Abstract
Capsule Parasitism has no apparent detrimental effect.
Aims To determine whether parasitism by a haematophagous nest ectoparasite, the Louse Fly Crataerina pallida has a detrimental effect on the reproductive success of its Common Swift Apus apus hosts.
Methods An association between C. pallida abundance and various host life‐history parameters indicative of host reproductive success were sought.
Results No relationship was found between measures of parasite load and clutch size, brood size, length of time required to reach nestling asymptotic weight, the length of time from hatching to fledging, fledging success per nest, fledgling weight, and fledgling size.
Conclusion Parasitism has no apparent detrimental effect upon its hosts. Louse Flies may have evolved low levels of virulence or the negative effects expected as a consequence of its parasitism may be expressed on life‐history traits other than those studied here.
INTRODUCTION
An explicit assumption of host–parasite relationships is that parasites cause some cost to their hosts (Poulin Citation2007). These costs influence host fitness or life‐history traits in a variety of ways. Parasites can, for example, increase nestling mortality (Merino & Potti Citation1995), reduce nestling body mass and growth rates (Richner et al. Citation1993, Brown & Brown Citation1986), influence clutch sizes (Moss & Camin Citation1970, Møller Citation1991), cause an increase in the time nestlings spend in the nest (reviewed by Møller [Citation2005]), or increase the amount of provisioning parents must provide (Christe et al. Citation1996).
The Louse Fly Crataerina pallida Latreille (Diptera: Hippoboscidae), is an obligate haematophagous nest ectoparasite of the Common Swift Apus apus Linnaeus (Apodiformes: Apodidae). Louse Flies feed regularly (Kemper Citation1951) and the cumulative effect of such parasitism should incur considerable costs on host fitness. However, there is no evidence that parasitism has such an impact (Lee & Clayton Citation1995, Tompkins et al. Citation1996). A limitation of these studies could be that parasite abundance seen at the colony studied, the Oxford University Museum colony (Lack Citation1956), may not truly reflect natural levels of parasite abundance.
Access to a previously unstudied swift colony provided an opportunity to examine the relationship between swift breeding success and parasitism anew and to attempt to find an effect upon hosts of such parasitism. Parasitic abundances at this site are considerably higher than those seen at Oxford, possibly more closely resembling natural parasitic levels and distributions. We compared swift breeding success between the years 2007 and 2008 at this colony and the association between parasitic abundance and several important swift life‐history traits were investigated.
METHODS
Study site
The colony was positioned within a concrete highway bridge (51′ 04′ 00′ N 07′ 81′ 00′ E) spanning the Bigge Reservoir, Olpe, Germany. The bridge was a large concrete structure 372 m long, 22.3 m wide and 19 m above the water surface. A pair of enclosed walkways ran the length of the underside of the bridge. These walkways were divided into eight chambers, each approximately 5 m wide and 40 m long. Swifts could access these chambers through ventilation holes with an approximate diameter of 10.5 cm on the floors of the chambers. There were a total of 264 holes at the bridge. The swifts built their nests on the floor of these chambers in close proximity to the entry holes.
The colony comprised 38 breeding pairs in 2007; 35 of which produced a total of 75 nestlings. Unfortunately, owing to poor weather only seven nestlings fledged. Nestlings were present from 10 June until 26 July. In 2008 there were 41 breeding pairs, 38 of which produced 89 nestlings, 38 of which fledged. Nestlings were present from 2 June to 31 July.
Swift nestling measurements
The colony was visited daily during both breeding seasons. Data on the dates of hatching and fledging were recorded. Clutch size could not be determined in 2007. The fledging date was determined as the last day on which a nestling was present at the nest. Nestling weight was measured using electronic scales accurate to 0.01 g (Scout Pro, Ohaus, USA). The asymptote weight was nestling mass on the date on which a nestling reached its maximum weight before subsequently fledging. Weight regression occurs in this species in the days prior to fledging. Nestling size parameters, left wing length (in both 2007 and 2008), and length of the longest left primary feather of nestlings (2008 only), were measured using electronic callipers (Lux‐tools, Germany) following the methods outlined by Svensson (Citation1992).
Parasite load
Louse Fly populations were censored regularly throughout swift breeding period. Following the methods used by Lee & Clayton (Citation1995) and Tompkins et al. (Citation1996) the highest number of Louse Flies seen on any single occasion was used as a measure of parasitic intensity for each nest. As parasite numbers can fluctuate on a day‐by‐day basis (Walker Citation2009), this measure provides an accurate indication of parasitic pressure and also allows easy comparison with previous studies.
Data analysis
Statistical analyses were used to investigate relationships between parasite load and life‐history traits. Possible differences in breeding and nestling traits between 2007 and 2008 were examined using Mann–Whitney U‐tests. The strength of associations between parasite abundance and host traits were gauged using Spearman rank correlation. A glm was conducted using parasite load as a dependent and year, brood size, and fledgling number per nest as variables.
Data were considered separately for 2007 and 2008. However, owing to the small size of the colony, and in particular the small number of fledglings in 2007, data were additionally pooled across years. Where more than one nestling was present within a single nest, and where appropriate for the analysis conducted, mean values per nestling per nest were calculated and used to avoid pseudo‐replication.
RESULTS
Parasite abundance
The level of parasite abundance at the 35 nests where nestlings hatched in 2007 was 8.94 ± 5.17 adult Louse Flies per nest. At the 38 nests inhabited by nestlings in 2008 mean parasite abundance was 12.05 ± 7.47. The overall mean parasite abundance over the two years was 10.61 ± 6.64 adult parasites per nest. There was a significant difference in parasite abundance between the years (U = 460.5, z = 1.91, P < 0.02).
The overall mean parasite abundance considering only the nests where nestlings fledged from in both 2007 and 2008 was 11.01 ± 6.74. There was a significant difference in levels of parasitism at nests where fledging occurred between years, with the parasite abundance in 2007 being higher (mean = 12.50 ± 4.41) than in 2008 (mean = 10.76 ± 6.96) (U = 461.50, z = 2.24, P < 0.01). An adult parasite prevalence of over 90%, and parasite pupae prevalence rates of over 70% were observed in both 2007 and 2008.
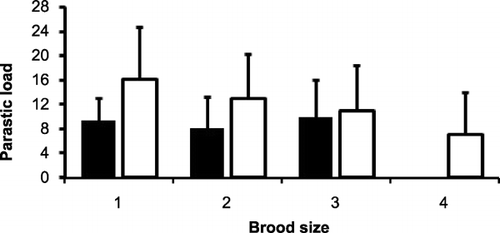
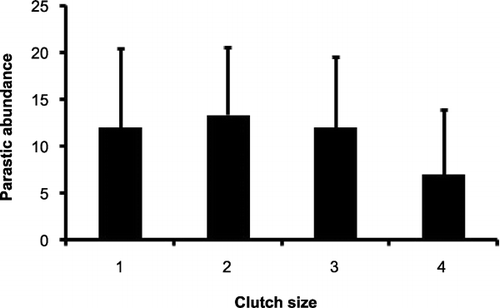
Clutch and brood size
The mean parasite load for broods and clutches of different sizes is shown in Figs & . There was no significant difference in brood sizes between years (U = 717.50, z = −0.044, P = 0.33). There was no significant difference in the parasite load at nests with different clutch sizes in 2008 (one‐way anova, F = 2.28, df = 3, P = 0.09). There was, however, a significant difference in levels of parasite abundance between broods of different sizes, with smaller broods having more parasites, both in 2007, (one‐way anova, F = 5.02, df = 3, P < 0.05) and in 2008 (one‐way anova, F = 5.66, df = 4, P < 0.05).
Nestling asymptotic mass
There was a significant difference in the asymptotic mass nestlings reached in 2007 (mean = 47.96 ± 6.87 g) and in 2008 (mean = 50.22 ± 6.87 g) (U = 59.00, z = 2.30, P ≤ 0.01) and also the time it took them to reach it (mean 2007 = 31.55 ± 4.18 days; mean 2008 = 26.67 ± 4.18 days) (U = 69.50, z = 1.97, P ≤ 0.01). This reflects the generally better weather conditions experienced in 2008, which enabled survivorship of smaller nestlings and quicker nestling development.
There was no association between asymptotic weight and parasite load in either year (2007, r s = −0.36, n = 7, P = 0.41; 2008, r s = −0.98, n = 38, P = 0.55). Using pooled data for 2007 and 2008 there was also no significant association between parasite load and either asymptotic weight (overall mean = 49.80 ± 4.93 g; r s = 0.19, df = 30, P = 0.29), or the number of days required to reach asymptotic weight (overall mean = 25.58 ± 4.94 days; r s = −0.07, df = 30, P = 0.70).
At the asymptote mass nestlings in 2007 had larger left wing lengths (mean = 126.72 ± 10.23 mm) than in 2008 (mean = 113.93 ± 10.22 mm), and this difference was almost significant (U = 85.50, z = 1.47, P = 0.07). There was, however, no significant correlation between parasite abundance and wing length over the two years (overall mean = 116.34 ± 15.73 mm; r s = −0.11, df = 30, P = 0.54), or between parasitism and primary feather length (overall mean = 82.83 ± 18.87 mm; r s = −0.08, df = 30, P = 0.64) in 2008.
Fledging mass and wing length
There were no significant differences in mean mass between fledglings in 2007 (mean = 37.60 ± 16.43 g) and 2008 (mean 40.40 ± 4.05 g; U = 146.00, z = −0.39, P = 0.34), but a significant difference in their wing lengths (mean 2007 = 116.98 ± 4.61 mm; mean 2008 = 157.97 ± 5.11 mm; U = 37.00, z = 2.99, P ≤ 0.01). The mean primary feather length in 2008 was 123.79 ± 5.31 mm).
There was no notable correlation between fledging mass and parasite abundance in either 2007 or 2008 (2007, r s = 0.22, df = 6, P = 0.63; 2008, r s = −0.005, df = 37, P = 0.97). There were also no significant associations between parasitism and left wing size in 2008 (r s = −0.121, df = 37, P = 0.471), or longest left primary length (2008, r s = 0.038, df = 37, P = 0.822). A significant effect on left wing size was seen in 2007 (r s = 0.85, df = 6, P < 0.01), but this was probably because of the small sample size. Using pooled data for both 2007 and 2008 there was likewise no noticeable association between parasite load and either fledgling weight (r s = 0.03, df = 30, P = 0.86), left wing size (r s = −0.04, df = 30, P = 0.80), or longest left primary length (r s = −0.01, df = 24, P = 0.96).
There was a significant difference in the age at which fledging occurred in 2007 (mean = 31.66 ± 3.94 days) and in 2008 (mean = 40.01 ± 2.57 days; U = 202.50, z = −2.16, P ≤ 0.01). Poor weather probably initiated earlier nestling fledging in 2007. The age taken to reach fledging was not significantly correlated with parasite abundance (r s = 0.02, df = 30, P = 0.91).
Fledging success
There was a significant difference in the per nest number of fledglings between 2007 (mean = 0.21 ± 0.48) and 2008 (mean = 1.00 ± 0.84) (U = 1038.00, z = −4.11 = 71, P ≤ 0.01), and in the number of nestlings which died per nest in 2007 (mean = 1.94 ± 0.84) and 2008 (mean = 1.34 ± 0.91; U = 420.00, z = 2.70, P ≤ 0.01), which given the high level of nestling mortality in 2007 is not surprising. There were no notable correlations between either parasitism and the number of fledglings per nest (overall mean = 0.62 ± 0.79; r s = 0.11, df = 71, P = 0.34), or parasite abundance and the number of nestlings dying before fledging per nest (overall mean = 1.63 ± 0.92; r s = 0.03, df = 71, P = 0.73).
The glm analyses revealed that there was no statistically significant interaction between variables on parasite ranking (R 2 = 0.18, df = 70, F = 0.84, P = 0.47). Year was the variable that most strongly influenced parasite rank (β = 2.30, se = ± 1.69, P = 0.17), followed by brood size (β = 0.80, se = ± 1.05, P = 0.44).
DISCUSSION
As in other studies examining this host–parasite system, no convincing association between parasite load and the breeding success of the host swifts was found. This is surprising given the considerable level of resources this parasite appears to extract from hosts and the constraints that swifts face in their reproduction. Swift breeding has to be completed within an extremely short period and given the vagaries of European summers, with the changeable weather conditions and fluctuating aerial insect abundances, parasite load would be thought to be an important factor influencing breeding success in this species.
That no effects on clutch size were found is perhaps not unexpected as adult parasites emerge from diapause once clutch size is already established. Parasitism could, therefore, only influence clutch size if swifts could anticipate parasite loads before incubation begins. Parasites could influence brood size if they disrupted adult behaviour during incubation; however, such disturbance has not been reported and, therefore, is unlikely (Lack Citation1956). That Common Swift nestlings exhibit flexibility in development rates in response to detrimental environmental conditions is well documented (Lack & Lack Citation1951, Lack Citation1956, Weitnauer Citation1947). Increased parasitism might, therefore, be thought to be a prime candidate leading to an extension of the nestling period. However, we found no connection between parasite load and the time required for nestlings to reach asymptotic weight or the time required to reach fledging. Nestling mass is an important predictor of fitness (Magrath Citation1991). Therefore, any detrimental effect of parasitism on asymptotic or fledging weight is likely to have substantial future fitness costs. No relationship between parasitism and either asymptotic or final fledgling size was found.
It might be expected that no influence of parasitism upon traits of pertinence to fledgling flight ability, such as final fledgling weight and size, would be found. Swifts are aerial specialists, spending the majority of their lives airborne. The physiological demands of flight impose strict constraints on body design. Fledging swifts have to be perfectly capable of flight immediately on leaving the nest. It has been shown that nestling fledging is dependent on specific wing loadings being reached, and that nestlings do not fledge until this is reached (Martins Citation1997). Any fledgling deviating from such constraints will have low survival chances. Effects of parasitism upon fledgling size should, therefore, be avoided at all costs. It may be more advantageous for such costs to be deferred onto traits with later lifetime consequences in order to maximize immediate survival chances.
The population abundance of C. pallida that we recorded was considerably greater than that reported by Lee & Clayton (Citation1995) and by Tompkins et al. (Citation1996). Lee & Clayton (Citation1995), in their examination of C. pallida population abundances, found a nest parasite prevalence of 67%, and a mean parasitic intensity, of 1.0 ± 0.2 larvae per nest (range 0–5), and a mean pupae number per nest of 1.7 ± 0.4 (range 0–9). Tompkins et al. (Citation1996) experimentally manipulated parasite abundances to create nests with enhanced parasite loads, and these had mean per nest parasite loads (based on the maximum number of larvae seen their study) of 7.39 ± 0.87.
Tompkins et al. (Citation1996) proposed that the vertical nature of Louse Fly transmission may account for lack of virulence observed. The development of reduced virulence is expected where host and parasite reproductive success is linked (Poulin Citation2007). The high level of host specificity exhibited by species within the Crataerina genus upon their respective hosts indicates that such co‐adaption may have occurred in these host–parasite systems. However, although vertical transmission between adult and nestling swifts is common, the extent of horizontal or phoretic transfer between nests and unrelated hosts and the implications this would have on parasite virulence remain unknown.
Studies on the Alpine Swift Apus melba Linnaeus and its related parasite, Crataerina melbae Róndani, have found detrimental effects upon such subtler host traits than those here studied, including nestling behaviour (Bize et al. Citation2003b), growth rates (Bize et al. Citation2003a) and parental lifetime reproductive success (Bize et al. Citation2004). Conversely, a study on a more obvious host life‐history trait – host condition – found no correlation with Louse Fly abundance (Tella et al. Citation1995). This may indicate that costs of such parasitism are indeed deferred upon more subtle traits. Likewise studies of other aerial insectivores have found parasitic effects upon a myriad of host traits, with, for example, parasitism being found to effect immune system investments and trade‐offs in the Barn Swallow Hirundo rustica (Møller et al. Citation2001). Studies of these and other life‐history traits in the Common Swift may be a promising avenue of further research.
A limitation of the present study was its purely observational nature. Although host–parasite systems are often studied in such a fashion, such studies do not provide conclusive evidence of parasitic effects. Experimental studies where parasitic abundance is artificially manipulated are required to reach more rigorous conclusions. Such an experimental study would be a logical next step in our research.
REFERENCES
- Bize , P. , Roulin , A. , Bersier , L.F. , Pfluger , D. and Richner , H. 2003a . Parasitism and developmental plasticity in Alpine Swift nestlings . J. Anim. Ecol. , 72 : 633 – 639 .
- Bize , P. , Roulin , A. and Richner , H. 2003b . Adoption as an offspring strategy to reduce ectoparasite exposure . Proc. R. Soc. Lond. B. , 270 : S114 – S116 .
- Bize , P. , Roulin , A. , Tella , J. , Bersier , L.F. and Richner , H. 2004 . Additive effects of ectoparasites over reproductive attempts in the long‐lived Alpine Swift . J. Anim. Ecol. , 73 : 1080 – 1088 .
- Brown , C.R. and Brown , M.B. 1986 . Ectoparasitism as a cost of coloniality in Cliff Swallows (Hirundo Pyrrhonota) . Ecology , 67 : 1206 – 1218 .
- Christe , P. , Richner , H. and Oppliger , A. 1996 . Begging, food provisioning, and nestling competition in Great Tit broods infested with ectoparasites . Behav. Ecol. , 7 : 127 – 131 .
- Lack , D. 1956 . Swifts in a Tower , London : Methuen publishing .
- Lack , D. and Lack , E. 1951 . The breeding biology of the swift . A. apus. Ibis , 93 : 501 – 546 .
- Lee , P.L.M. and Clayton , D.H. 1995 . Population biology of swift (Apus apus) ectoparasites in relation to host reproductive success . Ecol. Entomol. , 20 : 43 – 50 .
- Magrath , R.D. 1991 . Nestling weight and juvenile survival in the Blackbird, Turdus merula . J. Anim. Ecol. , 60 : 335 – 351 .
- Martins , T.L.F. 1997 . Fledging in the Common Swift: weight‐watching with a difference . Anim. Behav. , 54 : 99 – 108 .
- Merino , S. and Potti , J. 1995 . Mites and blowflies decrease growth and survival in nestling pied flycatchers . Oikos , 73 : 95 – 103 .
- Møller , A.P. 1991 . Ectoparasite loads affect optimal clutch size in swallows . Funct. Ecol. , 5 : 351 – 359 .
- Møller , A.P. 2005 . Parasites, predators and the duration of developmental periods . Oikos , 111 : 291 – 301 .
- Møller , A.P. , Merino , S. , Brown , C.R. and Robertson , R.J. 2001 . Immune defense and host sociality: a comparative study of swallows and martins . Am. Nat. , 158 : 136 – 145 .
- Moss , W.W. and Camin , J.H. 1970 . Nest parasitism, productivity and clutch size in Purple Martins . Science , 168 : 1000 – 1003 .
- Kemper , H. 1951 . Beobachtungen an Crataerina pallida Latr. und Melophagus ovinus L. (Diptera, Pupipara) . Zeitschr. F. Hygiene. (Zool.) , 39 : 225 – 259 .
- Poulin , R. 2007 . Evolutionary Ecology of Parasites , Princeton , NJ : Princeton University Press .
- Richner , H. , Oppliger , A. and Christe , P. 1993 . Effect of an ectoparasite on reproduction in Great Tits . J. Anim. Ecol. , 62 : 703 – 710 .
- Svensson , L. 1992 . Identification Guide to European Passerines , Thetford , , UK : British Trust for Ornithology .
- Tella , J.L. , Gortazar , C. , Gajon , A. and Osacar , J.J. 1995 . Apparent lack of effects of a high Louse‐fly infestation (Diptera, Hippoboscidae) on adult colonial Alpine Swifts . Ardea , 83 : 435 – 439 .
- Tompkins , D.M. , Jones , T. and Clayton , D.H. 1996 . Effect of vertically transmitted ectoparasites on the reproductive success of swifts (Apus apus) . Funct. Ecol. , 10 : 733 – 740 .
- Walker , M.D. 2009 . Daily fluctuations in the numbers of the Louse Fly Crataerina pallida (Latreille 1812) (Dip.: Hippoboscidae) seen at Common Swift Apus apus nests . Entomologists record , 121 : 157 – 158 .
- Weitnauer , E. 1947 . Am Neste des Mauerseglers A. apus apus (L.) . Der Ornithologische Beobachter , 44 : 133 – 182 .