Abstract
Capsule Pied Flycatchers are better able than Great Tits to adjust their feeding behaviour to varying conditions in the same area.
Aims Great Tits breeding in a mosaic of deciduous and coniferous forests in the northern temperate region exhibit consistently lower breeding success in their preferred deciduous habitat than in coniferous habitat. This was explained by the unexpectedly poor nestling feeding conditions in deciduous forests of this region. We studied whether the same paradox applies to Pied Flycatchers that occupy the same habitats in the same area.
Methods Parental provisioning behaviour was studied using video‐recording and experimental manipulation. Caterpillar abundance and basic breeding parameters were measured in different habitat types.
Results Parental provisioning frequency and the proportion of caterpillars in nestling diet was lower, while food objects were on average larger, in coniferous compared with deciduous habitat. However, the total volume of caterpillars and adult Lepidoptera delivered to nestlings did not differ between habitats. In contrast to Great Tits, offspring body parameters in Pied Flycatchers did not differ between habitat types.
Conclusions These results demonstrate how the relative suitability of particular habitat types varies between species and is dependent upon geographical location.
INTRODUCTION
Owing to the loss and degradation of habitats and the need to conserve remaining areas, ecologists and conservation biologists are becoming increasingly aware of the importance of estimating the quality and suitability of different habitats from the perspective of individual species. In this context, it is important to understand the degree of flexibility exhibited by species and the mechanisms by which they may cope with different breeding conditions in heterogeneous environments. Species’ responses to heterogeneous conditions may also be affected by climate change, which can change the timing of peaks in food abundance in breeding habitats (Both & Visser Citation2001, Both et al. Citation2004, Citation2006). As food availability is one of the most important proximate factors influencing reproduction in birds (Martin Citation1987), it provides a valuable indication of the relative suitability of a habitat for breeding. High synchrony with food availability is crucial for breeding success (Naef‐Daenzer & Keller Citation1999, Both & Visser Citation2001, Both et al. Citation2004). Nonetheless, a huge diversity of environmental and genetic factors are responsible for the variation in reproductive behaviour exhibited by birds inhabiting heterogeneous habitat mosaics. This has been expertly demonstrated by, among others, a 30‐year study of Blue Tits Cyanistes caeruleus in the Mediterranean area (Blondel et al. Citation2006, and references therein).
In some forest passerines, such as Pied Flycatchers Ficedula hypoleuca and Great Tits Parus major, breeding success has shown to be higher in deciduous than coniferous forest, and the former is preferentially selected by breeding birds (van Balen Citation1973, Lundberg & Alatalo Citation1992, Cramp Citation1993, Siikamäki Citation1995, Sanz Citation1998a). This pattern is what could be predicted for many forest bird species, given that deciduous forests are usually believed to be more food‐rich than coniferous forests (van Balen Citation1973, Perrins Citation1991, Sanz Citation1998b). However, Mänd et al. (Citation2005) recently showed that Great Tits breeding in a mosaic of deciduous and coniferous forests in the northern temperate region exhibited consistently lower breeding success in the preferred deciduous habitat than in the non‐preferred coniferous habitat. This apparent paradox was explained by the unexpectedly poor nestling feeding conditions in deciduous forests in this study area (Mägi et al. Citation2009). In addition to this study, others (Lundberg et al. Citation1981, Catalan & Haeger Citation1996) have found that the breeding success of Great Tits in terms of fledged young is slightly higher in coniferous forests. These results suggest that the relative suitability of a certain habitat type may significantly depending on geographical location and that in some regions deciduous habitat may even act as an ecological trap (Mänd et al. Citation2005, Citation2009).
Unlike Great Tits, which winter close to their breeding areas and often have two broods per season (at least in the current study area; Mägi & Mänd Citation2004), Pied Flycatchers are long‐distance migrants that return to their breeding grounds later and breed once during the season (Cramp Citation1993). However, there are also notable similarities between these two species, especially in their exploitation of forest habitats: both species occupy the same types of habitat, nest in cavities and feed their nestlings with very similar food items. Therefore, it would be of great interest to know whether the paradox described for Great Tits also applies to Pied Flycatchers breeding in the same area.
In this study we explored: (1) whether any habitat‐related differences exist in the reproductive performance of Pied Flycatchers; and (2) if so, can differences be explained by habitat‐specific variation in feeding conditions and parental provisioning behaviour during the brood‐rearing period. Both correlative and experimental approaches were used. First, we investigated the relationships between parental provisioning rates, the composition of delivered food, the seasonal dynamics of caterpillar abundance and various nestling body parameters in the different habitats. Second, we experimentally increased nestling hunger levels by temporarily depriving them of food, and recorded parental feeding behaviour before and after food deprivation. We assumed that the ability of parent birds to match the increased food demand of nestlings by increasing their provisioning rate is less limited in habitats with high breeding success than in habitats with low breeding success, where the provisioning rate of parents may already be close to the limit of their ability (as found, e.g. by Sanz et al. Citation2002, Tremblay et al. Citation2003, Stauss et al. Citation2005).
METHODS
Study area
Our study was conducted near Kilingi‐Nõmme (58°7′N, 25°5′E) in southwest Estonia in 2006. The study area covers approximately 50 km2 and contains a mosaic of coniferous and deciduous forest (see map of study area in Mägi & Mänd [Citation2004] and Mänd et al. [Citation2005]). Deciduous forests occur either as isolated patches within a largely agricultural landscape or as 250–500‐m wide riparian strips along stream valleys. They are mostly secondary forests (stands typically 45–55 years old) growing on fertile soils and containing a rich deciduous understorey. The most common deciduous species are Grey Alder Alnus incana and Silver Birch Betula pendula. Coniferous forests are typically managed, grow on nutrient poor sandy or peat soils and are dominated by Scots Pine Pinus sylvestris (stands typically 65–85 years old), which sometimes form mixed stands with Norway Spruce Picea abies or Downy Birch Betula pubescens.
Wooden nestboxes (500–600) with a cavity of 11 × 11 × 30 cm and an entrance hole diameter of 3.5–4.0 cm were erected in the deciduous woods and 1200–1300 nestboxes in the coniferous forests in the mid‐1970s. Damaged nestboxes have been annually repaired or replaced with new ones since then, and the number of boxes has remained approximately the same. Nestboxes were mounted on tree trunks at heights of 1.5–2.0 m and were arranged along linear transects, so that each transect generally consisted of some tens of nestboxes within homogeneous (either in coniferous or deciduous) habitat. Distances between neighbouring nestboxes were 50–60 m. Old nesting material was removed from nestboxes before the beginning of the breeding season.
Reproductive parameters
All nestboxes were checked to record the laying date of the first egg, clutch size and hatching date. In total 210 nestboxes were occupied by Pied Flycatchers: 72 in deciduous and 138 in coniferous habitat. The number of fledglings per nest and the body parameters of fledglings and adults were only recorded at filmed nests (see later; total 34). However, as these nests were distributed throughout the whole season and study area, we expect the results to be representative of the population. Adults were captured during the second half of the nestling period. Birds were weighed to the nearest 0.1 g using a Pesola spring balance, tarsus length measured to the nearest 0.1 mm and wing length measured to the nearest 1 mm using sliding callipers. The same measurements were taken from nestlings on day 13 post‐hatch. As Pied Flycatcher nestlings have almost achieved the size of fledglings at this age (Lundberg & Alatalo Citation1992, Cramp Citation1993), these measurements are hereafter referred to as fledgling parameters. For fledgling parameters, brood means were used as independent data points. No signs of polygamy were detected in studied pairs.
Filming and manipulation
In order to record adult provisioning rates and identify the food items delivered to nestlings, a setup was used that allowed us to monitor the entrance of the nestbox from inside. This consisted of a removable nestbox lid with a light and video camera (Sony DCR‐HC96E) on its top. Inside the nestbox a small mirror was attached to the back wall, so that it was possible to record food items at the moment when returning parent birds entered the box. A millimetre scale attached close to the entrance hole allowed the size of food items to be estimated. The entire construction with a camera cover was installed on nestboxes 24 hours before recording commenced, to allow parents to become accustomed to the setup. Subsequent examination of video‐recordings revealed no signs that the filming apparatus disturbed the birds.
A paired sampling design was used for filming, whereby two recordings were made simultaneously during each filming attempt: one in deciduous and the other in coniferous habitat. To eliminate the possible confounding effects of laying time and brood size on the results, nests were paired so that they had the same hatching date and number of nestlings. Recordings were only made in dry weather and took place between 08:00 and 18:30. Several recent studies on different passerine species, including Pied Flycatcher have found no significant diurnal variation in provisioning frequency (Moreno et al. Citation1995, Barba et al. Citation2009, more references in the latter).
Each nest was filmed on two consecutive days (65 min each) at the same time of the day. The first filming session took place when nestlings were eight days old, with the aim of recording the normal feeding activity of adults. The provisioning frequency recorded during the first filming session will hereafter be referred to as the ‘basic’ provisioning rate. On the following day, nestlings were covered with a net for two hours before filming, so that visiting parents were able to see and even touch the chicks but not feed them. The aim of this manipulation was to increase the hunger level of nestlings, thereby eliciting an increase in the parents’ feeding effort (Wright et al. Citation2002, Rosivall et al. Citation2005). Food deprivation lasting 1.5 hours has been claimed to increase the hunger level of passerine nestlings without inducing any remarkable stress or long‐term effects on their condition or survival (Wright et al. Citation2002, Goodship & Buchanan Citation2007). As all nestlings used in our experiment subsequently fledged, we have no reason to suspect that our 2‐hour food deprivations led to any serious negative consequences. No nests were abandoned as a result of the filming or manipulation procedures. When calculating the provisioning frequency for an individual parent, only the time interval between the first nest visit and the end of filming was taken into account. For each visit, the sex of the parent was determined from plumage characteristics. Two nest pairs were omitted from the manipulation analysis because of heavy rain during the second filming day.
Recording prey items
Prey items brought to nestlings were categorised as shown in Table . It was possible to identify most prey items to Order level. Although Pied Flycatcher nestling diet consists of a variety of prey items, caterpillars (and also adult Lepidoptera) are usually considered to be the most valuable and easily digestible food items for nestlings (Lundberg & Alatalo Citation1992, Cramp Citation1993). To estimate the amount of high‐quality food brought to nestlings, only the volume of the aforementioned prey items were considered (as in Mägi et al. [Citation2009]). The length and diameter of caterpillars and adult Lepidoptera were measured to the nearest millimetre and their approximate volumes calculated assuming cylinder‐like body shapes (Blondel et al. Citation1991, Als et al. Citation2002). The volumes of other prey objects were not calculated owing to their complicated body shapes. Most of them also contained larger proportions of inedible body parts than either of the first two categories of prey objects.
Table 1. Mean percentages* of different categories of prey items delivered to nests by Pied Flycatcher parents in different habitats before (basic level) and after temporarily depriving nestlings of food.
Caterpillar abundance
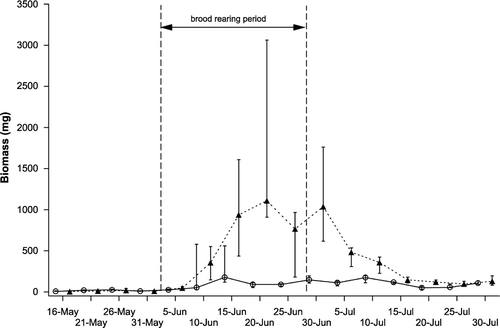
Since caterpillars are considered to be the most valuable food item for Pied Flycatcher nestlings (Lundberg & Alatalo Citation1992), changes in caterpillar abundance were monitored from 16 May to 30 July using the frass‐fall method, which has been widely used for this purpose in avian ecology (van Balen Citation1973, Tinbergen & Dietz Citation1994, Seki & Takano Citation1998, Visser et al. Citation2006). Six collecting sites in each habitat type were randomly selected. At each site, four collectors (round plastic funnels 30 cm in diameter) were placed beneath trees or bushes about 40 cm above the ground. Coffee machine filters at the end of the funnels were used to collect caterpillar excrement (frass) falling from the canopy. Filters were collected and replaced every five days. Filter contents were dried at 35–40°C and stored in plastic bags. Frass was then separated from litter, kept at 60°C for 48 hours and weighed to the nearest 0.1 mg. The mean of the four frass samples collected during five days was calculated for each collecting site. Caterpillar biomass was calculated from these data using the formula of Tinbergen & Dietz (Citation1994), which takes into account the daily variation in ambient air temperature, affecting caterpillar growth.
Data analysis
For data analysis, glm, pairwise t‐tests and Wilcoxon’s or Mann–Whitney U‐tests (whenever the condition of normality was violated) were used. Filming attempt – a variable that defined the pair of nests that were filmed simultaneously – was included to glm as a random factor to prevent pseudoreplication. Sex was included to the glm analysis as a fixed factor. For analysis of the frass fall data, date (taken as the mean date of the five‐day frass‐collecting period) was included in the models as repeated measure.
RESULTS
Basic parental provisioning and composition of nestling diet
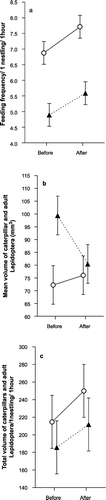
Overall parental provisioning rate was significantly higher in deciduous than coniferous habitat (t = 2.85, df = 16, P = 0.012; Fig. ), and female provisioning frequency was higher than that of males (glm [random factor design]; F 1,48 = 4.64, P = 0.036). Accordingly, the number of food items brought to nests per nestling was also higher in deciduous than coniferous habitat (t = 2.98, df = 16, P = 0.009).
Figure 1 Parental provisioning frequency (a); mean volume of caterpillars and adult Lepidoptera (b); and total volume of caterpillars and adult Lepidoptera brought to nestlings in time unit (c) in Pied Flycatchers in different habitats before (basic level) and after the temporary food deprivation. ○, deciduous habitat; ▴, coniferous habitat; error bars show ± se.
The proportions of different prey categories occurring among food items are presented in Table . Caterpillars constituted a significantly higher proportion of all food items in deciduous than in coniferous habitat (Wilcoxons’ test; Z = 2.39, n = 17, P = 0.017), while the proportions of adult Lepidoptera and Aranea were higher in coniferous habitat (Wilcoxon’s test; Lepidoptera: Z = 3.29, n = 17, P = 0.001; Aranea: Z = 3.05, n = 17, P = 0.002). There were no significant habitat‐related differences in the proportions of any other prey categories (although Ephemeroptera was represented in nestling diet only in deciduous habitat patches that were situated close to streams or ditches; Table ).
Although relatively more caterpillars were brought to nestlings in deciduous habitat (t = 3.33, df = 16, P = 0.004), the mean volume of individual caterpillars was significantly higher in coniferous habitat (t = −2.72, df = 16, P = 0.015). In the case of adult Lepidoptera both the number and mean volume of items were significantly higher in coniferous habitat (number: t = −2.19, df = 16, P = 0.01; volume: t = −2.53, df = 16, P = 0.022). Thus, nestlings in deciduous habitat gained more but smaller high‐quality food objects than nestlings in coniferous habitat (Fig. ). As a result, the total volume of caterpillars and adult Lepidoptera brought to nestlings per unit time did not differ significantly between habitats (t = 0.61, df = 16, P = 0.55; Fig. ).
The lack of significant habitat × sex interactions (glm [random factor design]; results not shown) indicated that the sexes did not behave differently with respect to habitat‐dependent provisioning behaviour. Once controlled for habitat, no significant relationships were found between overall provisioning frequency and either the mean or total volume of caterpillars and adult Lepidoptera delivered (glm [random factor design]; results not shown). None of the measured provisioning variables were significantly related to the body parameters of the parent birds (glm [random factor design]; results not shown).
Food deprivation experiment
Provisioning frequency increased significantly after nestlings were temporarily deprived of food, and the effects of the manipulation did not differ significantly between habitats (Table ; Fig. ). The same was true for the number of food items brought to nests per nestling (glm, results not shown).
Table 2. The effects of habitat type (HT) and food deprivation experiment (EXP) on parental provisioning frequency, the mean volume of individual caterpillars and adult Lepidoptera, and the total volume of caterpillars and adult Lepidoptera brought per nestling per hour in Pied Flycatchers (glm, the effect of filming attempt included as random factor; see ‘Methods’).
There was no significant change in the proportion of caterpillars and adult Lepidoptera brought to nestlings after the manipulation in either habitat (glm [random factor design]; results not shown). However, the proportion of Coleoptera in nestling diet increased (Table ; glm [random factor design]; habitat: F 1,42 = 2.62, P = 0.11; experiment: F 1,42 = 4.61, P = 0.038; experiment × habitat: F 1,42 = 0.32, P = 0.58).
The mean volume of individual caterpillars and adult Lepidoptera (Table ; Fig. ) and the total volume of this food category brought to nestlings per unit time were not significantly affected by the food deprivation experiment (Table ; Fig. ).
No significant differences between the sexes were apparent in their responses to the food deprivation experiment, as measured by the provisioning parameters listed previously (glm [random factor design]; results not shown).
Caterpillar abundance in the environment
Both frass mass and the calculated biomass of caterpillars showed significantly different seasonal dynamics in the different habitats (Fig. ; glm [date included as repeated measure]; habitat: F 1,10 = 6.01, P = 0.034; date × habitat: F 6,60 = 3.71, P = 0.003). The abundance of caterpillars in deciduous habitat increased only slightly and remained at a relatively constant level during the entire brood rearing period. Meanwhile, in coniferous habitat caterpillar abundance initially rose sharply before declining as the season advanced (Fig. ).
Reproductive parameters
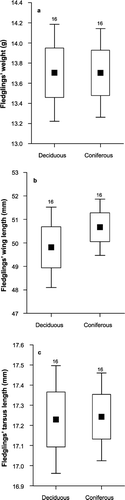
Neither the egg‐laying and hatching dates nor clutch or brood sizes differed significantly between the two habitats (Mann–Whitney U‐tests; lay‐date: Z = −0.48, P = 0.63, n = 170; hatching date: Z = −0.89, P = 0.37, n = 170; clutch size: Z = 0.81, P = 0.42, n = 170). The median laying date of the local population was 20 May and hatching date 9 June. The median clutch size was seven eggs (range 5–8). We did not record data about fledging for the whole local population, but in the sample used in filming (n = 34) the median number of offspring fledged per nest was six (range 5–7). None of the fledgling parameters (body mass, tarsus and wing length) showed significant between‐habitat variation (Fig. ; glm [random factor design], results not shown). Analysis of reproductive data for Pied Flycatchers covering five years prior to this study (V. Tilgar, unpubl.) also did not reveal significant habitat‐related differences in the number and mass of fledglings in our study area (glm; no. fledglings:F 1,598 = 0.03, P = 0.87; fledgling mass: F 1,516 = 0.05, P = 0.82). Of provisioning parameters, the total volume of caterpillars and adult Lepidoptera delivered was positively correlated with nestling weight (glm, β = 0.82, P = 0.047, controlled for habitat type and wing lengths).
DISCUSSION
Between‐habitat differences in caterpillar abundance and provisioning behaviour, but not in the nutritional state of nestlings
Although the overall provisioning frequency, number of food items delivered and even proportion of caterpillars among food were comparatively higher in Pied Flycatchers breeding in deciduous habitat, nestlings in this habitat did not receive more high‐quality food (measured as the total volume of caterpillars and adult Lepidoptera brought per unit time) than their counterparts in coniferous habitat. This is because the mean volume of individual caterpillars (and also that of adult Lepidoptera) that were delivered to nests by parents was significantly higher in coniferous habitat than in deciduous habitat. The latter finding is perhaps to be expected since the overall caterpillar biomass (judged by the frass fall data) during brood‐rearing period was far higher in coniferous compared with deciduous forests, which presumably allowed parents to be more selective in their choice of prey (see references below).
That fledgling weight correlated positively with the total volume of provisioned caterpillars and adult Lepidoptera supports the contention that this represents a particularly important high‐quality food category (Lundberg & Alatalo Citation1992, Cramp Citation1993). Thus, the fact that the nestlings in both habitats received equal amounts of this high‐quality food, despite obvious habitat‐related differences in its availability and in parental provisioning behaviour, is noteworthy. Given this, it is unsurprising that there were no differences between habitats in fledgling body parameters.
Hence, neither the caterpillar abundance in the environment nor parental provisioning frequency reliably predicted the actual nutritional state and growth of nestlings. Our findings are in accordance with the previously proposed idea that higher provisioning frequency may not indicate a greater volume of provisioned food, but may instead reflect the smaller size and/or lower‐quality of food items delivered to nestlings (Betts Citation1955, Royama Citation1970, Moreno et al. Citation1995, Stauss et al. Citation2005, Mägi et al. Citation2009, Wilkin et al. Citation2009). This may be because parents breeding in a low‐quality habitat do not have enough time to be choosy in selecting prey or there may not be enough high‐quality prey available in the vicinity (Naef‐Daenzer et al. Citation2000, Stauss et al. Citation2005). On the other hand, Tremblay et al. (Citation2005) and Grieco (Citation2002a) have shown that Blue Tit parents living in lower‐quality habitats nourished their chicks equally as well as parents in higher‐quality habitats, despite feeding them less often. Schwagmeyer & Mock (Citation2008) showed that in House Sparrows Passer domesticus the size of delivered prey was more important for offspring fitness than just the frequency of delivery.
No evidence that parental provisioning ability is differentially limited in the habitats studied
The food deprivation experiment investigated whether the ability of Pied Flycatchers to increase their provisioning effort in order to match the increased food demand of chicks varies between habitats. We assumed that the ability to increase provisioning effort might be relatively limited in a habitat where the provisioning rate of parents is already close to the upper limit of their ability (Sanz et al. Citation2002, Tremblay et al. Citation2003, Stauss et al. Citation2005). In fact, the effect of manipulation did not differ significantly between habitats, with provisioning frequency increasing in both to a similar extent. Moreover, despite increased provisioning frequency, the amount of high‐quality food (caterpillars and adult Lepidoptera) brought to nestlings was not significantly affected by the manipulation. We still cannot entirely rule out the possibility that some habitat‐related differences in provisioning rates might exist. The power of this analysis was relatively low owing to small sample sizes. It is also conceivable that the duration of food deprivation was not long enough to reveal habitat differences in food limitation, and that a longer‐term manipulation might have had a different effect. However, since our experimental setup had sufficient power to detect several other behavioural responses, we believe that if habitat‐related differences existed, they must have been slight.
Overall, this experimental result provides further evidence that, despite considerable habitat‐related variation in the abundance of high‐quality food and parental provisioning behaviour, the actual provisioning ability (or at least provisioning frequency) of Pied Flycatchers did not reach its upper limit in either habitat of our study system and, therefore, food was not a limiting factor for this species.
Other authors have claimed that when faced with unfavourable conditions, parent birds tend to switch either to bringing higher‐quality food with the same frequency or to increasing provisioning frequency by bringing chicks more abundant but less easily digestible food (Grieco Citation2002b, Eeva et al. Citation2005). Our results suggest that in the case of Pied Flycatchers in our study area the latter is probably true. While parents increased provisioning frequency when chicks were hungry, the only food category that was brought more often was Coleoptera, which is usually regarded as a low‐quality substitute mainly used when caterpillars, spiders and/or adult Lepidoptera are scarce (Eeva et al. Citation2005).
This raises the question of why parents increase their provisioning frequency in response to the increased hunger level of nestlings, when this does not have the effect of increasing the amount of high‐quality food supplied to nestlings. One possible answer may be that the principal function of the parental response is not so much to increase the supply of food but to silence the loud begging calls of hungry chicks that might otherwise reveal the nest to predators (Leech & Leonard Citation1997, Briskie et al. Citation1999). In such a case it may be optimal to be less selective and switch to less profitable but more abundant prey items. So again, our results highlight a long‐standing caveat (see previous references), but one which is often forgotten or ignored (Christe et al. Citation1996, Hurtrez‐Bousses et al. Citation1998, Tripet et al. Citation2002, Biard et al. Citation2005, Barba et al. Citation2009, Krist Citation2009): the provisioning frequency of parent birds, although an easily measurable variable, does not reliably reflect the actual nutrition level of chicks and should, therefore, be used with caution for that purpose.
Differences between Great Tits and Pied Flycatchers breeding in the same forest mosaic
The results of this study support our recent observation (Mägi et al. Citation2009, Mänd et al. Citation2009) that the deciduous forest in our northern temperate study area (mainly young, secondary alder and birch stands), which is considerably different from that in most other European study areas (e.g. mature oak forests), seems to offer less high‐quality food in comparison with coniferous stands in the same area. The pattern of caterpillar abundance of time in this study year closely resembled those in other monitored years in an important respect: overall caterpillar biomass in coniferous forests increased dramatically during the period from the second half of June until the beginning of July, but much less so in deciduous forests (Mägi et al. Citation2009, Remmel et al. Citation2009). This pattern is very different from those described in other regions in Europe, where the availability of caterpillars in deciduous forests first peaks and then declines markedly towards the end of breeding period, while it remains fairly stable in coniferous forests during the entire breeding season (van Balen Citation1973, Slagsvold & Lifjeld Citation1985, Gosler Citation1993) or peaks later in the breeding season (Eeva et al. Citation2000, Rytkönen & Orell Citation2001).
In common with Great Tits breeding in the same area (Mägi et al. Citation2009), Pied Flycatcher parents in deciduous habitat fed their chicks more frequently than those in coniferous habitat. However, in Pied Flycatchers this higher frequency did not result in a higher quantity of high‐quality food delivered. Moreover, unlike Great Tits, which perform consistently worse in deciduous compared with coniferous habitat in terms of the number and quality of fledglings produced (Mänd et al. Citation2005, Mägi et al. Citation2009, see also ‘Introduction’), no habitat‐related differences were detected in fledgling morphology or any other breeding parameters in Pied Flycatchers. Analysis of reproductive data for Pied Flycatchers covering five years prior to this study also failed to reveal significant habitat‐related differences in the number and mass of fledglings in our study area. Moreover, the year in which our current study was conducted did not appear to be exceptional in terms of climatic conditions, the dynamics of caterpillar abundance or basic breeding data when compared with other years for which the same data are available (unpubl. data). Thus, it seems reasonable to assume that the findings of this study are representative of a general phenomenon.
As fledgling mass has been shown to predict reliably the future survival of offspring (Naef‐Daenzer et al. Citation2001) and thus serves as a suitable fitness measure, it appears that Pied Flycatchers in our study system have adapted their breeding behaviour adequately to the highly different foraging conditions of different habitats. This is in accordance to the theoretical and empirical work performed during the second half of last century (Brown Citation1969, Fretwell & Lucas Citation1970, Krebs Citation1971), showing that birds, like many other animals, are first expected to occupy the preferred habitat until the point where density dependent effects reduce breeding success to that of the next best habitat, which subsequently starts to fill up. Hence, when all suitable habitats are saturated, the mean productivity of breeders should be equal in all habitat types. Indeed, this seems to hold for Pied Flycatchers in our study area. By contrast, Great Tits exhibit consistently lower breeding success in their preferred deciduous habitat, suggesting inadequate habitat selection or the existence of some kind of ecological trap (Robertson & Hutto Citation2006, Arlt & Pärt Citation2007, Mänd et al. Citation2009). It has been hypothesised that the preference of Great Tits for deciduous habitat evolved in the southern parts of the species’ range and is maladaptive in northern temperate regions where deciduous forest offers much poorer foraging conditions by comparison (Mägi et al. Citation2009, Mänd et al. Citation2009).
Why are Pied Flycatchers better able than Great Tits to cope with the less favourable breeding conditions in northern alder‐birch stands and avoid a potential ecological trap? One possible explanation lies in the different breeding times of the two species. Great Tits lay their first clutch earlier in the season (egg‐laying in our study area normally starts at the end of April) when the weather in our region is less predictable than during Pied Flycatchers’ breeding period (whose egg‐laying starts in the middle of May). In support of this idea, and in contrast to the pattern observed among first broods, the much later second broods of Great Tits (laying of second clutches usually starts at the middle of June) do not exhibit significant habitat‐related difference in nestling growth (Mägi & Mänd Citation2004, Mägi et al. Citation2009). Alternatively, the Pied Flycatchers may simply be a more opportunistic and flexible forager than Great Tits, which is known to be fairly conservative in its foraging preferences (Rytkönen & Krams Citation2003). This is probably also a reason why Pied Flycatchers can allow for a random nest‐site choice, in contrast to Great Tits and Blue Tits, which choose their nest‐sites actively (Goodenough et al. Citation2009). Finally, as noted previously, since Pied Flycatchers are smaller than Great Tits, it is perhaps not as limited by food availability in deciduous forests.
‘Deciduous’ versus ‘coniferous’ habitat – some implications
Although Pied Flycatchers seem to be better adjusted than Great Tits to breeding in the deciduous habitat within our study area, we found the relative suitability of the two habitats to be different from that described by most previous studies carried out in other regions. While it had generally been found that both foraging conditions and breeding success in Pied Flycatchers is higher in deciduous compared with coniferous habitat (Lundberg & Alatalo Citation1992, Siikamäki Citation1995, Sanz Citation1998b), no such difference was apparent in our study area. As described previously, the meaning of the term ‘deciduous habitat’ in our region differs significantly from that in earlier studied regions both in the sense of forest composition and the breeding performance of birds. In this context, it is noteworthy that a European‐wide study recently found a significant northward decreasing trend in some egg carotenoid levels of Pied Flycatchers in deciduous forests, but not in coniferous forests (T. Eeva et al. unpubl. data). Thus, the distinction commonly made in avian ecology – ‘deciduous versus coniferous’ – appears to be oversimplified when used over a wide geographical scale. Hence, region‐specific characteristics should be taken into account when evaluating different habitat types for conservation purposes or when trying to forecast the effects of climate change on avian populations.
ACKNOWLEDGEMENTS
We are grateful to Toomas Tammaru for valuable advice about data analysis, Lepiku Sass for fruitful discussions and John Davison for correcting the language. The study was financially supported by the Estonian Science Foundation (grant number 6908), the Estonian Ministry of Education and Science (target‐financing project number 0180004s09) and the European Union through the European Regional Development Fund (Center of Excellence FIBIR). The study complies with the current laws of Estonia.
REFERENCES
- Als , T.D. , Nash , D.R. and Boomsma , J.J. 2002 . Geographical variation in host–ant specificity of the parasitic butterfly Maculinea alcon in Denmark . Ecol. Entomol. , 27 : 403 – 414 .
- Arlt , D. and Pärt , T. 2007 . Nonideal breeding habitat selection: a mismatch between preference and fitness . Ecology , 88 : 792 – 801 .
- Barba , E. , Atiénzar , F. , Marín , M. , Monrós , J.S. and Gil‐Delgado , J.A. 2009 . Patterns of nestling provisioning by a single‐prey loader bird, Great Tit Parus major . Bird Study , 56 : 187 – 197 .
- Betts , M.M. 1955 . The food of titmice in oak woodland . J. Anim. Ecol. , 24 : 282 – 323 .
- Biard , C. , Surai , P.F. and Møller , A.P. 2005 . Effects of carotenoid availability during laying on reproduction in the Blue Tit . Oecologia , 144 : 32 – 44 .
- Blondel , J. , Dervieux , A. , Maistre , M. and Perret , P. 1991 . Feeding ecology and life history variation of the Blue Tit in Mediterranean deciduous and sclerophyllous habitats . Oecologia , 88 : 9 – 14 .
- Blondel , J. , Thomas , D.W. , Charmantier , A. , Perret , P. , Bourgault , P. and Lambrechts , M.M. 2006 . A thirty‐year study of phenotypic and genetic variation of Blue Tits in Mediterranean habitat mosaics . BioScience , 56 : 661 – 673 .
- Both , C. and Visser , M.E. 2001 . Adjustment to climate change is constrained by arrival date in a long‐distance migrant bird . Nature , 411 : 296 – 298 .
- Both , C. , Artemyev , A.V. , Blaauw , B. , Cowie , R.J. , Dekhuijzen , A.J. , Eeva , T. , Enemar , A. , Gustafsson , L. , Ivankina , E.V. , Järvinen , A. , Metcalfe , N.B. , Nyholm , N.E.I. , Potti , J. , Ravussin , P.A. , Sanz , J.J. , Silverin , B. , Slater , F.M. , Sokolov , L.V. , Török , J. , Winkel , W. , Wright , J. , Zang , H. and Visser , M.E. 2004 . Large‐scale geographical variation confirms that climate change causes birds to lay earlier . Proc. R. Soc. B , 271 : 1657 – 1662 .
- Both , C. , Bouwhuis , S. , Lessells , C.M. and Visser , M.E. 2006 . Climate change and population declines in a long‐distance migratory bird . Nature , 441 : 81 – 83 .
- Briskie , J.V. , Martin , P.R. and Martin , T.E. 1999 . Nest predation and the evolution of nestling begging calls . Proc. R. Soc. B , 266 : 2153 – 2159 .
- Brown , J.L. 1969 . The buffer effect and productivity in tit populations . Am. Nat. , 103 : 347 – 354 .
- Catalan , R.M. and Haeger , J.F. 1996 . Breeding patterns of the great tit (Parus major) in a pine plantation and a holm oak forest in a Mediterranean region (southern Spain) . Rev. Ecol. Terre Viel , 51 : 341 – 357 .
- Christe , P. , Richner , H. and Oppliger , A. 1996 . Begging, food provisioning, and nestling competition in great tit broods infested with ectoparasites . Behav. Ecol. , 7 : 127 – 131 .
- Cramp , S. 1993 . “ Ficedula hypoleuca, Pied Flycatcher ” . In The Birds of the Western Paleartic, Vol. 7. Flycatchers to Shrikes , Edited by: Perrins , C.M. and Brooks , D.J. 64 – 71 . Oxford , , UK : Oxford University Press .
- Eeva , T. , Veistola , S. and Lehikoinen , E. 2000 . Timing of breeding in subarctic passerines in relation to food availability . Can. J. Zool. , 78 : 67 – 78 .
- Eeva , T. , Ryömä , M. and Riihimäki , J. 2005 . Pollution‐related changes in diets of two insectivorous passerines . Oecologia , 145 : 629 – 639 .
- Fretwell , S.D. and Lucas , H.J. Jr. 1970 . On territorial behavior and other factors influencing habitat distribution in birds . Acta Biotheor. , 19 : 16 – 36 .
- Goodenough , A.E. , Elliot , S.L. and Hart , A.G. 2009 . Are nest sites actively chosen? Testing a common assumption for three non‐resource limited birds . Acta Oecol. , 35 : 598 – 602 .
- Goodship , N.M. and Buchanan , K.L. 2007 . Nestling testosterone controls begging behaviour in the Pied Flycatcher, Ficedula hypoleuca . Horm. Behav. , 52 : 454 – 460 .
- Gosler , A.G. 1993 . Great Tit , London : Hamlyn Ltd. .
- Grieco , F. 2002a . How different provisioning strategies result in equal rates of food delivery: an experimental study of blue tits Parus caeruleus . J. Avian Biol. , 33 : 331 – 341 .
- Grieco , F. 2002b . Time constraint on food choice in provisioning blue tits, Parus caeruleus: The relationship between feeding rate and prey size . Anim. Behav. , 64 : 517 – 526 .
- Hurtrez‐Bousses , S. , Blondel , J. , Perret , P. , Fabreguettes , J. and Renaud , F. 1998 . Chick parasitism by blowflies affects feeding rates in a Mediterranean population of blue tits . Ecol. Lett. , 1 : 17 – 20 .
- Krebs , J.R. 1971 . Territory and breeding density in the great tit, Parus major L . Ecology , 52 : 2 – 22 .
- Krist , M. 2009 . Short‐ and long‐term effects of egg size and feeding frequency on offspring quality in the collared flycatcher (Ficedula albicollis) . J. Anim. Ecol. , 78 : 907 – 918 .
- Leech , S.M. and Leonard , M.L. 1997 . Begging and the risk of predation in nestling birds . Behav. Ecol. , 8 : 644 – 646 .
- Lundberg , A. and Alatalo , R.V. 1992 . The Pied Flycatcher , London : Poyser .
- Lundberg , A. , Alatalo , R.V. , Carlson , A. and Ulfstrand , S. 1981 . Biometry, habitat distribution and breeding success in the Pied Flycatcher . Ficedula hypoleuca. Ornis Scand. , 12 : 68 – 79 .
- Mägi , M. and Mänd , R. 2004 . Habitat differences in allocation of eggs between successive breeding attempts in Great Tits (Parus major) . Ecoscience , 11 : 361 – 369 .
- Mägi , M. , Mänd , R. , Tamm , H. , Sisask , E. , Kilgas , P. and Tilgar , V. 2009 . Low reproductive success of Great Tits in the preferred habitat: a role of food availability . Ecoscience , 16 : 145 – 157 .
- Mänd , R. , Tilgar , V. , Lõhmus , A. and Leivits , A. 2005 . Providing nest boxes for hole‐nesting birds – Does habitat matter? . Biodivers. Conserv. , 14 : 1823 – 1840 .
- Mänd , R. , Leivits , A. , Leivits , M. and Rodenhouse , N.L. 2009 . Provision of nest‐boxes raises the breeding density of Great Tits Parus major equally in coniferous and deciduous woodland . Ibis , 151 : 487 – 492 .
- Martin , T.E. 1987 . Food as a limit on breeding birds: a life‐history perspective . Ann. Rev. Ecol. Syst. , 18 : 453 – 487 .
- Moreno , J. , Cowie , R.J. , Sanz , J.J. and Williams , R.S.R. 1995 . Differential response by males and females to brood manipulation in the pied flycatcher – energy expenditure and nestling diet . J. Anim. Ecol. , 64 : 721 – 732 .
- Naef‐Daenzer , B. and Keller , L.F. 1999 . The foraging performance of Great and Blue Tits (Parus major and P. caeruleus) in relation to caterpillar development, and its consequences for nestling growth and fledging weight . J. Anim. Ecol. , 68 : 708 – 718 .
- Naef‐Daenzer , L. , Naef‐Daenzer , B. and Nager , R.G. 2000 . Prey selection and foraging performance of breeding Great Tits Parus major in relation to food availability . J. Avian Biol. , 31 : 206 – 214 .
- Naef‐Daenzer , B. , Widmer , F. and Nuber , M. 2001 . Differential post‐fledging survival of Great and Coal Tits in relation to their condition and fledging date . J. Anim. Ecol. , 70 : 730 – 738 .
- Perrins , C.M. 1991 . Tits and their caterpillar food supply . Ibis , 133 : 49 – 54 .
- Remmel , T. , Tammaru , T. and Mägi , M. 2009 . Seasonal mortality trends in tree‐feeding insects: a field experiment . Ecol. Entomol. , 34 : 98 – 106 .
- Robertson , B.A. and Hutto , R.L. 2006 . A framework for understanding ecological traps and an evaluation of existing evidence . Ecology , 87 : 1075 – 1085 .
- Rosivall , B. , Török , J. and Szöllősi , E. 2005 . Food allocation in Collared Flycatcher (Ficedula albicollis) broods: do rules change with the age of nestlings? . Auk , 122 : 1112 – 1122 .
- Royama , T. 1970 . Factors governing the hunting behaviour and selection of food by the Great Tit (Parus major L.) . J. Anim. Ecol. , 39 : 619 – 668 .
- Rytkönen , S. and Krams , I. 2003 . Does foraging behaviour explain the poor breeding success of Great Tits Parus major in northen Europe? . J. Avian Biol. , 34 : 288 – 297 .
- Rytkönen , S. and Orell , M. 2001 . Great Tits, Parus major, lay too many eggs: experimental evidence in mid‐boreal habitats . Oikos , 93 : 439 – 450 .
- Sanz , J.J. 1998a . Effects of geographic location and habitat on breeding parameters of Great Tits . Auk , 115 : 1034 – 1051 .
- Sanz , J.J. 1998b . Effects of habitat and latitude on nestling diet of Pied Flycatchers Ficedula hypoleuca . Ardea , 86 : 81 – 88 .
- Sanz , J.J. , Moreno , J. , Arriero , E. and Merino , S. 2002 . Reproductive effort and blood parasites of breeding Pied Flycatchers: the need to control for interannual variation and initial health state . Oikos , 96 : 299 – 306 .
- Schwagmeyer , P.L. and Mock , D.W. 2008 . Parental provisioning and offspring fitness: size matters . Anim. Behav. , 75 : 291 – 298 .
- Seki , S.‐I. and Takano , H. 1998 . Caterpillar abundance in the territory affects the breeding performance of Great Tit Parus major minor . Oecologia , 114 : 514 – 521 .
- SiikaMäki , P. 1995 . Habitat quality and reproductive traits in the pied flycatcher – an experiment . Ecology , 76 : 308 – 312 .
- Slagsvold , T. and Lifjeld , J.T. 1985 . Variation in plumage color of Great Tit Parus major in relation to habitat, season and food . J. Zool. , 206 : 321 – 328 .
- Stauss , M.J. , Burkhardt , J.F. and Tomiuk , J. 2005 . Foraging flight distances as a measure of parental effort in Blue Tits Parus caeruleus differ with environmental conditions . J. Avian Biol. , 36 : 47 – 56 .
- Tinbergen , J.M. and Dietz , M.W. 1994 . Parental energy expenditure during brood rearing in the Great Tit (Parus major) in relation to body mass, temperature, food availability and clutch size . Funct. Ecol. , 8 : 563 – 572 .
- Tremblay , I. , Thomas , DW. , Lambrechts , M.M. , Blondel , J. and Perret , P. 2003 . Variation in Blue Tit breeding performance across gradients in habitat richness . Ecology , 84 : 3033 – 3043 .
- Tremblay , I. , Thomas , D. , Blondel , J. , Perret , P. and Lambrechts , M.M. 2005 . The effect of habitat quality on foraging patterns, provisioning rate and nestling growth in Corsican Blue Tits Parus caeruleus . Ibis , 147 : 17 – 24 .
- Tripet , F. , Glaser , M. and Richner , H. 2002 . Behavioural responses to ectoparasites: time‐budget adjustments and what matters to Blue Tits Parus caeruleus infested by fleas . Ibis , 144 : 461 – 469 .
- Van Balen , J.H. 1973 . A comparative study of the breeding ecology of the Great Tit Parus major in different habitats . Ardea , 61 : 1 – 93 .
- Visser , M.E. , Holleman , L.J.M. and Gienapp , P. 2006 . Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird . Oecologia , 147 : 164 – 172 .
- Wilkin , T.A. , King , L.E. and Sheldon , B.C. 2009 . Habitat quality, nestling diet, and provisioning behaviour in Great Tits Parus major . J. Avian Biol. , 40 : 135 – 145 .
- Wright , J. , Hinde , C. , Fazey , I. and Both , C. 2002 . Begging signals more than just short‐term need: cryptic effects of brood size in the Pied Flycatcher (Ficedula hypoleuca) . Behav. Ecol. Sociobiol. , 52 : 74 – 83 .