Introduction
In North County Dublin, Ireland, a region that has a relatively self-contained beekeeping regime with little movement of colonies or queens into the area, tolerance (co-adaptation) to varroa mites is becoming established. This has occurred without any mite treatment since 2010, and no bee breeding or special colony selection. The region is bounded by the Irish Sea to the east, counties Meath and Kildare to the west and the river Liffey and the inner suburbs of Dublin City to the south (Figure ). It has a strong Viking history and by 841 AD it was part of the Scandinavian settlement in Dublin. The Viking power was curtailed after the Battle of Clontarf in 1014 but today many of the surnames and place names are of Scandinavian origin. It is a fertile and relatively flat land about 25 miles (40 km) long and 15 miles (25 km) wide. Beekeeping has a long history in the region, and was reputed to have been introduced by a seventh century monk, St. Molaga, who built a church, Lann Beachaire (the Church of the Beekeeper) near Balbriggan, in North County Dublin. An arch stone dated 1689 in the adjacent Bremore Castle depicts a monk holding a skep or a bell with bees flying towards it (Figure ).
Varroa Infestation
In temperate locations untreated colonies will typically die three to four years after being infested with varroa for the first time (Büchler, Citation1994). From an early stage of varroa-mite infestation in Western Europe and North America, treatments were available that were effective in controlling mite numbers. Annual treatments of colonies on one or more occasions are now the norm though mite levels are still high. Varroa-mite infestation and its accompanying viruses are generally considered nowadays to be an important factor globally in honey bee colony deaths (Rosenkranz, Aumeier, & Ziegelmann, Citation2010). The varroa mite was first identified in Ireland in 1998 and by September 2003 it had spread to North County Dublin. It was found and identified in the author’s apiary in the centre of the county, north of Swords (Figure ) and was the result of heavily infested colonies being moved adjacent to the apiary from a varroa-infested region in the south east of the country. The strangeness of mite threat to the honey bees can be gauged when the initial response of the most affected colony was to build a thick screen of propolis across the hive entrance leaving only a small entry. Thereafter, infested colonies died out unless treated, which in the region was generally with a thymol-based product.
Beekeeping Regime in the Region
Migratory beekeeping is not common and overall there is limited movement of colonies into or within the region. An exception has been a low-level introduction of native honey bee queens Apis mellifera mellifera (Galtee) from strains in County Tipperary, particularly in the late 1990s and early 2000s. In fact, the large increase in beginner-beekeepers in the past decade in North County Dublin has been facilitated through a mentoring system that involves experienced beekeepers in the local association (Fingal North Dublin BKA) mentoring and providing startup-colonies from their own stocks (McMullan, Citation2012). This policy decision by the association was taken to reduce transmission of disease, specifically American Foulbrood, and also to restrict the introduction of non-native strains of Apis mellifera into the area. This ecological arrangement of a fairly settled population of colonies, where colony density is not high and colonies raise their own replacements, should yield reduced virulence in their parasites through vertical transmission, i.e., via the offspring (Fries & Camazine, Citation2001). This is the classic response to infestation by an exotic parasite in the absence of external treatments.
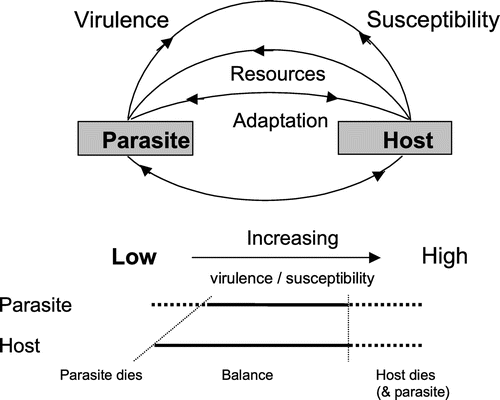
A demonstration of how this can occur is given in Figure . A parasite depends on its host for its existence and hence takes resources (nourishment) from the host, with virulence being its capacity to harm its host; on the other hand the susceptibility of the host being the likelihood of it being infested/infected by the parasite. According to Schmid-Hempel and Koella (Citation1994), “Variability in the host-parasite interactions has considerable impact on the ecology and evolution of parasites and on the epidemiology of disease”. This variability allows adaptation to occur. At low levels of parasite virulence and low levels of host susceptibility the parasite will be unable to sustain its existence and will die. At the other extreme, when virulence and susceptibility are high, the parasite can kill its host and both host and parasite die. This is the typical outcome when an exotic parasite, one that the host species has not encountered before, infests the host.
Figure 3. Schematic of parasite-host interaction. The diagram shows how a balance (or tolerance) can arise due to variability in parasite virulence and host susceptibility (from McMullan, Citation2012). The initial infestation of colonies by varroa mites will typically start on the right with colonies dying due to high mite virulence and low honey bee resistance (high susceptibility). Over time, in untreated colonies, reducing mite virulence and increasing honey bee resistance will be selected resulting in a balanced relationship.
By 2010 a more benign mite/honey bee relationship was becoming evident in the region. It became apparent that in colonies where annual varroa treatments were missed, typically where inclement weather late in the season prevented treatments, few adverse effects were observed. It is over seven years since I have given any treatments to my bees and increasingly fewer beekeepers in the region treat their colonies to control mites. By May 2017, almost two thirds had adopted a non-treatment approach. Colonies typically raise their own queens, as in the case of the parent hive in a swarming colony, after the beekeeper has removed a second colony with the old queen that then remains within the region. The large increase in beginner-beekeepers (Association membership has more than doubled since 2010) and the policy of providing starter colonies from within the region have resulted in a large increase in colonies, with almost all of them arising from within. In effect, the complex interactions between parasites (mites and the diseases they transmit) and their bee hosts are left to themselves to find their evolutionary path to tolerance (Blacquière & Panziera, Citation2018). These conditions are similar to those experienced by feral honey bees that have resulted in various populations of varroa-infested bees that survive without treatments (Fries, Imdorf, & Rosenkranz, Citation2006; Le Conte et al., Citation2007; Locke, Citation2016; Seeley, Citation2007).
Changes in Honey Bee Colonies
Beekeepers in this region have observed changes in the bee population subsequent to the arrival of varroa mites. Initially signs of Deformed wing virus (DWV) were commonly observed during hive inspections with groups of DWV-damaged bees in late summer crawling on the ground in front of the hives. These signs have decreased over time and today it is unusual to see signs of DWV. Other changes in colonies in North County Dublin have been observed over the period; more drones and more drone brood are located towards the centre of the brood nest, and colony winter mortality has declined from 15.8 to 11.2%, 2014–2017 using COLOSS data extracted for the North County Dublin region. The mortality rate for Ireland as a whole in 2015/16 was 29.5% (Brodschneider et al., Citation2016). Typically throughout the beekeeping world the initial infestation by the varroa mites resulted in rapidly increasing mite populations, sometimes dramatic, leading to a collapse of the colony (Büchler, Citation1994). In a tolerant or balanced relationship between mite and host honey bee it would be expected that while the mite population levels would fluctuate within each season, the levels at the same time each year would be similar. During the transition to a tolerant relationship, hosts and parasites may have undergone changes in biological and behavioural characteristics. Damage to mites may be contributing to this tolerance. Also, it has been shown that one or two regular dorsal dimples can occur on the mite’s idiosoma that can be considered as “defects” rather than “damage” caused by the bees (Davis, Citation2009). A study by Lodesani, Vecchi, Tommasini, and Bigliardi (Citation1996) showed that the presence of these regular dorsal dimples in the mother mite resulted in a 35.5% absence of first daughters compared with a 14.5% absence when no dimple defect was present. Lodesani et al. (Citation1996) also demonstrated that there was no difference between the prevalence of dimples on mites in the brood cells and mite-falls on the floor. Changes to the sealed-brood period of the honey bee would affect the time available to the varroa mites to develop in the brood cells and is therefore critical to the reproduction of female mite offspring (Büchler & Drescher, Citation1990). Also, the pupation time will be influenced by the brood-nest temperature and in this regard it has been demonstrated that a threat in the form of a parasite or pathogen will elicit a response in the colony in the form of a brood-temperature rise (Hou, Li, Deng, & Diao, Citation2016; Starks, Blackie, & Seeley, Citation2000).
Study Undertaken during 2016 and 2017
During 2016 and 2017 a study was undertaken to improve our understanding of the dynamics involved by identifying changes since the arrival of varroa mites over 14 years ago in North County Dublin. These changes may help to identify possible reasons for the mite tolerance that is being demonstrated. The study was undertaken during the period April 2016 to May 2017. The honey bees were A. m. mellifera from the author’s five-colony apiary in the middle of North County Dublin, and had not received any miticide or other treatments since autumn 2010. All colonies were in hives with “solid floors” and mesh between floor and brood box, i.e., floors were not “open mesh”. The hives were “modified commercial” that had a single brood-box comb-area approximately 20% greater than in a Langstroth brood-box. During April/May 2016 the colonies built up rapidly and reached an average of over 8 frames of brood in the 11 frame brood boxes. The main nectar flows were in April to mid June from spring flowers and tree blossom. There was a gap in the nectar flow in July/August and this corresponded with a drastic reduction in colony brood in the period. Later, from mid September to mid November 2016, strong flows mainly from ivy (Hedera helix) produced large populations of “winter” bees. Three of the colonies produced swarm queen-cells and re-queened during May/June 2016 and the nuclei with old queens were moved to apiaries in the surrounding area. All colonies over-wintered with >20 kg (45 lb) of largely ivy stores, typically <10 kg (22 lb) are consumed from November to late March as the A. m. mellifera strain tends to be frugal. During April/May 2017 the colonies again built up rapidly and reached an average of over 8 frames of brood in the 11 frame brood boxes. By mid May two colonies had produced queen-cells and were in the process of re-queening.
The study considered: the seasonal mite population levels; possible bee-damage to mites; changes to idiosoma dorsal-dimples; prevalence of clinical signs of DWV and the virus status using RT-PCR and changes to brood nest temperature.
Seasonal Mite-fall
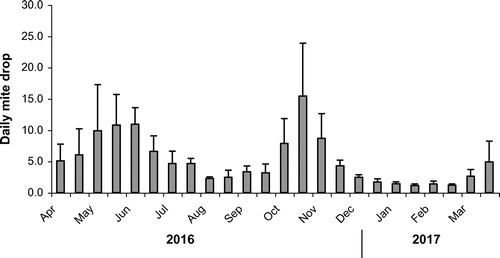
The natural mite fall was quantified as a means of estimating mite populations in the colonies (Branco, Kidd, & Pickard, Citation2006). The mites fall through the screened-floor onto the insert on the solid floor below. For the twelve-month period, April 2016 to March 2017, the natural mite-fall in the colonies was collected twice weekly. The natural mite-fall was aggregated into half-month periods over the twelve months April 2016 to March 2017 (Figure ). It shows a build up in mite-fall in spring and autumn and declines during summer and winter. The average colony mite-fall at the beginning and end of the twelve-month period was similar at ~5 mites per day.
Damage to Mites
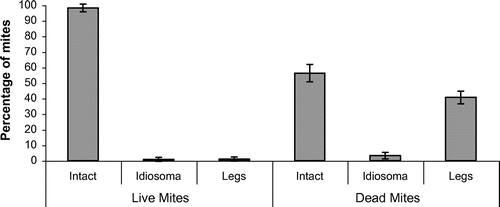
The natural mite fall was examined to check for evidence of a possible mechanism that is contributing to colony tolerance. Over a ten-day period in October 2016, mites were collected daily from the colonies in the apiary and examined for damage. Since there were no ants or other large arthropods present on the floor insert, any mite damage other than regular dorsal dimples was due to the bees. The mites were listed as live or dead, and condition was listed as intact, damaged idiosoma or damaged legs. Mites with one or two regular dorsal dimples are not considered as damaged (Davis, Citation2009). The majority of the mites in the natural mite-fall were dead and damage was observed on 44% of them. This included 41% leg damage and 3% non-regular damage to the idiosoma (Figure ). As no ants or large arthropods were present, this damage was inflicted by the bees. Leg damage on live mites was rare and was only observed on 8 dying mites out of the total mite-fall of 1,084. The dead mite count may have been inflated, as some of the mites listed as dead may have been alive earlier in the day when they dropped onto the insert.
Figure 5. Damage to naturally falling mites in five colonies in North County Dublin collected daily over a ten-day period in October 2016 (total mite-fall 1,084). Mites, live (431 mites) and dead (653 mites) are each categorised (% ±SD) as intact, damaged idiosoma or damaged legs. Damage to idiosoma can be crushed/broken, while leg damage can vary from the removal of the tip or apoteles to multiple legs leaving empty sockets.
A thin smear of Vaseline™ was applied around the perimeter of the floor insert, and the mite fall during the ten-day period described above examined and trapped mites in the smear counted. This is a measure of the potential number of mites that could leave the solid floor and return to the brood nest. A total of 36 mites out of the 1,084 mites (3.3%) were stuck to the Vaseline smear on the outer edge of the insert indicating low mite movement to the edge of the insert. Also, seldom was any of the mite-fall observed moving on the insert.
Idiosoma Dorsal-dimples
The incidence of regular dorsal dimples (single and double) on mite idiosoma was established for the ten-day mite drops in October 2016. The mites were grouped as one, two or no dimples present and broken down further into mother dark-coloured mites and daughter light-coloured mites. Also, to establish any change in the dimple incidence over time, an archived sample of mite-fall taken during an autumn varroa treatment in 2004, shortly after the mite infested the apiary, was examined and a similar grouping of mites and dimples was undertaken for this sample.
The incidence of dorsal-dimples for adult (dark-coloured) and daughter mites (light-coloured) was quantified for the October 2016 and the 2004 (archive) sample (Table ). It is notable that the 2004 (archive) sample contained a number of the bee lice, Braula coeca, which has since disappeared, probably due to chemical treatments. The incidence of dimples was significantly higher in 2016; 3.4 times for the old mother mites (χ2 = 21.33, p < 0.001) and 2.0 times for the daughter mites (χ2 = 12.32, p < 0.01).
Table 1. The regular dorsal dimple prevalence on the idiosoma of varroa mites from a 2004 (archive) treatment mite-fall in North County Dublin is shown. Also shown is the dimple prevalence from the October 2016 natural mite-fall sample over a ten-day period for five colonies from the same apiary. The mites are categorised as dark (mother) and light (daughter) and the following comparisons of dimple prevalence are made: 2004 dark mites vs. 2016 dark mites, and 2004 light mites vs. 2016 light mites.
Prevalence of DWV
On 2 April 2017 one frame of brood, which had a large area of sealed brood at all ages was chosen from each of the five colonies. After removing the adult bees each frame was inserted into a separate stainless steel (perforated) cage and transferred to an incubator at 34.5°C (50–60%, RH). Every eight hours the five cages were rotated one position to reduce temperature differences experienced by the brood. Emerging callow bees were collected at eight-hour intervals from the cages and examined for wing-damage indicative of DWV, counted, bagged and frozen. This continued until all the callow bees had emerged. The emerged bees from each of the five frames were tested for their viral status using RT-PCR, 40 cycles. Similarly, samples of flying bees from each of the five colonies in the field were tested.
Wing damage was observed on only 24 of the 7,552 (0.32%) emerged bees, and in no case was a full wing shrivelled. All samples of callow bees were negative for DWV. Two of the samples of flying bees were positive and these corresponded to the colonies with the two highest prevalences of clinical signs of DWV in the emerged bees.
Brood Temperature
Using probes inserted between combs, the central brood (max/min) temperatures were recorded in the colonies on a daily basis over ten consecutive days in April 2017. To assess the potential thermal impact if open mesh floors were used, temperature readings were taken below the brood nest, directly on top of and at the centre of the solid hive floors, and also of ambient temperature outside the hive. Readings were taken over four consecutive days in April 2017 for the five colonies.
The mean daily median temperature for all colonies over the ten-day period was 35.20°C (SD 0.08, n = 5). The mean daily median temperature on the centre of the solid floors in the hives was 28.2°C (SD 1.4, n = 5). The corresponding mean daily median ambient temperature was 9.1°C (SD 1.2, n = 5).
Conclusions
The natural mite-fall in all colonies has continued at high levels with the seasonal profiles related to the brood levels (Fries, Camazine, & Sneyd, Citation1994) and with similar mite-fall at the beginning and end of the twelve-month period (Figure ). It is remarkable that this tolerance is being demonstrated while mite-drops are still high, however, according to Moritz (Citation1981) the small daily resource-drain by a mite on a bee probably has little impact on a healthy bee. There are similarities to mite fall values found in tolerant feral colonies reported from the Arnot Forest, United States where mite infestation growth was in the same order as in those colonies with mite-susceptible bees (Seeley, Citation2007). High infestation rates have been reported in an island population in northeastern Brazil that has survived with varroa for over thirty years (De Mattos, De Jong, & Soares, Citation2016).
The impact of other parasites, principally DWV, would appear to be becoming attenuated. The observed incidence of DWV was high when varroa mites first arrived in the region, and even during the early days of treatment, was still high. During the early stages of the non-treatment period, clinical signs were high and late summer purges with dead and dying bees in front of the hives common. Today clinical signs of DWV are infrequent. The relatively high mite populations have not resulted in a high level of virulence. This is consistent with the findings of Gisder, Aumeier, and Genersch (Citation2009) where mite infestation alone was not correlated with malformed wings in a colony. A recent study (Brettell et al., Citation2017) concluded that a high DWV load was the only consistent factor associated with wing damage. This latter study also found that using RT-PCR, deformed bees contained the highest viral loads, which is consistent with the findings here.
The majority of the mites falling on the hive floor were dead. Damage observed on 44% of dead fallen mites included 41% leg damage and 3% non-regular damage to the idiosoma (Figure ). Leg damage on live mites was rare, only on a few dying mites (8/1,084). It would appear that leg damage is lethal to mites. Leg damage on dead mites varied widely from a tip (apotele) of one leg to mutilations where legs were missing, leaving leg sockets. On the other hand, regular damage (dimples) on the idiosoma was much greater than in 2004 at 3.5 and 2.0 times for old mother and daughter mites respectively. According to Lodesani et al. (Citation1996) this large increase is indicative of a substantial reduction in mite fecundity, which should contribute to varroa-tolerance.
There is no indication of an increase in the central brood nest temperature compared with the pre-varroa temperatures. This observation aligns with that of Levin and Collison (Citation1990). There is therefore no evidence that sealed-cell duration has reduced as a result of a rise in brood temperature since the arrival of varroa. Le Conte and Arnold (Citation1988) have demonstrated that varroa mites have a preference for cells cooler than 33°C, which would favour drone brood for mite reproduction as traditionally it was located on the outer edges of the brood comb (Winston, Citation1987). However, increasingly in North County Dublin drone brood is being observed higher up the brood combs and closer to the centre of the brood nest, thereby exposing it to a higher brood cell temperature. The expected reduction in the drone sealed-cell period and mite reproduction would appear to indicate selection at work, facilitating the critical role of the drones in varroa tolerance (Jandricic & Otis, Citation2003).
Similarly, the use of solid hive floors, as in this study may also be contributing to tolerance. It has been demonstrated that only a small proportion (3.3%) of mite-fall moved to the edge of the hive floor insert and would have potentially returned to the brood nest. Furthermore, Coffey (Citation2007) in a temperate area demonstrated that the initial reduction in mite population growth was not sustained when “open mesh” floors were used. A large temperature differential can exist in spring between the centre of the solid floor and ambient temperature, 19°C was measured in this study. In the presence of an open mesh floor the temperature at this point in the hive would tend towards ambient temperature, a reduction in temperature of 19°C. Hence, the use of open mesh floors exposes colonies to greater thermal risk in temperate areas during the critical spring build-up period, and from the foregoing also gives varroa mites a potential reproductive advantage.
Former Research Associate, Trinity College, Dublin, Ireland
Email: [email protected]
Acknowledgements
I would like to thank Prof Gard Otis for his helpful comments and suggestions. Also, Dr John McKillen AFBI, Northern Ireland for carrying out the virus analysis. I would also like to thank John and Dorothy Stapleton for access to colonies, and members of Fingal North Dublin Beekeepers’ Association for their ongoing assistance.
References
- Blacquière, T., & Panziera, D. (2018). A plea for use of honey bees’ natural resilience in beekeeping. Bee World, 95(2), 34–38. doi:10.1080/0005772X.2018.1430999.
- Branco, M. R., Kidd, N. A. C., & Pickard, R. S. (2006). A comparative evaluation of sampling methods for Varroa destructor (Acari:Varroidae) population estimation. Apidologie, 37, 452–461.10.1051/apido:2006010
- Brettell, L. E., Mordecai, G. J., Schroeder, D., Jones, I. M., da Silva, J. R., Vincent-Rubiano, M., & Martin, S. J. (2017). A comparison of deformed wing virus in deformed and asymptomatic honey bees. Insects, 8, 28. doi:10.3390/insects8010028
- Brodschneider, R., Gray, A., van der Zee, R., Adjlane, N., Brusbardis, V., Charrière, J.-D., … Woehl, S. (2016). Preliminary analysis of loss rates of honey bee colonies during winter 2015/2016 from the COLOSS survey. Journal of Apicultural Research, 55(5), 375–378. doi:10.1080/00218839.2016.1260240
- Büchler, R. (1994). Varroa tolerance in honey bees- occurrence, characters and breeding. Bee World, 49, 6–18.
- Büchler, R., & Drescher, W. (1990). Variance and heritability of the capped developmental stage in European Apis mellifera L. and its correlation with increased Varroa jacobsoni infestation. Journal of Apicultural Research, 29, 172–176.10.1080/00218839.1990.11101215
- Coffey, M. F. (2007). Biotechnical methods in colony management, and the use of Apiguard and Exomite Apis for the control of the varroa mite (Varroa destructor) in Irish honey bee (Apis mellifera) colonies. Journal of Apicultural Research and Bee World, 46, 213–219.
- Davis, A. R. (2009). Regular dorsal dimples on Varroa destructor - damage symptoms or developmental origin? Apidologie, 40, 151–162.10.1051/apido/2009001
- De Mattos, I. G., De Jong, D., & Soares, A. E. E. (2016). Island population of European honey bees in Northeastern Brazil that have survived Varroa infestations for over 30 years. Apidologie, 47, 818–827.10.1007/s13592-016-0439-5
- Fries, I., Camazine, S., & Sneyd, J. (1994). Population dynamics of Varroa jacobsoni – a model and a review. Bee World, 75, 5–28.10.1080/0005772X.1994.11099190
- Fries, I., & Camazine, S. (2001). Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie, 32, 199–214.10.1051/apido:2001122
- Fries, I., Imdorf, A., & Rosenkranz, P. (2006). Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie, 37, 564–570.10.1051/apido:2006031
- Gisder, S., Aumeier, P., & Genersch, E. (2009). Deformed wing virus: Replication and viral load in mites (Varroa destructor). Journal of General Virology, 90, 463–467.10.1099/vir.0.005579-0
- Hou, C. S., Li, B. B., Deng, S., & Diao, Q. Y. (2016). Effects of Varroa destructor on temperature and humidity conditions and expression of energy metabolism genes in infested honeybee colonies. Genetics and Molecular Research, 15 (3), 15038997.
- Jandricic, S. E., & Otis, G. W. (2003). The potential for using male selection in breeding honey bees resistant to Varroa destructor. Bee World, 84, 155–164.10.1080/0005772X.2003.11099597
- Locke, B. (2016). Natural Varroa mite-surviving Apis mellifera honey bee colonies. Apidologie, 47, 467–482.10.1007/s13592-015-0412-8
- Le Conte, Y., & Arnold, G. (1988). Etude du Thermopreferendum de Varroa Jacobsoni Oud [Study of temperature preference of Varroa jacobsoni Oud]. Apidologie, 19, 155–164.10.1051/apido:19880205
- Le Conte, Y., De Vaublanc, G., Crauser, D., Jeanne, F., Rousselle, J. C., & Bécard, J. M. (2007). Honey bee colonies that have survived Varroa destructor. Apidologie, 38(6), 566–572.10.1051/apido:2007040
- Levin, C. G., & Collison, C. H. (1990). Broodnest temperature differences and their possible effect on drone brood production and distribution in honey bee colonies. Journal of Apicultural Research, 29, 35–44.10.1080/00218839.1990.11101195
- Lodesani, M., Vecchi, M. A., Tommasini, S., & Bigliardi, M. (1996). A study of different kinds of damage to Varroa jacobsoni in Apis mellifera L. colonies. Journal of Apicultural Research, 35, 49–56.10.1080/00218839.1996.11100912
- Moritz, R. F. A. (1981). Altersabhängige Empfindlichkeit von Varroa jacobsoni Oudemans gegen K-79 (Chlordimeformhydrochlorid), Diagnose und Therapie der Varroatose [Age-related susceptibility of Varroa jacobsoni (Oudemans) to K-79 (Chlorodimeformhydrochloride). In Diagnosis and Therapy of Varroosis] (pp. 62–68). Bukarest: Apimondia.
- McMullan, J. B. (2012). Having healthy honey bees, an integrated approach. Dublin: FIBKA. ISBN 978-0-9571355-0-5
- Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103, S96–S119.10.1016/j.jip.2009.07.016
- Schmid-Hempel, P., & Koella, J. C. (1994). Variability and its implications for host-parasite interactions. Parasitology Today, 10, 98–102.10.1016/0169-4758(94)90007-8
- Seeley, T. D. (2007). Honey bees of the Arnot forest: A population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie, 38, 19–29.10.1051/apido:2006055
- Starks, P. T., Blackie, C. A., & Seeley, T. D. (2000). Fever in honey bee colonies. Naturwissenschaften, 87, 229–231.10.1007/s001140050709
- Winston, M. L. (1987). The biology of the honey bee. Cambridge: Harvard University Press.