf provinces enabled the provision of advance notice to landowners, who then identified where trees were located; it also enabled villagers to scout for mature trees for sampling. This gave confidence that the trees sampled from these areas were representative of the remaining populations. In Western Province, sampling was limited to known trees around Buzi, Berr and Sibidiri, where trees had mostly been found by landowners by chance. There was no systematic sampling or desire from villages in the South Fly District to locate trees, and therefore it is possible that more trees occur throughout that area.
The selection of sampled trees was restricted largely to those trees with a basal diameter >10 cm. Smaller trees were included in areas where trees were present but no larger specimens were available. In order to reduce the sampling of genetically related trees, particularly clonal root suckers, a minimum distance of 20 m was used between sampled trees. For planted specimens, some trees >10 cm were excluded when landowners advised that the seed used to plant multiple trees had come from the same parent tree. No other restrictions, beyond diameter size, were used to select trees because of the small number of candidates.
Landowners identified that 56% of sampled trees had been planted as seedlings or established via assisted natural regeneration (). The latter process included tending and/or transplanting naturally germinated seedlings (wildings) or vegetative suckers. All four trees sampled at the Kuriva District recreation area were planted. The percentage of planted trees was significantly (P < 0.05) higher in the Kairuku and Malalaua (approx. 80%) districts than in the Rigo (28%) and South Fly (0%) districts.
Tree description
Descriptions of sampled trees included total height, bole length, habit, diameter over bark at 0.2, 0.7 and 1.3 m, canopy spread from north to south and east to west, and count and measurement of fire scars. Tree form was assigned to one of four categories: (1) single stem; (2) trunk forking; (3) 2–3 primary stems from the base; or (4) multistemmed shrub. Stem taper calculations were based on stem diameter reduction between measurement points per linear metre of stem (i.e. (Dia0.2–Dia0.7)/(0.7–0.2 m)). Reproductive status was estimated by observing the density of buds, flowers, maturing fruits and mature fruits using a scale of 0 (nil) to 5 (high). Recruitment was assessed by counting suckers and seedlings within 10 m of sampled trees, and either basal or breast height diameter was measured for recruits >100 cm in height. Physical or biotic damage was recorded on a scale of 0 (nil) to 5 (high). Physical damage was further classified as deliberate or indiscriminate slashing of bark with a machete; and heartwood check was noted for severity, frequency and cardinal direction. Heartwood checking is a practice in which people make wedge-shaped cuts of various depths into tree stems to check for the presence of heartwood.
Ten fully expanded mature leaves without damage were collected. Images were taken of leaves on a flat white background with a 15 cm scale and clear acrylic on top. Image files were converted to 8-bit greyscale and leaves measured using Image-J 1.52a (National Institutes of Health). Measures were leaf area, length, width, roundness (4*area/(π*length2) and aspect ratio (length/width).
Heartwood and oil chemistry
Core sampling
One bark-to-bark wood core (5.15 mm diameter) was extracted (Haglöf Increment Borer 300–2 T) from each sampled tree at a height of 0.2 m. Any variance in this height (caused by the presence of hollows, rot, fire scars and heartwood checks that prevented coring at a consistent height) was recorded. The cardinal direction also varied to accommodate tree shape and damage. The length of sapwood, transition wood and heartwood was marked and measured using colour changes and smell to determine the transition boundaries. Not all wood types were present in all cores. A photograph was taken and the core placed in an airtight container containing indicator silica gel for quick drying to preserve the oil for later extraction.
Preparation of sandalwood samples for oil extraction
Core samples were size-reduced to 2–0.25 mm using a cutter mill. The cutter mill used was RX-04 (Mill Powder Tech, Taiwan) at 25 000 RPM, operated in pulse mode to maintain temperature at <40°C. The milled samples (1.0 g ±0.01 g) were transferred to separate 20 ml vials and 10 ml n-hexane containing camphor at 0.1 mg ml−1 as an internal standard. The monoterpene camphor (C10) was selected because it: does not interfere in the sandalwood sesquiterpenes (C15) range; is stable during the extraction process and gas chromatography (GC) analysis (injector temperatures); and shows a similar abundance between different detection methods (flame ionisation detector – FID, electron ionisation-mass spectrometry – EI-MS). The samples were sonicated for 30 min at 25°C using an ultrasonic water bath with total power of 245 W operating at a frequency of 42 Hz. The samples were then filtered with a 0.45 µm polypropylene filter and transferred to 2 ml amber glass vials for GC-MS analysis.
Calibration standards were prepared in the range 1–0.025 mg ml−1 by serial dilution using pure essential oil of S. album (ISO 3518). The standards were subjected to the same extraction and GC-MS analysis method. A calibration curve was plotted to extrapolate the concentration of oil in samples.
Gas chromatography-mass spectrometry parameters
Analysis was completed on a Thermo Fisher Scientific Q-Exactive orbitrap mass spectrometry system-Trace 1310. Separation was performed on a DB5 column, 30 m × 0.25 mm × i.d 0.25 µm (Thermo Scientific TG-5 MS) using a gradient temperature oven program: the oven was initially set at 100°C for 1 min and then increased at a rate 2°C min−1 up to 200°C and then at 20°C min−1 up to 300°C and maintained at this temperature for 1 min. The carrier gas was helium at a flow rate of 1.0 ml min−1. The injection volume was 0.2 µl and the injector temperature was 250°C with a split flow of 10 ml min−1. The mass spectrometer was operated in electron impact mode (70 eV). A mass range of 50–750 m/z was scanned with a resolution of 60 000 and automatic gain control target of 1 × 106. Filament delay was set at 4 min and the mass transfer line was maintained at 280°C. The compounds were identified using the NIST library and Kovat retention indices.
Statistics
Differences between sample sites for measured traits were tested using a general linear model. Where necessary, arcsine data transformations were performed on percentage data to ensure they conformed with equal variance (Levene’s) and normality (Kolmogorov-Smirnov). The number of samples collected in each population varied, making the data unbalanced. This was particularly the case for the Kuriva site, where only four trees were collected; these trees were located in a highly managed recreational area, in contrast to the other sites. Given the low numbers and distinct environment, the Kuriva samples were not included in the statistical analysis, but they are presented in the tables and figures. The variation in the number of samples in remaining sites could be accommodated in the model. Pairwise statistical comparisons among sites for particular variables were determined using Tukey’s test. Correlations between continuous numerical traits were evaluated using Pearson’s correlation coefficient. Principal component analysis (PCA) was undertaken based on a correlation matrix using seven key oil components ((Z)-α-santalol, (Z)-β-santalol, a-trans-bergamotol, t-t,farnesol, (Z)-nuciferol, β-curcumen-12-ol and (Z)-lanceol). A statistical comparison of binomial data was undertaken using Fisher’s exact test. The analysis of binomial data referred to the presence or absence of certain characteristics such as heartwood rot, sun/fire scalding, heartwood check and seedlings and suckers. Data analysis was carried out using jamovi software (The jamovi project Citation2020) based on R-language (R Core Team Citation2019), and the general linear model analysis used the GAMLj module (Gallucci Citation2019).
Results
Tree and leaf morphology
Stem size and tree height
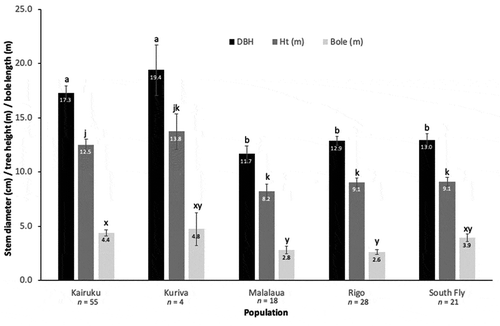
Trees from the Kairuku District had a statistically greater mean height than those from the Rigo, Malalaua and South Fly districts (). While trees from Kuriva had the greatest mean height, this group was statistically intermediate between the other two groupings, possibly because its small sample size (n = 4) means that comparisons involving this provenance are imprecise. Trees from the Kairuku and Kuriva districts had a statistically greater stem diameter (diameter at breast height – DBH) than those from the Rigo, Malalaua and South Fly districts, with no significant difference in mean diameter within each of these two groupings. The mean bole length of trees from the Kairuku District was statistically greater than that of trees from the Malalaua and Rigo districts. Trees from the Kuriva and South Fly districts were statistically intermediate between the other two groupings.
Figure 2. Mean stem diameter (DBH), tree height (Ht; m) and bole length (m) across five populations of Santalum macgregorii in southern Papua New Guinea
Tree form and stem taper
Across all populations, trees with forked trunks (65% of trees sampled) were the most prevalent form, followed by single-stemmed (28%), 2–3 primary stems (6%) and multistemmed shrubs (1%). Trees with single trunks were found to have a significantly (P > 0.05) greater bole length (4.8 m) but smaller canopy area (15.3 m2) than trees with forked trunks (3.4 m and 22.3 m2) and 2–3 primary stems (2.1 m and 26.6 m2). No difference in stem diameter (at 0.2 m) was found among the three tree forms (single – 17.2 cm, forked – 18.2 cm, and 2–3 stemmed – 20.0 cm). No significant difference was found between sites for stem taper, with stem diameter decreasing by 4.1 cm m−1 of stem between 0.2 m and 0.7 m above ground level (AGL) and by 1.8 cm m−1 between 0.7 m and 1.3 m. The high relative rate of taper between 0.2 and 0.7 m suggests that 0.2 m (where the first core was taken) can be considered part of the butt of the tree. Given the consistent stem taper between sites, no statistical difference was found for stem diameter at any of the three measurement points (0.2, 0.7 and 1.3 AGL).
Leaf morphology
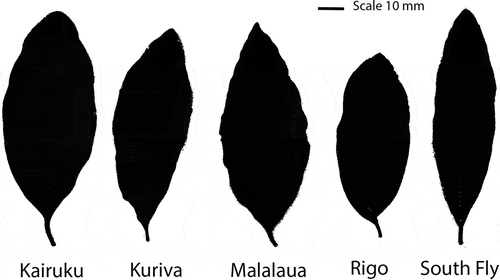
Significant variation in leaf morphology was found between sample sites (). Trees in Kairuku produced leaves with a significantly (P < 0.05) greater leaf area (2261 mm2) than each of Malalaua (1980 mm2) and Kuriva (1793 mm2), which in turn were significantly (P < 0.05) greater than each of Rigo (1499 mm2) and South Fly (1456 mm2). The leaves in Rigo were found to be significantly (P < 0.05) shorter in length compared with all remaining sites. The leaves in South Fly were significantly (P < 0.05) narrower in width compared with all remaining sites. South Fly trees were also found to be significantly different with the other sites based on the leaf shape measures of roundness and aspect ratio. Generally, leaves from South Fly trees were more narrow and elongated than the four eastern localities ().
Table 2. Leaf trait variation across five populations of Santalum macgregorii in southern Papua New Guinea
Heartwood
Heartwood percentage
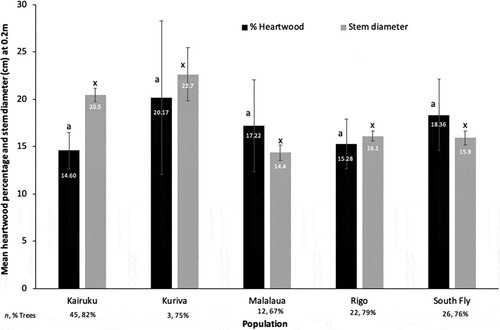
Overall, 78% of sampled trees contained between 14.6% (Kairuku) and 20.2% (Kuriva) (mean 15.9%) heartwood cross-sectional area percentage (). Stem diameter (at 0.2 m AGL) was moderately positively correlated with sapwood diameter (r = 0.62) but less so with heartwood diameter (r = 0.39) and not at all with heartwood percentage (r = 0.09). There was a poor correlation between stem diameter and heartwood percentage for both planted and natural trees. Excluding trees from the Kuriva District from the analysis (because the sample size was too small), diameter class was not a good indicator of heartwood percentage. Trees from the Kairuku District had the largest diameter and the lowest heartwood percentage, whereas trees from the South Fly District had the second-lowest average diameter but the highest heartwood percentage. Heartwood was found in all populations in trees with a stem diameter under 11 cm, with two containing <4% heartwood and two >20% heartwood. The lowest percentage of heartwood (<0.1%) was found in a tree with an 18.9 cm diameter, whereas the highest (61.4%) was in a 15.6 cm diameter tree.
Figure 4. Heartwood percentage in cores at 0.2 m that had heartwood present
Comparing the difference in heartwood percentage between planted and natural trees, planted trees in the Rigo District had twice as much heartwood as natural trees (24.8% vs 11.6%), natural trees in the Malalaua District had three times the heartwood as planted trees (40.1% vs 12.6%), and there was little difference in the Kairuku District, with natural trees containing 18.9% heartwood and planted trees 13.8%.
Heartwood oil chemistry
Mean heartwood oil content was recorded at less than 1% across all districts without any significant differences between districts (). Heartwood oil content was not correlated with any of the key oil constituents. Furthermore, stem diameter was not associated with oil constituents except for a weak correlation with (Z)-α-santalol (r = 0.36), (Z)-α-santalol (r = 0.31) α-trans-bergamotol (r = 0.38) and (Z)-lanceol (r = −0.28). Significant tree-to-tree variation was found for each of the six most abundant oil constituents (two constituents shown in ).
Table 3. Mean percentage for seven key oil constituents across five districts of Santalum macgregorii in Papua New Guinea
Figure 5. Tree-to-tree variation, by district, for area % (under peak for the compound in the gas chromotography-trace) (Z)-α-santalol (a) and (Z)-nuciferol (b). The 41% line represents the minimum (Z)-α-santalol required to meet the international standard for Santalum album oil
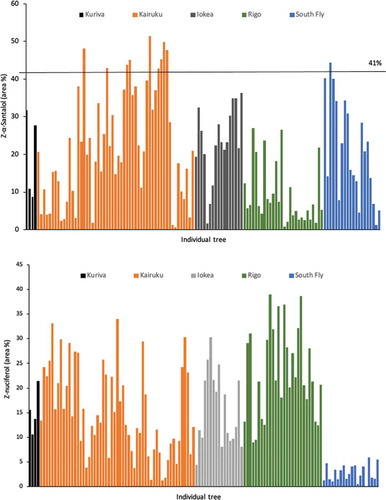
With only four heartwood cores sampled at Kuriva, it was not possible to detect statistical differences in percent mean oil components compared with other sites (). A significantly higher mean proportion of (Z)-α- and (Z)-β-santalol was found in Kairuku, Malalaua and South Fly compared with Rigo. The mean proportion of (Z)-nuciferol was significantly higher in Rigo and significantly lower in South Fly compared with Kairuku and Malalaua. For (Z)-lanceol, a significantly higher mean proportion was found in oil samples from South Fly compared with Kairuku, Malalaua and Rigo (). Low levels of farnesol were detected, ranging from a mean proportion of 0.5% in Kuriva to 2.1% in Rigo. A total of 31% of individuals across the sampled sites contained no farnesol.
There was a strong positive correlation between (Z)-α- and (Z)-β-santalol (r = 0.97) and the mean proportion of (Z)-β- to (Z)-α-santalol was 37%. Both these constituents were strongly correlated with α-trans-bergamotol and weakly to moderately negatively correlated with (Z)-nuciferol, β-curcumen-12-ol and (Z)-lanceol (). A much stronger negative correlation between (Z)-α-santalol and (Z)-lanceol was found for the South Fly samples (r = −0.77) compared with the other populations (r = −0.37). Similarly, a weaker negative correlation (r = −0.21) was found between (Z)-α-santalol and (Z)-nuciferol for South Fly compared with the other populations (r = −0.66). No association was found between any oil constituent and each of heartwood percentage and heartwood diameter.
Table 4. Correlation matrix for seven key sandalwood oil components
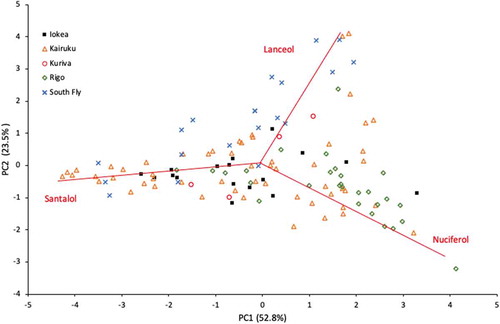
The PCA demonstrates that the proportions of (Z)-α-santalol and (Z)-nuciferol influences much of the variation in the first principal component (PC1) and (Z)-lanceol influences the second principal component (PC2) (). The Rigo samples cluster towards the (Z)-nuciferol end of PC1 and South Fly samples cluster along the PC2 according to their elevated levels of (Z)-lanceol. Kairuku, Malalaua and Kuriva are distributed across the PC1 based on their relative levels of (Z)-α-santalol and (Z)-nuciferol, but each site had some individuals influenced by (Z)-lanceol.
Figure 6. Two-dimensional principal component (PC) analysis ordination scores of seven key oil constituents explaining 76.3% of the total variance across 124 individual tree heartwood oil samples from five districts for Santalum macgregorii in Papua New Guinea. The oil constituents used in the analysis were (Z)-α-santalol, (Z)-β-santalol, a-trans-bergamotol, t-t,farnesol, (Z)-nuciferol, β-curcumen-12-ol and (Z)-lanceol
The proportions of commercially important components, (Z)-α- and (Z)-β-santalol, in individual trees ranged from 0.5% to 51% and from 0% to 24.2%, respectively, across all populations, and significant (P < 0.05) differences for each were found between individual populations. Continuous variation was found for all six main oil components: (Z)-α-santalol, (Z)-β-santalol, (Z)-nuciferol, (Z)-β-curcumen-12-ol, (Z)-lanceol and α-trans-bergamotol.
Pests and disease
Biological pests
No incidence of leaf galling, scale insects, root rot or leaf-eating caterpillars was found across all trees sampled in this study. An isolated pocket of trees in Ipaipana (Kairuku) had leaf-tying caterpillars (tiers), but this was not observed in any other area.
Heartwood rot
Heartwood rot affected 19.4% of sampled trees and, although there was some variation between populations (ranging from 12.5% to 33.3%), there was no statistical difference among them. Tree form influenced the prevalence of heartwood rot. A significantly (P < 0.05) greater proportion of trees with multiple stems (33%) had heartwood rot compared with forked (19%) and single-stemmed (16%) trees. No statistical differences in proportion of trees with heartwood rot were found among the latter two forms. The proportion of trees with trunk scald and heartwood check with heartwood rot was 33% and 26%, respectively, with the former, but not the latter, significantly higher than for all sampled trees combined (19%).
Human-induced damage
Damage to tree stems due to human action () was common, with evidence of bark slash (50%) and/or heartwood check (25%). Fire-scald damage was also common—found in 25% of surveyed trees.
Table 5. Incidence and severity score for bark slash, heartwood check and fire-scald scars found across five populations of Santalum macgregorii in southern Papua New Guinea. Severity score was determined through subjective evaluation on a scale of 1 (low) to 5 (high)
In the Central and Gulf Province locations (Kairuku, Malalaua and Rigo districts), 27–39% of trees had evidence of bark slash. In contrast, only 5% of trees in the South Fly District presented with bark slash. For those trees with bark-slash symptoms, the severity score was also lower for trees in the South Fly and Rigo districts (1.0–1.5) compared with trees in the Kairuku and Malalaua districts (2.7–2.8), although these differences were not significantly different. This was due mainly to the low number of trees affected in the South Fly and Rigo districts.
Heartwood check was most prevalent in the Kairuku, Malalaua and Rigo districts (27–39% trees affected), where a significantly (P < 0.05) greater proportion of trees in all three populations was affected compared with trees from the Kuriva (0%) and South Fly (5%) districts (). There was no difference in the severity of heartwood-check symptoms between any of the populations where this damage was found. The relative level of tree isolation (i.e. distance to village) had no consistent effect on the proportion of trees with heartwood check.
A significantly (P < 0.05) greater proportion of trees with fire scald was found in the sampled trees from the Rigo (54%) and South Fly (33%) districts compared with the Kairuku (15%), Kuriva (0%) and Malalaua (11%) districts (). Fire-scald damage was significantly (P < 0.05) lower in the Malalaua District (116 cm2) compared with the Kairuku (363 cm2), Rigo (534 cm2) and South Fly (467 cm2) districts. There were no significant differences between the three latter sites.
Reproduction
Phenology
All observed flowering trees in the South Fly District had white flowers, in contrast with all other populations, which had red flowers. No other differences were observed in floral form between the South Fly District and the other populations. Both bud and flower intensity in the South Fly District were statistically lower than in the Malalaua and Kairuku districts, and the Rigo District was intermediate between these groupings. No fruits were observed in the South Fly District, and the sample size in the Kuriva District was too small for comparison. There was no statistical difference in immature fruit abundance between the Malalaua, Kairuku and Rigo districts, but the Malalaua District had a greater abundance of mature fruits. Comparing planted against natural trees reveals that only one natural tree (n = 14) from the Kairuku and Malalaua districts had reproductive structures, compared with 71% (n = 59) of planted trees. In contrast, trees in the Rigo District had a high frequency of individuals with reproductive structures, regardless of their status (80% natural (n = 20), 87.5% planted (n = 8)).
Recruitment
Overall, recruitment was twice as likely to occur as vegetative suckers (42%) than as seedlings (21%) (). There was no seedling recruitment in the South Fly and Kuriva districts, and there was no significant difference in either the percentage of trees (14–29%) or the mean number of seedlings (10.4–13.25) () for the other three regions.
Table 6. Summary of seedling recruitment for five populations of Santalum macgregorii in southern Papua New Guinea
Table 7. Summary of root-sucker recruitment for five populations of Santalum macgregorii in southern Papua New Guinea
Discussion
This study clearly demonstrates significant variation within and between populations of S. macgregorii with regard to morphological and key heartwood and biochemical traits. The results represent a sample of trees of unknown age occurring in differing growing environments with variation in host species. Therefore, this study was unable to determine the relative contribution of genotype, environment, host or the interactions of these on the results. However, quantifying variation can be used to inform initial tree selection to provide germplasm for species domestication. Propagation of these selections and the establishment of replicated common garden trials can form the basis of quantifying genotypic variation in key commercial traits. This approach has been recommended for other sandalwood species, such as S. yasi (Bush, Thomson et al. Citation2020) and S. austrocaledonicum (Page et al. Citation2020).
The sampled sandalwood populations represent a point in time of available trees, and there is evidence that the populations have been modified by exploitative harvesting and opportunistic planting close to human settlements. The situation is different in the more isolated South Fly District, however, where the population was the least modified. Landowner discussions revealed that there has been no harvesting of S. macgregorii in the South Fly District in recent decades, and heartwood-check damage is rare. Despite this, mean tree diameter in the South Fly District was either equal to or lower than other sites where intensive harvesting has been a feature. This may be a reflection of differences in market potential between regions. For instance, in the South Fly District, little to no time is spent protecting trees that have no perceived value and that are affected by fire. In the eastern provinces (i.e. Central and Gulf), where there is an established market for sandalwood, there was evidence of active management of trees, particularly when they are proximate to settlements.
Heartwood percentage
The heartwood percentage varied considerably between trees and size classes, but there were no statistically significant patterns. Mean heartwood content of individual districts differed by <5% from the overall heartwood average of 15.9%. The contrasting results found for heartwood percentage between planted and natural trees across different sites is difficult to explain. Many planted trees are tended, and it is possible that they grow quicker with fewer biotic stresses than natural trees. Therefore, natural and planted trees with an equivalent size may be different with regard to age and, therefore, heartwood content may be greater in ‘older’ natural trees. However, there was no relationship between diameter and heartwood percentage for planted or natural trees, with examples of large natural individuals having little to no heartwood (e.g. 25.0 cm and 0.17%) and small planted individuals having high amounts of heartwood (e.g. 13.9 cm and 32.8%).
Increasing stem diameter positively influenced the diameter of heartwood in S. macgregorii, but this relationship only accounted for 15% of the variation. Despite this, no association was found between stem diameter and heartwood percentage. This result is consistent with findings for S. album and S. austrocaledonicum, where, despite a positive correlation between heartwood and stem diameter (r = 0.63 and 0.70 for the two species, respectively), percentage heartwood was weakly correlated with stem diameter in the former (r = 0.3) (Kumar et al. Citation2011) and varied independently of stem diameter in the latter (Page et al. Citation2010). Stem diameter was found to be positively correlated with heartwood weight (r2 = 0.88) in 16-year-old S. album trees in north-west Australia (Brand et al. Citation2012). In contrast, no such association was found between heartwood content and stem diameter in wild S. album in Sri Lanka (Subasinghe et al. Citation2017).
Kumar et al. (Citation2011) suggested that any association between stem diameter and heartwood percentage is likely to get stronger with tree age. The mean basal (20–50 cm) stem diameter growth rate across several species of sandalwood, including S. austrocaledonicum, S. insulare and S. yasi, is around 1 cm y−1 (Butaud Citation2010; Page et al. Citation2012; Bush, Thomson et al. Citation2020). Assuming that S. macgregorii has a similar growth rate, the sampled trees may range in age from 7 to 27 years. The width of the sapwood band is likely to reflect the size of the crown and may vary over time, depending on prevailing environmental conditions and crown vigour. The heartwood is accumulated over time, so its proportion increases relative to that of the sapwood. Genotype-by-environment interactions may influence the timing of onset for heartwood. In S. album, the onset of heartwood development can vary: 10–13 years in India (Rai Citation1990), 16 years in northern Western Australia (Brand et al. Citation2012) and 14–46 years in Timor (Haffner Citation1993).
In wild trees of S. album sampled in West Timor, where mean stem diameters were much larger (DBH 22.5) than those of trees sampled in this study (DBH 11.7–19.4 cm), a moderate correlation (R2 = 0.51) was found between stem diameter and heartwood percentage (Fox et al. Citation1994). Mean percentage heartwood in the West Timor trees (17–42% at 1.3 m) was also higher than that for S. macgregorii in this study (14.6–20.2% at 0.2 m). Compared with S. austrocaledonicum sampled in Vanuatu, the S. macgregorii trees in this study were of similar mean diameter (approx. 15–22 cm at 0.15 m for S. austrocaledonicum and 14.4–22.7 cm at 0.2 m for S. macgregorii), but S. macgregorii had a lower heartwood percentage (14.6–20.2% compared with 19–32% for S. austrocaledonicum). In 44 individual wild tree samples of S. yasi, Bush, Brophy et al. (Citation2020) reported a mean heartwood proportion of 23% in trees ranging in diameter from 7.0 cm to 26 cm at 0.3 m AGL. In the present study, the sampled trees were quite small, with possible variation in heartwood initiation at the sample points. Genetic and environmental variations are also likely to have a significant influence on heartwood development in sandalwood.
Heartwood oil chemistry
The heartwood oil of S. macgregorii is characterised by extreme tree-to-tree variation in key fragrant compounds. Concentrations ranged from negligible to significant proportions of the total oil for (Z)-α-santalol (0.5–51%), (Z)-β-santalol (0–24.2%), nuciferol (0–40.5%) and lanceol (0–72%). Although there were provenance-related differences in the mean values between populations, the high variation within sites means that selection needs to be undertaken on the basis of individual trees. The Kairuku population had the highest proportion of individuals with elevated levels of the fragrant santalols, with 16% of trees meeting the international standard for santalols set for S. album (ISO Citation2002). Only one other individual (from South Fly) was found to meet the international standard. This study demonstrates that relative santalol content in this species is highly variable between individual trees, which may be a contributing factor to the low market value of S. macgregorii (Thomson Citation2020). Similar variation in santalol content was described between individuals and populations for S. austrocaledonicum (Page et al. Citation2010). The mean value for both (Z)-α-santalol (19.2%) and (Z)-β-santalol (7.3%) for S. macgregorii in this study was found to be lower than for S. yasi (29.5 and 18.1%, respectively) (Bush, Brophy et al. Citation2020) and S. album (44–50% and 18–20%, respectively) (Brand et al. Citation2012; Subasinghe et al. Citation2017).
The PCA ordination and contrasting associations among constituents between populations found in this study demonstrate that trees from the eastern provinces were influenced by the level of (Z)-nuciferol, whereas trees from the Western Province site (South Fly) were more strongly influenced by (Z)-lanceol. A small number of individuals from Central and Gulf provinces had elevated levels of (Z)-lanceol, but no individuals from Western Province had elevated levels of (Z)-nuciferol. It appears that S. macgregorii comprises both (Z)-nuciferol-rich and (Z)-lanceol-rich individual trees. Brophy et al. (Citation2009) made similar findings among 14 S. macgregorii trees sampled in Central, Gulf and Western provinces, recording higher levels of (Z)-nuciferol in Central and Gulf provinces. Brophy et al. (Citation2009) detected farnesol only in the eastern provinces, but, in our study, farnesol was present in about 69% of trees across all sites. Farnesol is a recognised allergen in cosmetics (Baldovini et al. Citation2011), and so the low levels (0.5–2.1%) found in this study is positive. Continuous variation was found for all six major oil constituents, thus discounting any presence of chemotypes in this sample of S. macgregorii, which is again consistent with Brophy et al. (Citation2009) for the same species.
The relatively low levels of heartwood oil content detected in our study was due to the solvent extraction method, which underestimates heartwood oil yield, combined with included transition wood to ensure an adequate sample size for analysis. The results are indicative of the relative content among samples, and no significant differences were found between localities for oil content. In studies of oil concentration in S. yasi (Bush, Brophy et al. Citation2020) and S. austrocaledonicum (Page et al. Citation2010), solvent extraction gives lower estimates than steam extraction, suggesting that solvent extraction is not representative of commercial yields.
Heartwood rot
Heartwood rot is a considerable problem that significantly reduces the volume of heartwood within a tree. All the sampled trees in our research would be considered to be unmanaged from the standpoint of limiting heartwood rot. This has implications for woodlots, where almost one in every five trees may be expected to have heartwood rot if they are not managed. Heartwood rot can be introduced into the tree through damage to the bark that permits the entry of water and other biological agents into the main stem. This can be due to the direct action of people (e.g. heartwood check) and fire or other biotic (pest) or abiotic (wind) damage to the branches and trunk. In this study, we found that trees with trunk scald (fire/sun) had an elevated level of heartwood rot relative to the rest of the population. Interestingly, trees with heartwood-check damage had levels of heartwood rot that were similar to all other sampled trees. Given the severity of both trunk scald and heartwood check, it was surprising that many (75%) trees did not have symptoms of heartwood rot at the base (0.2 m). Within S. album plantings in Australia, Barbour et al. (Citation2010) found that wood-rot fungi entered the trees either by the roots or where bark protection was lost through mechanical damage or trunk scald. In the present study, we also found heartwood rot in outwardly healthy trees. The prevalence of heartwood rot in sandalwood trees growing in agroforestry systems is an area for further research.
Pests and disease
Fire is the second-biggest risk (after harvesting) to mature trees in the wild; nevertheless, the trees themselves are quite robust and can survive as annual root suckers after fire. In this study, it was not uncommon to find only small suckers in grasslands, and trees above waist height were found predominantly in fire-protected landscape positions (e.g. gullies, the tops of high ridges, and riparian vine thickets). The presence of larger trees in grasslands is attributable to the efforts of landowners in tending the trees by clearing adjacent grass and thereby protecting them from annual fires.
A high incidence of bark slashing was found in S. macgregorii populations located close to or within villages (Malalaua and Kairuku districts) or in public areas (Kuriva District) where there was considerable human activity. In contrast, there was a low incidence of bark slashing in trees located in areas that were more distant from villages with a relatively low level of human activity (Rigo and South Fly districts). The prevalence of fire-damaged trees was greater in isolated areas (Rigo and South Fly districts). This higher frequency of fire damage was probably due to the lower management of fires in these isolated areas compared with village and garden areas. The severity of fire scald was lower in Malalaua than in all other sites; most of the trees in Malalaua were located in village ornamental plantings and home gardens, where there was active fire management.
No relationship was found between distance to village and the amount of heartwood-check damage. Trees, therefore, do not necessarily have a reduced risk of damage because of either their closeness to villages or their isolation. There was a low percentage and level of heartwood check in South Fly, which may be due to the lack of active buyers in the area and therefore a low incentive for people to check for heartwood. Heartwood-check damage can be caused by tree owners, as has been found in Sri Lanka (Subasinghe et al. Citation2017); in PNG, however, owners report that it is likely performed by prospective thieves.
Reproduction and recruitment
Phenology data provide single-point-in-time records that may only be indicative of seasonal conditions at the time of sampling. It is interesting to note, however, that all types of reproductive structures were observed in the Kairuku, Malalaua and Rigo districts, suggesting that several environmental cues may induce flowering in this species. The higher propensity of reproduction found in cultivated specimens in the Kairuku and Malalaua districts suggests that water, possibly nutrition, and the diversity of host plants near villages could have a positive influence on seed production. There may also be a tendency for selection by humans of reproductively fecund individuals—if the trait is heritable, the planted population will, on average, be more fecund. Flower colour was red in all populations except the South Fly District (which was white), supporting the similar observations of Bosimbi (Citation2006).
Landowners in the South Fly District reported that they had never observed mature fruits, and none was observed in this survey. Although flowering was observed on 14 trees (64%), it was sparse (only one or two inflorescences per tree) on all but one tree. This could reflect seasonal variation, but, coupled with no observed seedlings, and the fact that no landowners had ever observed fruits, it is indicative of reproductive failure in this population. Sexual reproductive failure, low fruit production, and poor seed viability have been recorded in several other species of sandalwood (Ratnaningrum et al. Citation2017); in S. lanceolatum, such failure was related to population fragmentation and clonal reproduction, combined with self-incompatibility and pollen sterility (Warburton et al. Citation2000; Brunton Citation2019; Lee et al. Citation2019).
Because of their harvesting and fire practices, people are the primary cause of sandalwood decline in PNG, but they are also the key to sandalwood survival and income generation through tree farming. In Central and Gulf provinces, resource owners suggested that most of the mature trees were removed in the early 2000s when there were high levels of trade (Rome et al. Citation2020). With competition for resources and difficulties in protecting trees from fire, heartwood checking and poaching, resource owners are establishing trees within and near to their villages to afford some level of protection. This practice is reflected in the results of this survey, which revealed that over half (56%) of the sampled trees were cultivated, and this was as high as 80% in the Kairuku and Malalaua districts. Every effort was made to survey natural trees, and the figures therefore reflect the very low numbers of trees growing beyond village areas in more natural settings. In the Rigo District, most sampled trees (72%) were located in natural vegetation beyond immediate village areas, with many having regenerated as root suckers from previously harvested trees. Although sandalwood trees were found within village areas, they were too small to be included in this study. The South Fly District was unique in that the trees were largely undisturbed, with only two examples of villagers have moved them to village areas.
Clonal recruitment through root suckering was found to be a prominent form of reproduction in S. macgregorii across all study sites. Seedling recruitment occurred in the populations of Central and Gulf provinces (approx. 20% of trees had approx. 10 seedlings each), but not in the isolated population in Western Province. Most (approx. 80%) seedlings were within 5 m of a mature seed tree and were generally less than 1 m in height. Very few saplings were taller than 1 m or occurred beyond the seed trees. With frequent grass fires in natural sandalwood areas (i.e. beyond village areas), seedling recruitment was minimal throughout the sampled populations of S. macgregorii.
Speciation and domestication
Sandalwood trees in Western Province were distinct from those in the eastern sites for oil composition, leaf shape, flower colour and potential reproductive failure. Trees in Western Province are geographically closer to populations of S. lanceolatum in Cape York Peninsula (approx. 250 km) than to the other PNG populations of S. macgregorii (approx. 450 km), and the species is reported to occur on some Torres Strait islands. It is possible that the trees in Western Province form part of a cryptic species also occurring in Cape York Peninsula, or that both are S. lanceolatum. Although the phenotypic features do not necessarily discriminate a new species, molecular genetic research is required to test the hypothesis. Determining the sandalwood species within the region will inform strategies for their conservation and domestication. If the Western Province population turns out to be a different species (i.e. not S. macgregorii), an improvement program could potentially generate hybrids between the two. Alternatively, it may be important for the Western Province population to be infused with germplasm from the trees in Cape York Peninsula. Either way, the Western Province population appears to be in need of genetic infusions to reinvigorate its reproductive fecundity and conserve its remaining natural stands.
Landowners in PNG are actively managing trees and are interested in establishing agroforestry sandalwood plantings (Rome et al. Citation2020). Domestication programs can help develop improved germplasm to support these initiatives. This study provides a broad evaluation of heartwood characters for S. macgregorii and identified heartwood oil variation as the basis for selecting high-quality trees. A total of 36 trees had (Z)-α-santalol concentrations of 30–51% and (Z)-β-santalol of 9–24%. These trees are representative of four populations: Kairuku (20 individuals), Kuriva (1), Malalaua (5) and South Fly (10). The top five individuals in Rigo had concentrations of 23–26% of (Z)-α-santalol and 8–12% of (Z)-β-santalol and may be included among selected individuals to maximise genetic diversity.
The clonal propagation of the top 36 trees, plus potentially five individuals from Rigo, and their establishment as a clonal archive, could provide the nucleus for the initial domestication of this species. The mean concentration of the 41 selected individuals was 36.7% (Z)-α-santalol, 14.4% (Z)-β-santalol, 8.4% α-trans-bergamotol, 7.8% (Z)-nuciferol, 4.3% (Z)-lanceol and 0.6% t-,t-farnesol. The level of (Z)-α-santalol is equivalent to the breeding objective for S. austrocaledonicum, with Page et al. (Citation2020) suggesting a range of 30–35%. Although this clonal approach may lead to genetic gain, it assumes that oil quality remains relatively stable as trees age and is heritable in subsequent generations. Given that these assumptions are yet to be established for any sandalwood species, there would be value in making single-tree seed collections to form the basis of replicated provenance-progeny trials. Such trials, which could comprise collections from the 41 selected individuals, as well as random collections, would help determine the relative contributions of genotype, environment and host on the expression of key commercial traits such as heartwood percentage, oil concentration and oil quality.
The mean heartwood percentage (0.2 m) of the selected trees was 14.2%, which is similar to that for all sampled trees (15.8%). Therefore, independent selection of heartwood-percentage and oil-quality traits is required for their simultaneous improvement. Of the trees selected for high oil quality, the top 20 of these for heartwood percentage had a combined mean of 22.3% and included individuals from each of the sample sites (Malalaua 2, Kairuku 11, Malalaua 1, Rigo 1 and South Fly 5). The selection of up to 41 individuals is likely to support improvements in the short term, but long-term domestication will rely on further surveys to identify additional selections.
Conclusion
This study provides the most comprehensive evaluation of phenotypic and heartwood oil quality for S. macgregorii achieved to date. Significant variation in oil composition was found within and between sample sites. Across all sampled sites, the tree-to-tree variation in the (Z)-α- and (Z)-β-santalol ranged from 0.5% to 51% and from 0% to 24.2%, respectively, with a strong positive correlation between them (r = 0.97). Each of these compounds was negatively correlated with (Z)-nuciferol for sites in Central and Gulf provinces and (Z)-lanceol for the site in Western Province, giving a clear distinction in oil profiles between these areas. A total of 79% of the surveyed trees with a basal diameter of >10 cm had heartwood. Mean heartwood percentage was 15.8% of the basal area, with no significant difference between sites.
The trees in Western Province were found to be phenotypically distinct from those in the eastern sites and may represent a new cryptic species and/or a relationship with S. lanceolatum in Cape York Peninsula. Further molecular genetic work is recommended to determine the relationships between S. lanceolatum and S. macgregorii to support efforts in the conservation and domestication of both species.
It is clear that the distribution of S. macgregorii has been heavily modified by the actions of people through harvesting, fire, assisted natural regeneration and tree planting. This was particularly evident in two districts (Kairuku and Malalaua) where there was a high incidence of trees within or proximal to settlements. This study has demonstrated that people are interested in managing the PNG sandalwood resource to increase tree numbers. Potential exists to further support communities in their endeavours to manage and restore these resources through participatory domestication and agroforestry production.
Acknowledgements
This study was conducted with the generous support of the Australian Centre for International Agricultural Research (FST/2014/069). The study acknowledges the support of landowners in PNG, who agreed to participate in the study and assisted the research team in locating sandalwood trees. PNG Forest Authority staff members who supported and assisted this study include Ruth Turia, Wake Yelu, Dambis Kaip, Jacob Pange, Jason Oa, Jason Wapi, Keina Kaivisi and Markop Kil. Recognition to Cameron and Austen Page for conducting leaf image analysis. Thanks to Richard Burns and John Doran, and two anonymous reviewers, who provided critical reviews of the manuscript.
Disclosure statement
The communicating author has a small sandalwood planting of fewer than 100 Santalum album.
Additional information
Funding
References
- Applegate GB, Chamberlain J, Feigelson J, Hamilton L, McKinnell FH, Neil PE, Rai SN, Statham PC, Stemmermann L. 1990. Sandalwood in the Pacific: a state-of-knowledge synthesis and summary from the April 1990 symposium. In: Hamilton L, Conrad CE, editors. Proceedings of the Symposium on Sandalwood in the Pacific; 1990 Apr 9 –11; Honolulu, Hawaii. Berkeley (CA): USDA Forest Service; p. 1–11.
- Baldovini N, Delasalle C, Joulain D. 2011. Phytochemistry of the heartwood from fragrant Santalum species: a review. Flavour and Fragrance Journal. 26:7–26. doi:10.1002/ffj.2025.
- Barbour L, Norris L, Burgess T. 2010. Heartwood rot identification and impact in sandalwood (Santalum album). Canberra (Australia): Rural Industries Research and Development Corporation.
- Bosimbi D. 2006. Sandalwood research, development and extension in Papua New Guinea. Bulolo (PNG): National Tree Seed Centre.
- Bosimbi D, Bewang IF. 2007. Country papers: Papua New Guinea. In: Thomson L, Bulai P, Wilikibau B, editors. Proceedings of the regional workshop on sandalwood research, development and extension in the Pacific Islands; 2005 Nov 28–Dec 1; Nadi, Fiji. Suva (Fiji): Secretariat of the Pacific Community, Australian Agency for International Development Assistance and German Agency for Technical Cooperation; p. 63–70.
- Brand JE, Norris LJ, Dumbrell IC. 2012. Estimated heartwood weights and oil concentrations within 16-year-old Indian sandalwood (Santalum album) trees planted near Kununurra, Western Australia. Australian Forestry. 75:225–232. doi:10.1080/00049158.2012.10676406.
- Brophy JJ, Goldsack RJ, Doran JC, Niangu M. 2009. Heartwood oils of Santalum macgregorii F. Muell. (PNG Sandalwood). Journal of Essential Oil Research. 21:249–253. doi:10.1080/10412905.2009.9700161.
- Brunton A. 2019. Population genetics of Santalum lanceolatum (Northern sandalwood) [honours thesis]. Sunshine Coast (Australia): University of the Sunshine Coast.
- Bush D, Brophy J, Bolatolu W, Dutt S, Hamani S, Doran J, Thomson L. 2020. Oil yield and composition of young Santalum yasi in Fiji and Tonga. Australian Forestry. 83(4):238–244.
- Bush D, Thomson LAJ, Bolatolu W, Dutt S, Hamani S, Likiafu H, Mateboto J, Tauraga J, Young E. 2020. Domestication provides the key to conservation of Santalum yasi – a threatened Pacific sandalwood Australian Forestry. 83(4):186–194.
- Butaud J-F. 2010. Conservation strategy for sandalwood (Santalum insulare) in French Polynesia and findings after 10 years of its implementation. In: Thomson L, Padolina C, Sami R, Prasad V, Doran J, editors. Sandalwood Resource Development, Research and Trade in the Pacific and Asian region. Proceedings of the regional workshop; 2010 Nov 22-25; Port Vila, Vanuatu. Suva (Fiji): Secretariat of the Pacific Community, James Cook University and the Australian Centre for International Agricultural Research; p. 46–56.
- Dawson IK, Leakey RRB, Clement CR, Weber JC, Cornelius JP, Roshetko J, Barbara V, Antoine K, Tchoundjeu Z, Masters E, et al. 2014. The management of tree genetic resources and the livelihoods of rural communities in the tropics: non-timber forest products, smallholder agroforestry practices and tree commodity crops. Forest Ecology and Management. 333:9–21.
- Doran JC, Thomson L, Brophy JJ, Goldsack B, Bulai P, Faka’osi T, Mokoia T. 2005. Variation in heartwood oil composition of young sandalwood trees in the south Pacific (Santalum yasi, S. album and F1 hybrids in Fiji, and S. yasi in Tonga and Niue). Sandalwood Research Newsletter. 20:3–7.
- Eddowes PJ. 1998. Santalum macgregorii. [accessed 2014 Sep 2]. Available from: http://www.iucnredlist.org
- Fick S, Hijmans R. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology. 37(12):4302-4315.
- Fox JED, Brand J, Barrett DR, Effendi M. 1994. Heartwood and tree size in Santalum album in Timor, Indonesia. In: Gjerum L, Fox JED, Ehrhart Y, editors. Sandalwood Seed Nursery and Plantation Technology: Proceedings of a Regional Workshop for Pacific Island Countries. Noumea (New Caledonia): CIRAD Foret, Australian Centre for International Agricultural Research and Food and Agricultural Organization; p. 193–208.
- Gallucci M. 2019. GAMLj: general analyses for linear models. [jamovi module]. Available from: https://gamlj.github.io/.editors
- Gunn BG, Bewang IF, Bunn Y. 2002. A strategy for conserving, managing the genetic resources of Santalum macgregorii (PNG sandalwood) in Papua New Guinea. Canberra (Australia): CSIRO Forestry and Forest Products.
- Haffner D. 1993. Determining heartwood formation within Santalum album and S. spicatum. Sandalwood Research Newsletter. 1:4–5.
- Harbaugh DT, Baldwin BG. 2007. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. American Journal of Botany. 94:1028–1040. doi:10.3732/ajb.94.6.1028.
- ISO. 2002. 3518:2002(E) Oil of sandalwood (Santalum album L.). Geneva (Switzerland): International Organization for Standardization.
- Kiapranis R. 2012. Papua New Guinea. In: Thomson L, editor. Regional Workshop on Sandalwood Resource Development, Research and Trade in the Pacific and Asian Region; 2010 Nov 22; Port Vila, Vanuatu. Suva (Fiji): European Union, Secretariat of the Pacific Community, James Cook University and the Australian Centre for International Agricultural Research; p. 27–31.
- Kumar ANA, Srinivasa YB, Joshi G, Seetharam A. 2011. Variability in and relation between tree growth, heartwood and oil content in sandalwood (Santalum album L.). Current Science. 100:827–830.
- Leakey RRB, Weber JC, Page T, Cornelius JP, Akinnifesi FK, Roshetko J, Tchoundjeu Z, Jamnadass R. 2012. Tree domestication in agroforestry: progress in the second decade (2003–2012). In: Nair PKR, Garrity D, editors. Agroforestry – the future of global land use. Berlin: Springer; p. 145–174.
- Leakey RRB. 2019. From ethnobotany to mainstream agriculture: socially modified Cinderella species capturing ‘trade-ons’ for ‘land maxing’. Planta. 250:949–970.
- Lee DJ, Burridge A, Brunton A, Conroy GC, Ogbourne SM, Thompson N. 2019. Selection, breeding and development of Cape York Sandalwood. Paper presented at the Sandalwood Regional Forum; 2019 Nov 11 –13; Port Vila, Vanuatu.
- Miller AJ, Gross BL. 2011. From forest to field: perennial fruit crop domestication. American Journal of Botany. 98:1389–1414.
- Page T, Southwell I, Russell M, Leakey RRB. 2007. Evaluation of heartwood and oil characters in seven populations of Santalum lanceolatum from Cape York. In: Thomson L, Bulai S, Wilikibau B, editors. Regional Workshop on Sandalwood Research, Development and Extension in the Pacific Islands and Asia; 2005 Nov 28–Dec 1; Nadi, Fiji. Suva (Fiji): Secretariat of the Pacific Community, Australian Agency for International Development Assistance, German Agency for Technical Cooperation; p. 131–136.
- Page T, Doran J, Tungon J, Tabi M. 2020. Participatory domestication strategy for Santalum austrocaledonicum in Vanuatu. Australian Forestry. 83(4):216–226.
- Page T, Southwell I, Russell M, Tate H, Tungon J, Sam C, Dickinson G, Robson K, Leakey RRB. 2010. Geographic and phenotypic variation in heartwood and essential oil characters in natural populations of Santalum austrocaledonicum in Vanuatu. Chemistry & Biodiversity. 7:1990–2006. doi:10.1002/cbdv.200900382.
- Page T, Tate H, Tungon J, Tabi M, Kamasteia P. 2012. Vanuatu sandalwood: growers’ guide for sandalwood production in Vanuatu. Canberra (Australia): Australian Centre for International Agricultural Research.
- R Core Team. 2019. R: a language and environment for statistical computing. (Version 3.6) [Computer software]. Available from: https://cran.r-project.org/.editors
- Rai SN. 1990. Status and cultivation of sandalwood in India. In: Hamilton L, Conrad CE, editors. Proceedings of the Symposium on Sandalwood in the Pacific; 1990 Apr 9-11; Honolulu, Hawaii. Berkeley (CA): USDA Forest Service; p. 66–71.
- Ratnaningrum YWN, Indrioko S, Faridah E, Syahbudin A. 2017. Gene flow and selection evidence of sandalwood (Santalum album) under various population structures in Gunung Sewu (Java, Indonesia), and its effects on genetic differentiation. Biodiversitas. 18:1493–1505
- Rome G, Turia R, Oa L, Page T, Applegate G, Saliau C. 2020. Sandalwood development in Papua New Guinea. Australian Forestry. 83(4):208–215.
- Simons AJ, Leakey RRB. 2004. Tree domestication in tropical agroforestry. Agroforestry Systems. 61–2:167–181.
- Subasinghe SMCUP, Samarasekara SC, Millaniyage KP, Hettiarachchi DS. 2017. Heartwood assessment of natural Santalum album populations for agroforestry development in Sri Lanka. Agroforestry Systems. 91:1157–1164. doi:10.1007/s10457-016-0001-5.
- The jamovi project. 2020. Jamovi (Version 1.2) [Computer Software]. Available from: https://www.jamovi.org.editors
- Thomson LAJ. 2020. Looking ahead – global sandalwood production and markets in 2040, and implications for Pacific Island producers. Australian Forestry. 83(4):245–254.
- Warburton CL, James EA, Fripp YJ, Trueman SJ, Wallace HM. 2000. Clonality and sexual reproductive failure in remnant populations of Santalum lanceolatum (Santalaceae). Biological Conservation. 96:45–54. doi:10.1016/S0006-3207(00)00049-5.