Abstract
Capsule Slavonian Grebe nests are used as a focal point for feeding chicks in the early stages of growth, with considerable variation in feeding rates diurnally and across the duration of post‐hatch nest use.
Slavonian Grebes Podiceps auritus nest on partially floating platforms constructed from emergent vegetation (Fjeldså Citation2004). Clutch sizes vary from three to ten eggs, although larger clutches tend to be found in continental inland breeding populations compared with the North Atlantic populations (Ferguson & Sealy Citation1983, Fjeldså Citation2004). Incubation starts after the first egg is laid (Fjeldså Citation2004). Consequently, hatching is asynchronous. This provides a parental dilemma between optimising the number of eggs that hatch, while brooding and feeding growing chicks, and guarding against the threat of nest predation (Simmons Citation1974, Kloskowski Citation2001).
Grebe chicks are semi‐nidifugous: they can leave the nest shortly after hatching, but sometimes remain longer in the nest (Fjeldså Citation2004). At this stage, they do not have the ability to forage, fully thermoregulate or avoid potential predators. Consequently, chick mortality is high during the first few days after hatching (Fjeldså Citation1973, Citation2004). Adult grebes can carry young chicks on their backs on the water, where they are fed and brooded. This may provide greater security than the nest, but reduces the time devoted to continued incubation of unhatched eggs. In addition, if the off‐duty adult is caring for the hatched chicks away from the nest, while the other incubates, there is also a risk of brood separation and predation. This is particularly acute when the adult attending the chicks has to dive to forage. An alternative is to use the nest as a focal point for the care of hatched chicks and the incubation of unhatched eggs. Thus, the off‐duty adult is free to forage and provision the chicks without the need to maintain continual brooding and protection of the chicks.
Dillon et al. (Citation2008) showed that Slavonian Grebes may continue to use the nest for many days after the hatch of the first egg. During this period, the nest serves as a focal point for both incubation and chick care. Here we present data on the provisioning rates of grebe chicks in the nest during the period after hatching of the first egg. Our aim was to quantify the intensity of feeding behaviour during this critical period.
Data were available on the feeding rates at three nests in 2001 from video taken using a miniature camera and infra‐red illumination, which permitted recording during darkness. Photographs were taken at a rate of five per second, 24‐hours a day (Perkins et al. Citation2005). Nests A and B were at Loch Ruthven, Inverness‐shire (57° 19′ N, 4° 15′ W), a 152‐ha upland mesotrophic loch. Nest C was at a 0.5‐ha pool at a gravel workings in Morayshire (approximately 57° 25′ N, 2° 4′ W). All three nests were in Bottle Sedge Carex rostrata beds. There was no evidence that the presence of the camera influenced the likelihood of clutch predation or desertion, and adults showed no apparent response to nest cameras (Perkins et al. Citation2005). Nests A and C were thought to be first breeding attempts, whereas the laying dates at Nest B suggested that this was a replacement clutch (Dillon et al. Citation2008).
The period of video analysis was from the moment the first egg hatched until chicks or adults did not use the nest for 24 hours, or when the nest was depredated. This provided 154, 418 and 514 hours of observation at Nests A, B and C respectively. These 1086 hours took approximately 330 hours to transcribe. There was one malfunction of the recording equipment at Nest B, resulting in the loss of images on 19 August 2001 between 13:00 and 18:28. All times are given as British Summer Time, with the timing of dawn and dusk calculated using twilight tables (Royal Observatory Edinburgh Citation2010).
A provisioning visit was defined as a visit to the nest when the bills of both the adult and a chick appeared to touch or the necks of both the adult and a chick were extended towards each other. The provisioning rate was recorded as the number of provisioning visits to a nest by both adults, per chick, per day. It was often impossible to be certain which chicks were on the nest. This was because chicks hid under the wings or around the incubating adult, or there was poor image quality. The diurnal provisioning pattern was calculated as the mean number of feeds per chick during each hour between dawn and dusk, with all days and all nests combined. The only prey items that could be identified were small fish, assumed to be Three‐spined Sticklebacks Gasterosteus aculeatus. All other food items were too small to be identified. These were most likely to be aquatic invertebrates such as chironomids, crustaceans, aerial insects and aquatic coleoptera, which feature commonly in the typical grebe diet (Cramp & Simmons Citation1977, Fjeldså Citation2004).
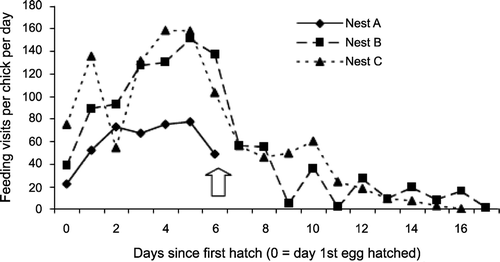
As expected, hatch was asynchronous at all nests (Dillon et al. Citation2008). Nest A was predated by an Otter Lutra lutra on the sixth day after the first egg hatched (Hancock et al. Citation2002). The rate of provisioning visits per chick increased from first hatching to a peak of 70–160 visits/chick/day on day five or six after hatching (Fig. ). After this, provisioning rates declined as chicks began to spend time away from their nests. The maximum visit rate to the nest, irrespective of the number of chicks using the nest, was 47 visits/hour. This occurred at Nest C between 11:00 and 12:00 on day four and five after first hatch.
Figure 1 The number of feeding visits per chick per day made by adult Slavonian Grebes at three nests in Scotland. The arrow indicates when Nest A was depredated.
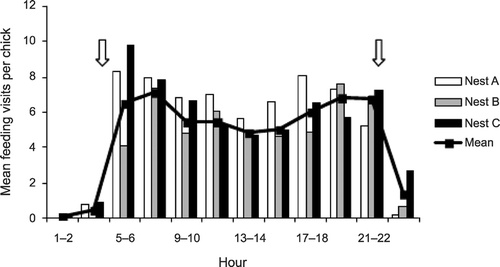
There was diurnal variation at all nests in the number of visits made by adult grebes during each 24‐hour period, with peaks during the first and last hours of daylight (Fig. ). At all nests, there were occasional night visits (Fig. ). A small number of fish were fed to the chicks, comprising a minimum of 2.7%, 2.4% and 1.3%, respectively, of the visits at each nest. Being much the largest food items, these would have represented a higher percentage of the biomass fed to chicks. For example, Fjeldså (Citation1973) found that despite invertebrates representing 71% of items in terms of abundance of stomach contents of Slavonian Grebes, small fish represented 69% by mass. There were also between one and two visits when fish presented by the adults to the chicks were rejected and the adult ate them. The adults also fed small numbers of feathers (primarily breast) to chicks while on the nest.
Figure 2 The diurnal pattern of feeding visits at three nests by adult Slavonian Grebes in Scotland. Arrows indicate approximate times of dawn and dusk. The three sets of columns represent the three nests.
The rapid increase in feeding visits (Fig. ) can be regarded as an adjustment to the increasing food demand as chicks grow and exhaust their residual reserves from the egg. The subsequent decline in feeding rate at the nest reflects increasing time spent away from the nest as chicks develop and are fed on the open water (Summers et al. Citation1994). The frequent feeding visits in the first five to six days, and the small number of fish identified as the prey, suggests that the chicks are fed mainly aquatic invertebrates gathered close to the nest during this period.
During the extended hatching period the nest is an important focal point both for brooding (Dillon et al. Citation2008) and for feeding (this study). High numbers of nest visits during this period increased the risk of nest predation owing to predators locating and following the adults’ approach route to the nest even in dense Bottle Sedge beds. A lower provisioning rate may cause slower chick growth rates, which could potentially lead to chicks remaining in the nest for longer. This longer attendance could itself lead to greater risk of predation because it increases the time available for predators to locate the nest, especially if the chicks become more vocal as they beg for food (Hakkarainen et al. Citation2002, Moreno‐Rueda Citation2005). It has been postulated, however, that the asynchronous hatch, rather than being an adaptation to cope with potential predation, is in fact an adaptation to mitigate possible starvation owing to food scarcity at hatching, with the advantage being proffered to the chicks that hatch first (Simmons Citation1974, Kloskowski Citation2001, Fjeldså Citation2004).
In addition to brood size, the quality and quantity of prey that is delivered may influence the adult provisioning rate and chick growth‐rate (Jackson Citation2003, Kloskowski Citation2004). Given the greater biomass per item of sticklebacks compared with other potential food items such as corixid bugs and emerging chironomids (Fjeldså Citation1973, Citation2004), it is surprising that fish were not provided more regularly during the early stages of chick development, especially as large numbers were available in both lochs (Summers et al. Citation1994). The feeding constraint hypothesis suggests that complex interactions may occur between prey size, energy demands and foraging efficiency (Slagsvold & Wiebe Citation2007). The ability to capture abundant prey close to the nests may also be an issue determining prey choice in the early days of chick development and may influence the provisioning rate. Fjeldså (Citation1973) showed that adult Slavonian grebes fed closer to the nest at all stages of the breeding attempt on rich lakes compared with poor lakes. The rapid provisioning rates may suggest that the two lochs in this study may be considered rich as measured by benthic abundance and hydrochemistry (Fjeldså Citation1973).
The small percentage of fish delivered to the chicks may also reflect an inability of young chicks to swallow large food items (Sjölberg Citation1985, Ulenaers & Dhondt Citation1994, Fjeldså Citation2004). This is supported by the rejection of fish by chicks on 7% of occasions when fish were presented during this study. For larger chicks being fed out on the open water, the percentage of food items that were sticklebacks was 34% and 46% during two years at Loch Ruthven and 65% at the Morayshire loch (Summers et al. Citation1994). Further, studies on Western Grebes Aechmophorus occidentalis in Canada (Forbes & Sealy Citation1990), Great Crested Grebes Podiceps cristatus in the Netherlands (Ulenaers & Dhondt Citation1994), and Red‐necked Grebes Podiceps grisegena in Poland (Kloskowski Citation2004) also showed that the size of food items offered increased with chick age. Alternatively, it may indicate a rapid depletion of optimal prey items near the nest, as has been found to occur with Hoodless Grebes Podiceps gallardoi in Argentina (Fjeldså Citation1986).
It appears, therefore, that aquatic invertebrates may be an important food source during the first days of chick development, with sticklebacks becoming important as the chicks become older. Grebe studies, and perhaps those of similar semi‐nidifugous species, based only on observations away from the nest, may underestimate the importance of small prey items in the early chick period (Kloskowski Citation2004). A gradual change to increasing proportions of fish in the diet has previously been found in Black‐throated Divers Gavia arctica in Scotland (Jackson Citation2003) and Red‐necked Grebes in Poland (Kloskowski Citation2004). Jacobsen (Citation1991) reported peaks of chironomid availability on emergent vegetation around dawn and dusk, whereas during the middle part of the day their availability was reduced because the chironomids swarmed above the vegetation. It is plausible that such a pattern also occurs around emergent vegetation in Scottish lochs. This would explain the post‐dawn and dusk peaks in feeding visits to the nests (Fig. ), although it is also possible this is related to rapid feeding of the chicks after a period of night starvation and to mitigate against hunger during the night. Periods of high winds could also considerably reduce the abundance and availability of chironomids on emergent vegetation. Indeed, day two after first hatch at Nest C was a windy day and the number of feeding visits recorded, taking into account the number of chicks and age of the chicks, was considerably lower than the preceding and following days (Fig. ). Such difficulties in delivering large numbers of invertebrates during periods of poor weather may lead to starvation (Kloskowski Citation2004).
The high number of feeding visits suggests that a large percentage of the food delivered to the chicks is found easily and close to the nest, thereby providing a focal point for feeding (‘central‐place foraging’ [Stevens & Krebs Citation1986]) rather than attempting to feed chicks dispersed around the sedge bed or loch. This may have the advantages of reducing foraging time, better enabling the adults to protect and thermoregulate their brood during the critical first few days of life (Ulenaers & Dhondt Citation1994, Summers & Mavor Citation1998). The fifth day after the first egg hatched was when continuous incubation ceased at all nests even though unhatched eggs remained in the nests (Dillon et al. Citation2008). This may have been the critical point when the advantage of hatching further eggs was outweighed by the continued predation risk at the nest, together with the increasingly difficult task of brooding all of the growing chicks. Despite this, provisioning continued, albeit increasingly sporadically, at the nest for many days, further highlighting the possible advantage of having a focal point for provisioning rather than attempting to provision a dispersed brood on the open water.
This study, together with Dillon et al. (Citation2008), highlights the importance of the nest beyond the time when chicks hatch. This behaviour has not previously been recorded in Slavonian Grebes, but has been recorded in other grebes, e.g. White‐tufted Grebes Rollandia rolland, Tachybaptus spp. and Podilymbus spp. (Fjeldså Citation2004). We have also shown the potential importance of aquatic invertebrates as chick food in the early days of development, complementing other work showing the importance of small fish to slightly older chicks (Summers et al. Citation1994).
ACKNOWLEDGEMENTS
Two anonymous referees are thanked for their helpful comments, which greatly improved the manuscript. Many thanks are also due to Stuart Benn for constant encouragement to study and conserve Slavonian Grebes in Scotland.
REFERENCES
- Cramp , S. and Simmons , K.E.L. , eds. 1977 . The Birds of the Western Palearctic , Vol. 1 , Oxford , , UK : Oxford University Press .
- Dillon , I.A. , Hancock , M. and Summers , R.W. 2008 . Post‐hatch nest use by Slavonian Grebes in Scotland . Br. Birds , 101 : 201 – 203 .
- Ferguson , R.S. and Sealy , S.G. 1983 . Breeding ecology of the Horned Grebe, Podiceps auritus, in Southwestern Manitoba . Can. Field Nat. , 97 : 401 – 408 .
- Fjeldså , J. 1973 . Feeding and habitat selection of the Horned Grebe, Podiceps auritus (Aves), in the breeding season . Videnskabelige Meddelser fra Dansk Naturhistorik Forening , 136 : 57 – 95 .
- Fjeldså , J. 1986 . Feeding ecology and possible life history tactics of the Hooded Grebe Podiceps gallardoi . Ardea , 74 : 40 – 58 .
- Fjeldså , J. 2004 . The Grebes , Oxford , , UK : Oxford University Press .
- Forbes , L.S. and Sealy , S.G. 1990 . Foraging roles of male and female Western Grebes during brood rearing . Condor , 92 : 421 – 426 .
- Hakkarainen , H. , Yli‐Tuomi , I. , Korpimaki , E. and Ydenberg , R. 2002 . Provisioning response to manipulation of apparent predation danger by parental Pied Flycatchers . Ornis Fennica , 79 : 139 – 144 .
- Hancock , M. , Summers , R. and Butcher , N. 2002 . Predation of Slavonian Grebe nests by Otters . Br. Birds , 95 : 390 – 394 .
- Kloskowski , J. 2001 . Temporal patterns of parental resource distribution in the Red‐necked Grebe: equalizing the share of the survivors . Behaviour , 138 : 1355 – 1370 .
- Kloskowski , J. 2004 . Food provisioning in Red‐necked Grebes (Podiceps grisegena) at Common Carp (Cyprinus carpio) ponds . Hydrobiologia , 525 : 131 – 138 .
- Jackson , D.B. 2003 . Between‐lake differences in the diet and provisioning behaviour of Black‐throated Divers Gavia arctica breeding in Scotland . Ibis , 145 : 30 – 44 .
- Jacobsen , O.W. 1991 . Feeding behaviour of breeding Wigeon Anas penelope in relation to seasonal emergence and swarming behaviour of chironomids . Ardea , 79 : 409 – 418 .
- Moreno‐Rueda , G. 2005 . A trade‐off between predation risk and sibling competition in the begging behaviour of Coal and Great Tits . J. Field Ornithol. , 76 : 390 – 394 .
- Perkins , A.J. , Hancock , M.H. , Butcher , N. and Summers , R.W. 2005 . Use of time‐lapse video cameras to determine causes of nest failure of Slavonian Grebes Podiceps auritus . Bird Study , 52 : 159 – 165 .
- Royal Observatory Edinburgh . 2010 . “ Rise and set times for the sun and moon ” . http://www.roe.ac.uk/info/srss.html (accessed 7 May 2010)
- Simmons , K.E.L. 1974 . Adaptations in the reproductive biology of the Great Crested Grebe . Br. Birds , 67 : 413 – 37 .
- Sjöberg , K. 1985 . Foraging activity patterns in the Goosander (Mergus merganser) and the Red‐breasted Merganser (M. serrator) in relation to patterns of activity in their major prey species . Oecologia , 67 : 35 – 39 .
- Slagsvold , T. and Wiebe , K.L. 2007 . Hatching asynchrony and early nestling mortality: the feeding constraint hypothesis . Anim. Behav. , 73 : 691 – 700 .
- Stevens , D.W. and Krebs , J.R. 1986 . Foraging Theory , Princeton , NJ : Princeton University Press .
- Summers , R.W. and Mavor , R.A. 1998 . Nest site selection and the time of breeding by Slavonian Grebes Podiceps auritus in Scotland . Wildfowl , 49 : 219 – 227 .
- Summers , R.W. , Mavor , R.A. and Hogg , S. Factors affecting loch selection and breeding success of Slavonian Grebes in Scotland . RSPB report to Scottish Natural Heritage . Inverness : RSPB .
- Ulenaers , P. and Dhondt , A.A. 1994 . Great Crested Grebe Podiceps cristatus chick mortality in relation to parental fishing . Bird Study , 41 : 211 – 220 .