Abstract
Capsule Foraging habitats are determined by vegetation characteristics rather than the availability of insect prey.
Aims To determine the diet composition of Little Owls in relation to the availability of insects at foraging sites, and to elucidate the main factors determining the owls’ habitat choice.
Methods The feeding ecology of Little Owls was studied during the 2002 breeding season in the agricultural landscape of western Bohemia (Czech Republic), where its population is in decline. Diet composition was determined by pellet analysis. Insect availability was studied using pitfall traps in the two most important Little Owl foraging habitats. For both habitats, we assessed the main vegetative characteristics (average and maximum vegetation height, vegetation density).
Results Based on number, insects were the most dominant prey, followed by small mammals; based on weight, insects comprised only a minor part of the diet. Among insect prey, Carabidae beetles were the most abundant. The proportion of insect numbers was strongly positively correlated with advancing day of the season and negatively correlated with the proportion of vertebrates. Although the highest densities of Carabidae were found in cornfields Little Owls significantly preferred grassland habitats, probably because of the lower vegetation cover.
Conclusions The availability of short sward vegetation in grassland habitats during the breeding season may play a key role in the conservation of Little Owls in central European farmland.
Rapid population declines of some farmland birds has concerned conservationists in many countries across Europe (Møller Citation1983, Fuller et al. Citation1995, Donald et al. Citation2001, Newton Citation2004, Batáry et al. Citation2007, Lemoine et al. Citation2007). Little Owls Athene noctua are a species of agricultural landscapes whose distribution and population have declined during the last 60 years (Cramp Citation1985, Tucker & Heath Citation1994) leading to extinctions in many regions (van Nieuwenhuyse et al. Citation2008). Large‐scale changes and habitat loss in agricultural landscapes, especially the losses of grasslands, which are its most important feeding habitat in central Europe, are thought to be responsible for the owls’ decline (Šálek & Schröpfer 2008, Schönn et al. 2001, van Nieuwenhuyse et al. Citation2008). Intensive grassland management, such as increasing nitrogen availability or reseeding with competitive species (e.g. Lolium sp.), result in taller and denser swards and may reduce the availability of the Little Owls’ principle prey species. Although the abundance of small mammals and insect prey is assumed to be relatively high, these prey may be unavailable to owls and other birds in tall and dense grassland (Southern Citation1954, Goodwin & Hungerford Citation1979, Devereux et al. Citation2004, Whittingham & Devereux Citation2008). On the other hand, though shorter vegetation may promote better access to food for predators such as the Little Owl, longer vegetation may support higher abundances of invertebrates (Devereux et al. Citation2004) or small mammals (Jacob & Brown Citation2000).
Little Owls are nocturnal predators that predominantly hunt ground‐dwelling prey from an elevated perch (Cramp Citation1985, Fajardo et al. Citation1998). Dietary studies of Little Owls in agricultural landscapes usually indicate a high proportion of insect prey (Génot & Bersuder Citation1995, Schmid Citation2003). However, small passerine birds and small rodents comprise a significant part of Little Owl diets in particular study areas (Schönn et al. 2001, Laiu & Murariu Citation1997, Hounsome et al. Citation2004). Ille (Citation1992) reported that these owls consumed more than 82% insects (especially ground‐dwelling beetles) and small mammals, while other prey items were taken only sporadically. In comparison with other owls inhabiting central Europe, the proportion of insects in the diet of Little Owls diet is very high (Cramp Citation1985), especially during the breeding season (Ille Citation1992, Schönn et al. 2001). Further, the proportion of insects in the diet of Little Owls increases from central Europe toward southern latitudes (Mikkola Citation1983) owing to the lower availability of M.crotinae rodents in the Mediterranean region. The contribution of insects to the diets of Little Owls may increase when voles become rare (Schönn et al. 2001) or may reflect seasonal variations in insect availability (Zerunian et al. Citation1982).
While these recent studies on the feeding ecology of Little Owls have focused mainly on diet composition (Cramp Citation1985, Manganaro et al. Citation2001, Obuch & Krištín Citation2004) and seasonal variations (Génot & Bersuder Citation1995, Schmid Citation2003), they have not simultaneously assessed food availability. In this study, we present these data from a region where populations of Little Owls are declining rapidly (Šálek 2004, Šálek & Schröpfer 2008). The main goals of this study were to describe seasonal changes in diet composition with respect to natural insect densities and to assess prey availability with respect to vegetation characteristics that may influence habitat choice. This information should provide an important framework for the conservation and management of this species in farmland regions.
METHODS
Study area
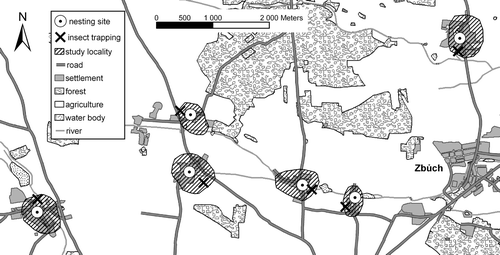
This study was carried out at six sites occupied by Little Owls in western Bohemia, southwest of the city of Plzen (Czech Republic) within a 50‐km2 area (350–486 m asl; 49.40° N, 13.9° E; Fig. ). Sites were chosen based on previous research on the distribution of Little Owls with a minimum distance between adjacent sites of 2.3 km (average = 3.9 km). In this study area Little Owls utilise cavities in old buildings of small villages and they hunt in areas surrounding their nests. Population density is 2.9 pairs/10 km2 (Šálek 2004). The main habitats of the surrounding areas are intensely managed fields (45%), forests (32%) and grassland patches (10%). The grasslands are dominated by Festuca spp., Phleum spp., Trisetum spp., Alopecurus spp. and Lolium spp., among many others.
Pellet analysis
Pellets and prey remains from nests were collected from four nests and six roosting sites every 14 days during the 2002 breeding season (April–July). All older pellets were removed from the nests or roosting sites before sampling began to avoid mixing pellets from different periods. In total, we identified 898 prey items from 563 pellets. The pellets were dried and all feathers and other remains were identified. Small mammals were identified from skulls according to Anděra & Horáček (1982), and birds from beaks and humeri using a reference collection. All insect remains were identified according to structure, colouration, legs and size of exoskeletons. The proportion of earthworms was not assessed due to minor content of their remains in pellets. The total number of prey individuals was determined by the most numerous body parts (e.g. upper jaw). The data on weight of vertebrate prey were taken from Hudec & Černý (Citation1977) and Anděra & Horáček (Citation1982). Weight of Carabidae beetles was calculated from length using the formula Weight = 0.0307 × Length2.64 (Jarošík Citation1989) and for other insect groups the formula Weight = 0.0305 × (Length)2.62 (Rogers et al. Citation1976) was used.
Foraging habitat preferences of Little Owls
Data on foraging habitat preferences was acquired using telemetry carried out during the 2002 breeding season. Adult birds were caught before the monitoring period at the beginning of March. A mist‐net placed near the nest sites, using a dummy combined with a territorial voice playing from a tape‐recorder, was used. Birds were equipped with ‘back‐pack’ transmitters (Biotrack TW 4, 3.5 g, battery lifespan of up to 8.5 months), which were then tracked using a three‐piece Yaggi aerial and an ICOM R‐10 wideband receiver. The locations of radiotracked birds were recorded at 15‐minute intervals from one hour after dusk to (one hour after) midnight, which coincides with the period of highest foraging activity of Little Owls (Exo Citation1989, Fajardo et al. Citation1998).
From April to July, we recorded a total 307 locations of six adult males, which provided information on their foraging areas. To assess habitat preferences, we randomly selected the same number of control locations within Little Owl home ranges. We distinguished two main habitats, meadows and cereal fields (together 86% of all Little Owl locations). For each habitat we assessed the actual average vegetation height, maximum vegetation height and vegetation density for each 14‐day period.
Insect trapping
During spring and summer 2002, we estimated the composition of ground‐dwelling insects in the Little Owls’ foraging areas (Fig. ). Ground‐dwelling beetles were collected using non‐baited pitfall traps (buried plastic bottles with the neck cut off, covered by a raised lid, and filled with a mixture of water and glycol) placed at the six localities from 5 May to 5 July. Traps were placed in two main feeding habitats – meadows and cereal fields (chosen based on the previous telemetry research) within the Little Owl foraging areas at each monitored locality. In both habitat types we installed a linear transect, 50 m from the habitat edge, consisting of three traps at 10‐m intervals. In total, 36 traps at six localities (two habitats, six localities, three traps per locality) were installed. The sampling period was divided into six 14‐day periods and the traps were controlled at 1‐week intervals in the morning hours.
Statistical analyses
We used principal component analysis (pca) in canoco for Windows (Braak & Šmilauer Citation1998) for analysis of the multivariate diet component data, treating proportions of prey components as ‘species’ data. glmms (lmer function, r software) were used to assess the effect of factors on diet composition and habitat preferences of Little Owls, as well as for analysis of insect trappings. We developed all combinations of independent variables. The suitability of each model was assessed according to aic criteria and we chose the models with the lowest aic values. We used locality as a random factor to avoid spatial pseudo‐replications. statistica software (Statsoft, Inc.) was used to compute 2 × 2 tables and percentage differences.
RESULTS
Overall diet composition
Insects were the most numerous prey based on number (64.4%), but comprised only 1.2% by weight, being far outweighed by mammalian prey (96.8% by weight). Vertebrate prey consisted predominantly of small mammals; birds were represented only occasionally (Table ). Among mammals, Common Voles Microtus arvalis, were the most dominant prey species. Its total proportion was 24.4% by numbers (n = 219) and 69.1% by weight; the genus Apodemus, the second most abundant mammal prey, formed 2.1% of all prey items (7.5% by weight). Insect prey consisted mostly of the order Coleoptera (see Appendix, which is available via the Supplementary content tab of the article’s online page at http://dx.doi.org/10.1080/00063657.2010.494717).
Table 1. Weight, frequency and biomass of each component in the diet of Little Owls, based on pellet analysis.
The pca analysis of insect number percentages clearly showed that insects were the most important component of the diet (Fig. ). The proportion of insects was negatively correlated with that of vertebrates. A positive correlation was found between the abundance of Microtus and other rodents. Muridae, Soricidae and Aves were rather occasional supplemental food items. Further, the day of the season was strongly positively correlated with mean temperature (Spearman rank correlation, df = 27, r s = 0.91, P < 0.05) and precipitation (Spearman rank correlation, df = 27, r s = 0.75, P < 0.05) for the pellet‐collection period. Therefore, we included day of the season and locality into the subsequent glmm. The proportion of insects (as the dependent variable) in the diet (by numbers) was positively correlated with increasing day of the season (glmm gamma model, correlation 0.656; Table ), and negative for invertebrates.
Figure 2 Projection scores of the main components of the diet of Little Owls in western Bohemia expressed as numbers (principle component analysis, I and II axis explained 72.4 % of total variation). Horizontal axis, I; vertical axis, II.
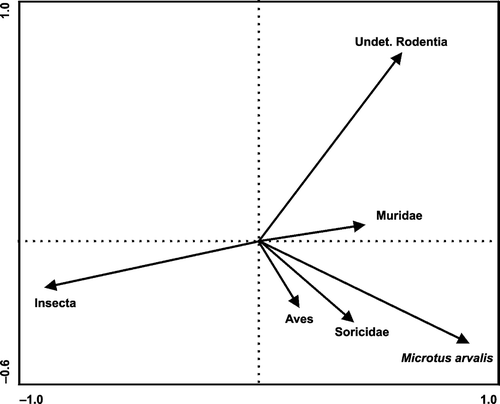
Table 2. Comparison of the composition of trapped insects and Little Owl insect diet composition (χ2test).
Insects in the diet and their availability
Insect prey consisted of 578 items belonging to 3 orders, 14 families and 31 species. Coleoptera were the most abundant invertebrate prey (67.1% by numbers, 69.4% by weight, n = 550), while Dermaptera and Hymenoptera comprised only 4.8% by number (n = 28). The most dominant Coleoptera were Carabidae (67.1%), followed by Curculionidae (9.7%) and Staphylinidae (7.3%). The most dominant species were Pterostichus melanarius (35.5%) and Harpalus affinis (12.3%). Poecilus cupreus was not a frequent species in the diet (0.5%), but dominated the insect‐trapping data (81.2%; Table ). Most of these insect prey were likely to have been caught on the ground (69.9%, n = 404) and only a few items taken in flight (2.4%, n = 14). Most of the Carabidae species are ground‐active, and occupy a variety of habitats.
We tested factors that affect the availability (abundance) of Carabidae prey (dependent variable). We included the 14‐day trapping periods (six in total), trapped species (nine) and habitat (two) into a glmm. All three parameters showed significant relationships with Carabidae abundance (Table ). Most Carabidae prey was trapped in the first 14‐day period (34.5%), and P. cupreus dominated in the overall trapping data (81.9%). Most carabids were trapped in cornfields (73.2%).
Table 3. Factors affecting the availability of insect prey on Little Owl hunting areas and the proportion of insect prey in the diet (Generalized linear mixed model with Poisson and Gamma distribution, respectively). BIC, Bayesian information criterion.
Pterostichus melanarius were the main insect prey in contrast with its low numbers in hunting areas, especially from meadows (Table ). Moreover, Little Owls mainly preferred meadows, and P. melanarius was most abundant in fields (Fig. ); these differences were significant (2 × 2 tables, χ2 = 47.5, df = 1, P < 0.0001).
Figure 3 Hunting habitat preferences of Little Owls in meadows and fields based on telemetry (n = 307 locations), and the proportional abundance of Pterostichus melanarius (n = 212 individuals) from traps in meadows and fields within Little Owl territories (2 × 2 tables, χ2 = 47.5, df = 1, P < 0.0001).
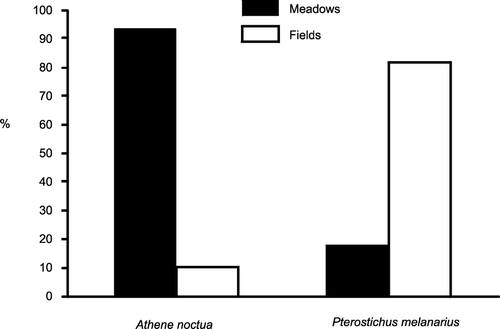
Because of the fact that Little Owls mainly preferred grasslands – particularly meadows and pastures – when foraging (Fig. ), we tried to explain which factors could affect the choice of hunting habitat. We included three factors in the glm: mean vegetation height, maximal vegetation height and density of vegetation cover. The density of vegetation was positively correlated with mean vegetation height (r s = 0.64) and was excluded from the analysis. The control locations had significantly higher mean vegetation cover than did Little Owl locations (binomial glm, 35% of explained variability, F = 18.7, P < 0.0001).
DISCUSSION
Little Owls are generalist predators feeding on a variety of prey species (Cramp Citation1985, Ille Citation1992, Schönn et al. Citation1991). In central Europe, vertebrate species (mainly mammals and birds) are the most important prey during winter, and insect prey dominates during the breeding season (Ille Citation1992, Schönn et al. 2001). In contrast, insects play a major role throughout the year in the Mediterranean area (Mánez Citation1983). Our results, consistent with earlier results obtained in central Europe, show the diet markedly changed during the breeding season. Small mammals were mostly captured at the beginning of breeding, with Microtus arvalis being the most frequent species. The proportion of this species in Little Owl diets can reach up to 80% (Laiu & Murariu Citation1997), but in central Europe generally ranges from 25 to 50% (Ille Citation1992, Schmid Citation2003). Higher proportions of vertebrate prey are probably linked with continental climatic conditions, especially during cold winters (Mlíkovský Citation1996, Obuch & Krištín Citation2004) when insects are not available.
Birds do not usually play an important role in the diet of Little Owls (Cramp Citation1985), though there can be exceptions (Hounsome et al. Citation2004). Hell (Citation1964) found a higher proportion of birds (especially passerines) during the harsh winter 1962/63 in Czechoslovakia. Our results show a low frequency of birds in the diet during the breeding season.
Invertebrates were the most dominant prey, comprising almost 65% of all prey remains by numbers. However, there was a great difference between invertebrate and vertebrate prey biomass (1.2% versus 98.8%) as has been demonstrated previously (Bon et al. Citation2001, Schmid Citation2003). This proportion of invertebrates in the diet is comparable with other studies from central and western Europe; Germany (Haensel & Walther Citation1966), Switzerland (Schmid Citation2003), France (Génot & Bersuder Citation1995), but is far below values from the Mediterranean region (Zerunian et al. Citation1982, Mánez Citation1983, Fattorini et al. Citation2001) and the Middle East (Obuch & Krištín Citation2004). The difference might stem from a greater abundance and availability of invertebrates in southern regions. Carabidae beetles have been found to be the most frequent prey species (Ille Citation1996, Schmid Citation2003), while Carabidae and Lumbricidae represent the main invertebrate prey in the Little Owl diet throughout Europe (Cramp Citation1985, Schönn et al. Citation1991). In our material, the proportion of Carabidae was 67.1% of the total insect prey numbers. We neglected Lumbricidae because of its sporadic occurrence in pellets; however, our analysis is likely to be biased toward prey items whose remains can be found after digestion. Camera monitoring of prey species brought to Little Owl nestlings have shown that Lumbricidae proportions are variable, but could be up to 65% of the diet (van Nieuwenhuyse et al. Citation2008). Similarly, the high proportion of Carabidae in pellets may also be because of the fact that those species have highly chitinised elements like elytra and are thus difficult to digest.
Little Owls have been described as ground hunters, which generally hunt from a perch and only occasionally take prey in the air (Cramp Citation1985, Mlíkovský Citation1998, Fajardo et al. Citation1998). These findings are in agreement with the Little Owls’ hunting habits in our study area, where most strikes are carried out from perches to the ground (Cramp Citation1985, Šálek Citation2004). Based on the known ecology of insect prey, we were able to infer some ecological characteristics of insect prey groups. At the family level, most Carabidae beetles taken by Little Owls were ground‐dwellers with nocturnal or intermediary activity.
Pterostichus cupreus, the main insect species found in traps, was not a frequent prey species, probably because it is most active during the day. Pterostichus melanarius, the most numerous insect prey, was most affiliated with cornfield habitats in our study. In contrast, Thiele (Citation1977) and H˚rka (Citation1996) describe this species as a typical inhabitant of flat open agricultural landscapes (fields and grasslands), with a slight preference towards moist habitats. Our analyses of the Little Owls’ hunting preferences demonstrate that one of the major factors determining habitat choice were vegetation height and the density of vegetation cover. Little Owls preferred grassland patches (especially grassy pastures, short sward lawns and mowed hayfields), which enabled efficient hunting on surface‐active soil insects. Hunting beetles in tall and dense swards is likely to be more difficult and energetically more costly, and may result in lower breeding success (Gassman & Bäumer Citation1993) or higher adult mortality (Exo Citation1988). During summer, especially during breeding, parents are likely to pay high costs to nourish themselves and their offspring. Although this period is characterised by increasing insect abundance, tall and dense vegetation will decrease its availability to the owls.
This importance of grasslands and pastures for Little Owls in the breeding season has been demonstrated by many studies (Loske Citation1986, Schönn et al. Citation1991, Dalbeck et al. Citation1999, Šálek & Berec Citation2001, Šálek & Schröpfer Citation2008), and has also been shown for many other grassland birds (Vickery et al. Citation2001, Atkinson et al. Citation2004, Devereux et al. Citation2006). The availability of short‐sward grassland patches in Little Owl territories (close to nesting sites) appears to be a limiting factor for the persistence of Little Owls in central European farmland areas. Although the amount of grasslands in the region has increased during the last two decades (Miko & Hošek Citation2009), current management practices (in most cases mowing twice per year) are not favourable for many animal taxa, including butterflies (Konvička et al. Citation2008), mammals (Šálek et al. Citation2009, Šálek et al. unpubl. data) and birds (Atkinson et al. Citation2004, Whittingham & Devereux Citation2008). Although the species richness of some of the ground‐dwelling beetles and other insects found in the Little Owl diet may be positively correlated with fertilisation levels or longer swards (Morris Citation2000, Söderström et al. Citation2001), tall and structural vegetation may result in changes to hunting practices and a dietary shift to alternative prey (Southern Citation1954, Bertolino et al. Citation2001), resulting in critically higher energy costs during the Little Owl’s breeding season.
tbis_a_494717_sup_16319128.pdf
Download PDF (18.6 KB)tbis_a_494717_sup_0001.zip
Download Zip (11.7 KB)ACKNOWLEDGEMENTS
We would like to thank J. Rajchard, J. Procházka, J. Hruška, L. Schröpfer, J. Wertigová for help with field work and A. Bezděk for identifying insects. We thank M. Konvička for valuable comments of the manuscript. D. Hardekopf, F. Sedláček and L.E. Blood improved the English. This study was supported by the grant MSMT6007665801 of the Czech Ministry of Education and by the research aim of the Institute of Systems Biology and Ecology AV0Z60870520.
REFERENCES
- Anděra , M. and Horáček , I. , eds. 1982 . Poznáváme naše savce Czech Republic Mladá Fronta, Prague
- Atkinson , P.W. , Buckingham , D. and M.rris , A.J. 2004 . What factors determine where invertebrate‐feeding birds forage in dry agricultural grasslands? . Ibis , 146 : 99 – 107 .
- Batáry , P. , Báldi , A. and Erdös , S. 2007 . Grassland versus non‐grassland bird abundance and diversity in manager grasslands: local, landscape and regional scale . Biodivers. Conserv , 16 : 871 – 881 .
- Bertolino , S. , Ghibeti , E. and Perrone , A. 2001 . Feeding ecology of the Long‐eared Owl (Asio otus) in northern Italy: is it a true specialist? . Can. J. Zool. , 79 : 2192 – 2198 .
- Bon , M. , Ratti , E. and Sartor , A. 2001 . Variazione stagionale della dieta della civetta Athene noctua (Scopoli, 1769) in una localita agricola della gronda lagunare veneziana. . Boll. M.s. Civ. Stor. Nat. Venez. , 52 : 193 – 212 .
- Braak , C.J.F. and Šmilauer , P. 1998 . CANOCO release 4. Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination , NY : Ithaca . Microcomputer Power
- Cramp , S. 1985 . Birds of the Western Palearctic Vol. 4. Terns to Woodpeckers , Oxford , , UK : Oxford University Press .
- Dalbeck , L. , Bergerhausen , W. and Hachtel , M. 1999 . Habitatpräferenzen des Steinkauzes Athene noctua SCOPOLI, 1769 im ortsnahen Grünland . Charadrius , 35 : 100 – 115 .
- Devereux , C.L. , M.Ceever , C. , Benton , T. and Whittingham , M.J. 2004 . The effects of sward height, density and drainage on starlings and lapwings foraging on grassland habitats . Ibis , 146 : 116 – 123 .
- Devereux , C.L. , Whittingham , M.J. , Krebs , J.R. , Fernández‐Juricic , E. and Vickery , J.A. 2006 . What attracts birds to newly mown pasture? Decoupling the action of mowing from the provision of short swards . Ibis , 148 : 302 – 306 .
- Donald , P.F. , Green , R.E. and Heath , M.F. 2001 . Agricultural intensification and the collapse of Europe’s farmland bird populations . Proc. R. Soc. Lond. B , 268 : 25 – 29 .
- Exo , K.M. 1988 . Jahreszeitliche ökologische Anpassungen des Steinkauzes (Athene noctua) . J. Ornithol. , 129 : 393 – 415 .
- Exo , K.M. 1989 . Tagesperiodische Aktivitätsmuster des Steinkauzes (Athene noctua) . Vogelwarte , 35 : 94 – 114 .
- Fajardo , I. , Pividal , V. , Tringo , M. and Jiménez , M. 1998 . “ Habitat selection, activity peaks and strategies to avoid road mortality by the Little Owl Athene noctua ” . In Alauda Vol. 66 , 49 – 60 . New methodology on owls research
- Fattorini , S. , M.nganaro , A. and Salvati , L. 2001 . Insect identification in pellet analysis: implications for the foraging behaviour of raptors . Buteo , 12 : 61 – 66 .
- Fuller , R.J. , Gregory , R.D. , Gibbons , D.W. , Marchant , J.H. , Baillie , S.R. and Carter , N. 1995 . Population declines and range contractions among lowland farmland birds in Britain . Conserv. Biol. , 9 : 1425 – 1441 .
- Gassmann , H. and Bäumer , B. 1993 . Zur Populationsökologie des Steinkauzes (Athene noctua) in der westlichen Jülicher Börde. Erste Ergebnisse einer 15 jährigen Studie . Vogelwarte , 37 : 130 – 143 .
- Génot , J.C. and Bersuder , D. 1995 . Le régime alimentaire de la chouette cheveche, Athene nocua, en Alsace‐Lorraine . Ciconia , 19 : 35 – 51 .
- Goodwin , J.G. and Hungerford , C.R. 1979 . Rodent Population Densities and Food Habits in Arizona Ponderosa Pine Forests USDA Forest Service Research Paper RM‐2 14. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO
- Haensel , J. and Walther , H.J. 1966 . Beitrag zur Ernährung der Eulen in Nordharz. – Vorland unter besonderer Berücksichtigung der Insekternverhaltung . Beitr. Vogelkd , 11 : 345 – 358 .
- Hell , P. 1964 . Prispevok k poznaniu potravy niektorych dravcov a sov v mimoriadne krutej zime 1962–63 . Zoologicke listy , 13 : 207 – 220 . [in Slovak]
- Hounsome , T. , O’M.hony , D. and Delahay , R. 2004 . The diet of Little Owls Athene noctua in Gloucestershire, England . Bird Study , 51 : 282 – 284 .
- Hudec , K. and Černý , W. , eds. 1977 . Fauna ČSSR, Vol. 23. Birds – Aves II , Czech Republic : Academia, Prague .
- H˚rka , K. 1996 . Carabidae of the Czech and Slovak Republics Czech Republic Kabourek, Zlín
- Ille , R. 1992 . Zum Biologie und Ökologie des Steinkauzes (Athene noctua) im Marchfeld: Aktuelle Situation und mögliche Schutzmanahmen . Egretta , 35 : 49 – 57 .
- Ille , R. 1996 . Zur Biologie und Ökologie zweier Steinkauzpopulationen in Ostösterreich . Abh. Zool.‐Bot. Ges. Österreich , 129 : 17 – 31 .
- Jacob , J. and Brown , J.S. 2000 . Microhabitat use, giving‐up densities and temporal activity as short and long‐term anti‐predator behaviours in common voles . Oikos , 91 : 131 – 138 .
- Jarošík , V. 1989 . Mass vs. length relationship for carabid beetles (Col., Carabidae) . Pedobiologia , 33 : 87 – 90 .
- Laiu , L. and Murariu , D. 1997 . Diet of the Little Owl (Athene noctua) during summer in a sub‐Carpathian depression of Moldovia – Romania . Trav. Mus. Natl Hist. Nat. ‘Grigore Antipa’ , 37 : 319 – 326 .
- Lemoine , N. , Bauer , H.G. , Peintinger , M. and Böhning‐Gaese , K. 2007 . Effects of climate and land‐use change on species abundance in a central European bird community . Conserv. Biol. , 21 : 495 – 503 .
- Loske , K.H. 1986 . Zum Habitat des Steinkauzes (Athene noctua) in der Bundesrepublik Deutschland . Die Vogelwelt , 107 : 91 – 101 .
- Konvička , M. , Beneš , J. , Čížek , O. , Kopeček , F. , Konvička , O. and Vitaz , L. 2008 . How too much care kills species: grassland reserves, agri‐environmental schemes and extinction of Colias myrmidone butterfly from its former stronghold . J. Insect Conserv. , 12 : 519 – 525 .
- Mánez , M. 1983 . Espectro alimentacio del Mochuelo común (Athene noctua) en Espána . Alytes , 1 : 275 – 290 .
- M.nganaro , A. , Ranazzi , L. and Salvati , L. 2001 . Diet overlap of Barn Owl (Tyto alba) and Little Owl (Athene noctua) in a mediterranean urban area . Buteo , 12 : 67 – 70 .
- Mikkola , H. 1983 . Owls of Europe T. & A.D. Poyser Calton UK
- M.ko , L. and Hošek , M. 2009 . Příroda a krajina České republiky. Zpráva o stavu 2009 Czech Republic Agentura ochrany přírody a krajiny ČR, Prague
- Mlíkovský , J. 1996 . Winter food of the Little Owl (Athene noctua) at Jablonná, central Bohemia . Buteo , 8 : 113 – 114 .
- Mlíkovský , J. 1998 . [Food Ecology of Native Raptors and Owls] Czech Republic (in Czech) ZO ČSOP Vlašim, Vlašim
- Møller , A.P. 1983 . Changes in Danish farmland habitats and their populations of breeding birds . Holarct. Ecol. , 6 : 95 – 100 .
- Morris , M.G. 2000 . The effects of structure and its dynamics on the ecology and conservation of arthropods in British grassland . Biol. Conserv. , 95 : 129 – 142 .
- Newton , I. 2004 . The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions . Ibis , 146 : 579 – 600 .
- Obuch , J. and Krištín , A. 2004 . Prey composition of the Little Owl Athene noctua in an arid zone (Egypt, Syria, Iran) . Folia Zool. , 53 : 65 – 79 .
- Rogers , L.E. , Hinds , W.T. and Buschbom , R.L. 1976 . A general weight vs length relationship for insects . Ann. Ent. Soc. Am. , 69 : 387 – 389 .
- Šálek , M. 2004 . Ekologie sýčka obecného (Athene noctua) v zemědělské krajině , University of South Bohemia . Master’s Thesis
- Šálek , M. and Berec , M. 2001 . Distribution and biotope preferences of the Little Owl (Athene noctua) in selected areas of the Southern Bohemia (Czech Republic) . Buteo , 12 : 127 – 134 .
- Šálek , M. and Schröpfer , L. 2008 . Recent decline of the Little Owl (Athene noctua) in the Czech Republic . Pol. J. Ecol. , 56 : 527 – 534 .
- Šálek , M. , Kreisinger , J. , Sedláček , F. and Albrecht , T. 2009 . Corridor vs. hayfield matrix use by mammalian predators in an agricultural landscape . Agric. Ecosyst. Environ. , 134 : 8 – 13 .
- Schmid , P. 2003 . Gewoellanalyse bei einer Population des Steinkauzes Athene noctua im Grossen Moos, einer intensiv genutzten Agrarlandschaft des schweizerischen Mittellandes . Ornithol. Beob. , 100 : 117 – 126 .
- Schönn , S. , Scherzinger , W. , Exo , K.M. and Ille , R. 1991 . “ Der Steinkauz ” . In Die Neue Brehm‐Bücherei , Vol. 606 , Germany : A. Ziemsen Verlag, Wittenberg Lutherstadt .
- Söderström , B. , Svensson , B. , Vessby , K. and Glimskär , A. 2001 . Plants, insects and birds in semi‐natural pastures in relation to local habitat and landscape factors . Biodivers. Conserv , 10 : 1839 – 1863 .
- Southern , H.N. 1954 . Tawny Owls and their prey . Ibis , 96 : 384 – 410 .
- Thiele , H.‐U. 1977 . Carabid beetles in their environments Vol. Springer‐Verlag , Berlin , , Germany
- Tucker , G.M. and Heath , M. F. 1994 . Birds in Europe: Their Conservation Status Cambridge , , UK BirdLife International
- van Nieuwenhuyse , D. , Génot , J.C. and Johnson , D.H. 2008 . The Little Owl: conservation, ecology and behaviour of Athene noctua , Cambridge , , UK : Cambridge University Press .
- Vickery , J.A. , Tallowin , J.R. , Feber , R.E. , Asteraki , E.J. , Atkinson , P.W. , Fuller , R.J. and Brown , V.K. 2001 . The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources . J. Appl. Ecol. , 38 : 647 – 664 .
- Whittingham , M.J. and Devereux , C.L. 2008 . Changing grass height alters foraging site selection by wintering farmland birds . Basic Appl. Ecol. , 9 : 779 – 788 .
- Zerunian , S. , Franzini , G. and Sciscione , L. 1982 . Little Owls and their prey in a Mediterranean habitat . Boll. Zool. , 49 : 195 – 206 .