Abstract
Capsule In most years, changes in numbers are associated with variations in breeding success.
Aims To describe the annual variation in numbers and breeding success of Capercaillies Tetrao urogallus and Black Grouse Tetrao tetrix at Abernethy Forest, and their inter‐relationships.
Methods Numbers and breeding success of Capercaillies and Black Grouse were recorded annually at Abernethy Forest (a Scots Pine Pinus sylvestris forest comprising ancient native, or semi‐natural, pinewood and plantations) during 1989–2009. Indices of abundance and densities of Capercaillies were obtained along transects, while counts of males at leks were obtained for both species. Breeding success (number of chicks per female) was obtained using dogs to locate females and chicks.
Results Capercaillie: the index of abundance increased to a peak in winter 1996/97 (2.7 times greater than in 1992/93) and then declined. There was no long‐term trend. In winters 2003/04 and 2004/05, the mean density was 4.2 per km2 (95% CLs 3.1–5.7). The total number was 140 (95% CLs 100–220) for 2003/04 and 170 (95% CLs 110–280) for 2004/05, comprising about 8% of the Scottish population. The number of males at leks peaked in 1995 (to 46 males) and again in 2004 (41 males), and there was no long‐term trend. The annual breeding success varied from 0 to 2.93 chicks per female (mean = 0.64). The mean was within the 95% CLs of an independent estimate of the productivity required to maintain numbers. In a free‐running model, annual breeding success and survival rates (which were assumed to improve when mortality owing to fence collisions was removed) largely accounted for the annual variation in the index of abundance, as measured from winter counts along transects during 1990/91 to 2002/03. If mortality associated with collisions with fences had continued, the index would have declined, assuming no immigration.
Black Grouse: The number of male birds at leks increased to a peak in 1997 (to 165 males), before falling back to a smaller number (about 50 males) in the early 2000s. There was a smaller peak in 2007. The annual breeding success varied from 0 to 4.71 chicks per female (mean = 1.76).
Conclusion Numbers of Capercaillies and Black Grouse varied over a 19‐year period at Abernethy Forest, but did not show either upward or downward trends, while the national population of Capercaillies dipped to a low level in 1998/99, and the Black Grouse population continued to decline. In most years, changes in numbers of both species were associated with variation in breeding production. Mortality caused by collisions with fences would have led to a decline in Capercaillie numbers if fences had not been removed.
In Scotland, the Capercaillie Tetrao urogallus population has declined substantially since the mid‐1970s (Moss Citation1994). Surveys showed there were only 2200 (95% CLs 1500–3200) during winters 1992/93 and 1993/94, and 1073 (95% CLs 549–2041) in winter 1998/99 (Catt et al. Citation1998, Wilkinson et al. Citation2002). There then followed an apparent recovery to 1980 birds (95% CLs 1284–2758) in 2003/04, though this estimate was not significantly different from the 1998/99 value (Eaton et al. Citation2007). Therefore, the Capercaillie remains on the red list of birds of conservation concern within the UK (Eaton et al. Citation2005). The Black Grouse Tetrao tetrix is also a species of conservation concern (Anon Citation1995, Eaton et al. Citation2005). In Britain, the number of lekking males (the only sex that can be monitored accurately by spring counts at leks) declined from an estimated 25 000 during 1989–1993, to 6500 in 1995–1996 and 3300 in 2005 (Baines & Hudson Citation1995, Hancock et al. Citation1999, Sim et al. Citation2008).
Within these national patterns, it is possible that numbers may show different patterns at individual sites, particularly where there have been attempts to stem the declines. Here, we describe the fluctuations in numbers of Capercaillies and Black Grouse at Abernethy Forest, an RSPB reserve where there has been conservation action to safeguard them by removing deer fences, which kill full‐grown birds, and controlling predators of ground‐nesting birds: Carrion Crows Corvus corone, Hooded Crows C. cornix and Red Foxes Vulpes vulpes during 1992–96 and from 2000 onwards (Catt et al. Citation1994, Summers, Green et al. Citation2004). In addition, deer densities have been reduced to encourage tree regeneration and reduce browsing pressure on shrub vegetation that is important in the diet of woodland grouse (Beaumont et al. Citation1995). Therefore, we expected grouse numbers to increase or at least be maintained. We also report on the annual variations in breeding success and how these relate to changes in numbers. Finally, annual changes in numbers of Capercaillies were modelled and compared with observed counts to examine the extent to which Abernethy Forest can be regarded as a ‘sink’ or ‘source’ for Capercaillies (Pulliam Citation1988).
STUDY AREA AND METHODS
Numbers of Capercaillies in winter
The study was carried out at Abernethy Forest (57° 15′ N, 3° 40′ W), Scotland, from 1989 to 2009. The forest (about 38 km2) comprises both ancient native pinewood (24.5 km2) and Scots Pine Pinus sylvestris plantations. There was no commercial extraction of timber though some young plantations were thinned up to the 1990s. During winters 1990/91 to 2002/03, Capercaillies were counted along 20 transects set randomly in the forest. Each was about 2 km long, U‐shaped and orientated north–south. The transects were walked in December, January and February or March in winter 1990/91, and in December, January, February and March in winters 1991/92 to 2002/03. Indices of abundance were derived from a Poisson regression analysis, with count as the y variable, and taking account of the effects of transect, month and year as fixed categorical effects. The coefficients of the year term provided indices of abundance. When testing for significant effects in the Poisson regression analyses, any over‐dispersion was accounted for using Pearson’s χ2 (Crawley Citation1993). Linear regression analysis of counts or indices against year was used to detect trends (Buckland et al. Citation2004).
From winter 2003/04, the sampling design was changed, partly because the size of the reserve had increased slightly, partly to obtain information on Capercaillie distribution, and also to improve the sighting rates of birds to obtain estimates of density using distance sampling (Buckland et al. Citation1993). Thus, 35 parallel transects, totalling 144 km, were set on an east–west orientation and 250 m apart across the forest. The change in sampling meant that the two sets of counts in winter were not comparable, though the difference was likely to be small because the increase in the size of the reserve was small. When a bird was sighted, the angle to the bird from the transect line was measured with a compass, and the radial distance measured with a laser range‐finder. These two values allowed calculation of the perpendicular distance from the transect line using trigonometry. The following models were fitted to the distribution of perpendicular distances (half‐normal, hazard‐rate and uniform, with appropriate expansions – cosine, Hermite polynomial and simple polynomial) and the best fit was based on the smallest aic. The best‐fitting model provided an estimate of the effective transect width and density (Thomas et al. Citation2005).
Lek counts of Capercaillies and Black Grouse
The numbers of male Capercaillies at all seven leks were counted on single visits at dawn in late April from hides or vehicles. Rain and strong winds were avoided because it is difficult to hear the calls of the males in these conditions. It is likely that some sub‐adult males were not present at the leks each morning (Wegge & Larson Citation1987). Counts of females were not analysed because the number seen at the leks may not reflect their true numbers, owing to their short period of attendance during the lekking season and because they visit more than one lek each spring (Storch Citation1997, K. Kortland pers. comm.). Data from one lek where a single male was recorded in only one year were omitted. In the early years, two lek sites were not known. In order to account for these presumed missing data, a Poisson regression model was used to derive lek and year terms from the existing data. The count at each lek in each year was the y variable, and lek and year were categorical effects. This allowed interpolation of missing data, which were then added to the counts for the leks that were visited.
Numbers of male Black Grouse were counted during early May at all 11 lek sites. Three leks were believed to be unknown during the early years of monitoring, so interpolated data were derived from a Poisson regression, as described previously, to obtain a more realistic pattern of change.
Breeding success of Capercaillies and Black Grouse
During late July and early August, a team of people walked line abreast with trained dogs through sections (totalling 5–6 km2) of the ancient native woodland that were regarded as good habitat for Capercaillie and Black Grouse broods, in order to flush and count females and any associated chicks. The median number of female Capercaillies encountered was 16 (range 10–34) and the median number of broods was four (range 0–13). For Black Grouse, the median number of females was 15 (range 7–27) and the median number of broods was eight (range 0–24). From 2001, brood searches were made in systematically chosen 1‐km squares or stands across the whole forest. The mean number of chicks per female (including those with no chicks) gave a measure of productivity. Bootstrap analyses were used to derive 95% CLs for each year (Efron Citation1982). At this time of year, the chicks are about a quarter to half the mass of adult birds, and suffer little additional mortality before independence (Baines Citation1993, Moss et al. Citation2000).
Changes in abundance in relation to breeding success
The effect of the productivity of Capercaillie and Black Grouse on the numbers of full‐grown birds was investigated. Changes in numbers at leks and indices of abundance along transects were expressed as a percentage change from one spring or winter (year n) to the next (year n + 1), and compared with productivity in the intervening summer in a linear regression analysis. For the lek data, it was assumed that one‐ and two‐year‐old males could also be part of the count (Wegge & Larsen Citation1987), though male Capercaillie do not breed until three or more years old (Storch Citation2001). Evidence for density dependence in population changes was tested by measuring the additional effect of the number in year n in the regression analysis. The residuals were plotted against time in order to see whether there was any visual indication of residual autocorrelation. In addition, the results from the standard linear regression model were compared against those obtained by fitting a linear regression model with first‐order autoregressive (AR(1)) normal errors using the autoreg procedure in sas (SAS Institute Citation2000). The lek data referred only to males, while the transect data included both sexes.
Modelling change in numbers
Capercaillie numbers were modelled using the measured breeding success (chicks per female) and estimates of survival (Moss et al. Citation2000) to examine annual changes in numbers at Abernethy Forest, assuming a closed population. It was assumed that there was an even sex ratio (based on initial counts) and that females breed at one‐year old, though some breed later than this (Storch Citation2001). If the model departed markedly from the observed counts, then migration could be important in explaining observed changes, or random error was affecting one of the demographic parameters. In a free‐running model, the number in a given year was used to predict the number in the next, so that any departure from the model was maintained in subsequent years (Rothery et al. Citation2002). Moss et al. (Citation2000) estimated juvenile and adult annual survival at 49.8% and 71.6%, respectively, when mortality owing to collisions with forest fences was common. In the absence of fence deaths, the survival rates were 71.6% and 78.1%, respectively. Fences at Abernethy Forest were largely removed by winter 1991/92 (Summers, Green et al. Citation2004), leaving only a boundary fence which was marked (Summers & Dugan Citation2001). Therefore, the higher survival rates were applied after this date. To determine the changes in Capercaillie numbers if fences had been retained, an additional free‐running model was applied, using the lower survival rates throughout the study period. To assess the goodness‐of‐fit of the model, a linear regression was fitted in which the difference between observed and modelled numbers in each year was examined as a function of the modelled number. Observations were weighted by the inverse of the square of the standard errors from the Poisson regression, and errors were assumed to be normally distributed with an AR(1) dependence structure. A significant slope would indicate a systematic discrepancy between the observed and modelled values.
A similar model for Black Grouse numbers has already been produced (Grant et al. Citation2009).
Weather data
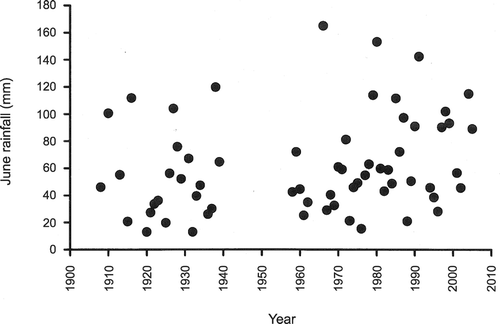
Data on June rainfall were obtained from the Meteorological Office, Edinburgh, for Grantown‐on‐Spey (about 10 km north of Abernethy Forest) for 1958–2006, and Aviemore (about 10 km southwest of Abernethy Forest) for 1983–2009. Earlier data were obtained from the local newspaper (Strathspey Herald) in which local chemists or the minister published rainfall data for Grantown‐on‐Spey (for 16 years during 1908–1939) or Nethybridge (for 6 years during 1915–1923). The latter is on the northern border of Abernethy Forest.
RESULTS
Winter counts of Capercaillies
Analysis of the counts of Capercaillies along the transects during winters 1990/91 to 2002/03 showed that there were significant differences among years (χ2 = 33.7, df = 12, P < 0.001), as well as differences among transects (χ2 = 517.5, df = 19, P < 0.001). There was no significant month effect. The index of abundance increased to a peak in winter 1996/97, and then declined to values similar to the start of the study period (Fig. ). Linear regression analysis showed that there was no long‐term trend (slope of regression line = 0.27, se = 0.27, P = 0.34).
Figure 1 Indices of abundance for Capercaillies (•) at Abernethy Forest during winter. The vertical lines show the 95% CLs. Abundance is also shown for a free‐running model (□) in which there was varying productivity (see Fig. ) and survival rates that improved after fence removal. An additional model (Δ) shows the pattern if fences had not been removed and Capercaillie survival had remained low.
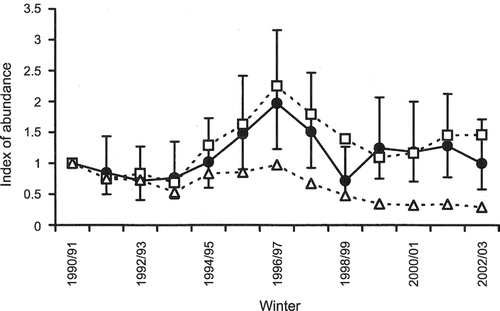
In winters 2003/04 and 2004/05, the locations of Capercaillies were recorded along the parallel transects across Abernethy. A half normal model with cosine adjustments was fitted to the distribution of perpendicular distances, giving an effective transect width of 39 m (78 m for both sides of the transect). Thus, the mean density of Capercaillies was 4.2 birds per km2 (95% CLs, 3.1–5.7) for the two winters combined. The values for the two winters were: 3.7 per km2 (95% CLs, 2.5–5.7) for winter 2003/04 and 4.6 per km2 (95% CLs, 2.9–7.3) for winter 2004/05. These densities translate to total numbers of 140 (95% CLs, 100–220) for 2003/04 and 170 (95% CLs, 110–280) for 2004/05.
Lek counts
During 1991–2009, counts of male Capercaillies varied between 0 and 15 at the different leks. In a Poisson regression analysis, there were marked differences in the number of males among leks (χ2 = 159, df = 6, P < 0.001), but only marginal differences between years (χ2 = 27.0, df = 18, P = 0.08). The fitted values from this model gave interpolated values for missing data for two leks in the first six years, assuming that these leks were occupied. These were added to the counts for the other leks to give a more realistic pattern for the early counts (Fig. ). Peak numbers occurred in 1995 (46 males) and 2004 (41 males). The data showed no long‐term trend (slope = −0.14, se = 0.31, P = 0.67).
Figure 2 Total numbers of male Capercaillies and Black Grouse at leks in Abernethy Forest. Some leks were not known in the early 1990s, and interpolated estimates were added (solid line). The open symbols show the actual counts in these years.
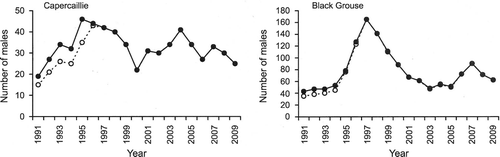
Eleven lek sites were used by Black Grouse, though three were not known in the early 1990s. In a Poisson regression analysis, there were significant differences in the number of males among leks (χ2 = 539, df = 10, P < 0.001) and years (χ2 = 134, df = 18, P < 0.001). The total number of males in the early 1990s was about 50, or about 40 without correction for presumed missing data. Numbers rose to 165 in 1997, and then fell to around 50–60 in the early 2000s. There was then a smaller peak in 2007 (Fig. ). There was no long‐term trend (slope = −0.35, se = 1.50, P = 0.82).
Breeding success
Annual breeding success for Capercaillies from 1989 to 2009 ranged from 0 to 2.93 chicks per female, and the mean was 0.64 (Fig. ). In the last nine years, when there was a programme of crow and Red Fox removal, the mean productivity was 0.54 chicks per female (range = 0–1.5).
Figure 3 Mean productivity (chicks per female) for Capercaillies and Black Grouse at Abernethy Forest. Vertical lines show the 95% CLs.
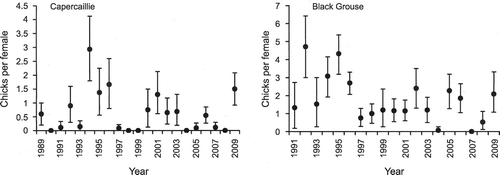
Annual breeding success for Black Grouse varied from 0 to 4.71 chicks per female between 1991 and 2009 (Fig. ) and the mean was 1.76 chicks per female. In the last nine years, the mean was 1.28 chicks per female (range = 0–2.4).
Changes in numbers in relation to breeding success
Changes in the indices of abundance for Capercaillies between winters, and between lek counts, were related to breeding success in the intervening summer. Both these relationships were positive, especially in the case of changes in winter indices when one outlying value was omitted (Fig. ). In this period (from winters 1998/99 to 1999/2000), there was a large increase in the index after a summer when there was no chick production. This indicates that, either the winter indices were incorrect, or that there had been an influx of birds. Nevertheless, based on the majority of years, there is evidence that changes in numbers at Abernethy were associated with the breeding success. There was no evidence of a density‐dependent effect (t = −0.66, P = 0.53, analysed without the outlier).
Figure 4 Percentage changes in the index of abundance for Capercaillies on transects and numbers of male Black Grouse at leks in relation to productivity (chicks per female) at Abernethy Forest. The regression equation for Capercaillies is: Change = −7.6 + 15.9 × Productivity (n = 12, P = 0.20); deleting the outlier (○): Change = −23.4 + 25.2 × Productivity (n = 11, P = 0.006); the regression equation for Black Grouse is: Change = −17.3 + 12.8 × Productivity (n = 18, P = 0.005); deleting the outlier (○): Change = −25.4 + 19.7 × Productivity (n = 17, P = 0.001); the fitted regression lines either exclude (solid lines) or include (dashed lines) the outliers.
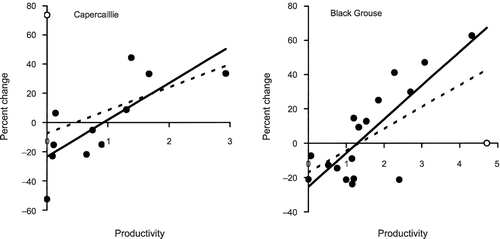
For Black Grouse, there was a significant correlation between the percentage change in the number of males at the leks and the breeding success in the intervening summer (Fig. ). There was one unusual value, where there was no change in numbers after the best ever breeding season (1992). Despite this, the significant relationship remained. There was no evidence of a density‐dependent effect (t = −1.54, P = 0.15, analysed without the outlier).
The basic regression models did not take into account that both change in numbers and productivity are time‐series. Thus, there was a possibility that the residuals of successive years were autocorrelated. However, visual inspection did not suggest that residuals of successive years were autocorrelated. Similar results were obtained whether errors were assumed to be independent or to have first‐order autocorrelation (for Capercaillies, P = 0.002 for the autoregressive model versus P = 0.006 for the basic regression model; and for Black Grouse, P = 0.020 for the autoregressive model versus P = 0.005 for the basic regression model). Therefore, we present only the results of the regression models without an autoregressive error structure.
The regression equations in Fig. provided estimates for the productivity required to maintain numbers (i.e. where the regression line cuts the x‐axis). For Capercaillies, these estimates were 0.5 chicks per female (including the outlier) and 0.9 (deleting the outlier). For Black Grouse, the regression lines indicates that productivity needs to be 1.4 chicks per female (including the outlier) or 1.3 (deleting the outlier) to maintain numbers.
Modelled change in numbers
Changes in the winter index for Capercaillies were modelled using the varying productivity (Fig. ) and fixed survival rates, the latter changing to account for the removal of fences after winter 1991/92. Variations in the modelled indices closely followed changes in the observed indices (as derived from the Poisson log‐linear regression) initially, but the match became poorer after the decline from the peak in winter 1996/97 (Fig. ). A weighted linear regression between the observed minus modelled indices and the modelled indices gave a slope that was significantly different from zero (−0.35, se = 0.12, t = −2.87, P = 0.015, assuming AR(1) errors), suggesting that there are some systematic discrepancies between the modelled and observed indices. It should be noted that any discrepancy in one year will be carried over into the following years because of the nature of the free‐running model, making a formal comparison of the observed and modelled indices difficult. However, many of the differences between the observed and modelled indices were relatively small and the modelled indices were well within the 95% CLs of the observed indices for all but one value (Fig. ). The correlation between modelled and observed indices was also high (r = 0.84, df = 11, P < 0.001). These results suggest that changes in the index of abundance for Capercaillies at Abernethy are quite strongly associated with variations in local breeding success.
The free‐running model was re‐run, but the low survival associated with additional mortality owing to fence collisions was retained throughout. In this case, the index declined to 29% of the observed figure by 2002/03 (Fig. ).
DISCUSSION
Numbers
Counts and indices of abundance for Capercaillies and Black Grouse at Abernethy Forest have shown variation between years, but no trends. Thus, numbers were maintained against a background of declines in both national populations (Eaton et al. Citation2007, Sim et al. Citation2008). The Scottish Capercaillie population declined from 2200 in winters 1992/93 and 1993/94 to 1100 in 1998/99, and the estimate in winter 2003/04 was 1980 individuals, though not significantly different from 1100 (Eaton et al. Citation2007). The c. 150 Capercaillies at Abernethy Forest in winters 2003/04 and 2004/05 represented about 8% of the Scottish population. The Black Grouse population in Britain fell to 3300 males by 2005 (Sim et al. Citation2008), so the number at Abernethy (51 males in 2005) represented about 1.5% of the British population.
The mean winter density of Capercaillies at Abernethy was less than densities of Capercaillies (5–10 per km2) in some Scandinavian woodlands (Sjöberg Citation1996). However, a proportion of Abernethy Forest was young woodland or clear‐fell, which are not preferred habitats for Capercaillies (Rolstad & Wegge Citation1989, Summers, Proctor et al. Citation2004) and much of the western part of Abernethy is unoccupied. Therefore, there is scope for the numbers and average density to increase as more of the forest becomes old‐growth woodland (Rolstad & Wegge Citation1989).
Changes in numbers in relation to breeding success
It may be expected that when breeding success is high, the number of full‐grown birds will increase, and when breeding success is low, the number will decline. However, radio‐tagged female Capercaillies have been known to leave Abernethy, with records from Strathavon (18 km from Abernethy), Revack (9 km) and Glenmore (9 km) (RSPB unpubl. data). Also, Moss et al. (Citation2006) reported natal dispersal distances of 1–30 km (median = 11 km) for first‐year females in Deeside. Therefore, there is inter‐change between neighbouring woods. This means that it is possible for the change in number from one winter to the next at Abernethy to be unrelated to breeding success in the intervening summer. Elsewhere in Scotland, for example, the breeding density of females in parts of the ancient native pinewoods at Glen Tanar (Deeside) and winter density of Capercaillies in selected parts of Kinveachy (Strathspey) were unrelated to the success of the previous breeding season (Moss & Oswald Citation1985, Moss & Weir Citation1987). It was thought that density‐dependent movements of young birds were important in determining numbers. However, the densities at Glen Tanar (4–8 females per km2) and Kinveachy (8–31 per km2 in November) were higher than at Abernethy, making density‐dependent emigration more likely. In addition, it is possible that some of the movements occurred just within these forests, which comprise different stand types. Thus, when changes in a whole forest were considered, as in our study at Abernethy Forest, there was a relationship between the change in the winter index and breeding success (Fig. ). Further, these changes in the index of abundance could be modelled using fixed survival values and local breeding success (Fig. ). Therefore, immigration and emigration with neighbouring woods must have been small or balanced, and Abernethy Forest has been neither a net ‘source’ nor ‘sink’ for Capercaillies. However, if fences had not been removed and survival had remained low (Moss et al. Citation2000), then the number would have declined, or immigrants would have been required to maintain numbers.
The relationship between changes in the number of Capercaillie males at the leks and breeding success was poorer than the relationship with transect counts. This may have been because of changes in lek attendance by young males (Wegge & Larsen Citation1987), as well as the difficulty of counting birds at leks within woodland.
Migration also appears to be of minor importance for explaining Black Grouse numbers at Abernethy because changes in their numbers were also associated with annual variation in productivity (at least for lekking males) (Grant et al. Citation2009). A model incorporating fixed survival rates for adults and young birds and the measured productivity followed the observed changes in their numbers.
Estimates were made of the productivity necessary to maintain numbers at Abernethy; 0.5 or 0.9 chicks per female for Capercaillie, and 1.4 or 1.3 for Black Grouse, depending on whether or not an outlying point in each data set was included (Fig. ). The values for Capercaillie are close to those obtained from a demographic model in which the requirements for maintenance were 1.1 chicks per female (95% CLs, 0.7–1.8) during a period when fence mortality was a major factor, and 0.62 (95% CLs, 0.3–1.0) in the absence of fence mortality (Moss et al. Citation2000). In the past nine years when predator control has been part of the management at Abernethy, breeding success has averaged 0.54 chicks per female for Capercaillie, close to the productivity required to maintain numbers when fence mortality is absent.
Between‐year variation in breeding success of Capercaillies and Black Grouse at Abernethy is partly related to rainfall in June (Summers, Green et al. Citation2004), as found in studies elsewhere on Capercaillie (Slagsvold & Grasaas Citation1979, Moss Citation1986) and Black Grouse (Ellison & Magnani Citation1984). Wet weather is thought to make chicks call more to the female for brooding, but this also alerts predators (Wegge & Kastdalen Citation2007). In addition, models indicate that breeding success is reduced by predation, either through a combination of crows plus mammalian predators, or crows on their own (Summers, Green et al. Citation2004). These results led to the reinstating of crow control at Abernethy Forest as a management prescription, and fox control was supported from findings from a Swedish study (Marcström et al. Citation1988).
With crows and Red Foxes being culled, it was expected that Capercaillie and Black Grouse breeding success would improve at Abernethy Forest, especially in years when rainfall was low (Summers, Green et al. Citation2004). However, there was above‐mean June rainfall in five of the last six years (2004–2009). Wetter Junes appear to be part of a long‐term climatic trend (Fig. ). Whether it is possible to mitigate against the effect of rainfall wetting chicks by habitat change, either by reducing the height of the shrubs (short‐term), or indirectly changing the shrub layer by changing the stand structure (long‐term), is the subject of ongoing research. In addition, it appears that the Pine Marten Martes martes has become a significant predator of Capercaillie clutches since marten numbers have increased at Abernethy (Summers et al. Citation2009). Therefore, it is proving difficult to improve the breeding success of woodland grouse.
ACKNOWLEDGEMENTS
We thank other staff and volunteers at Abernethy Forest for taking part in lek and brood counts. Brood counts were also carried out by D. Baines, M. Canham, D. Clements, S. Franks, D. Gladwin, R. Moss and R. Parr. S. Bierman and A. Butler helped with statistical analysis. L. Chambers and M. Duckett provided access to weather data at the Meteorological Office, Edinburgh and archived Strathspey Heralds at Grantown‐on‐Spey Museum, respectively. A. Dhondt, M. Grant, M. Hancock, K. Kortland, H. Lindén, W. Peach, N. Ratcliffe and J. Wilson commented on the drafts.
REFERENCES
- Anon . 1995 . “ Biodiversity: The UK Steering Group Report ” . In Action Plans , Vol. 2 , London : HMSO .
- Baines , D. 1993 . “ The Black Grouse report: first approaches towards the restoration of Black Grouse numbers in Britain ” . Report to English Nature and Scottish Natural Heritage
- Baines , D. and Hudson , P.J. 1995 . The decline of Black Grouse in Scotland and northern England . Bird Study , 42 : 122 – 131 .
- Beaumont , D. , Dugan , D. , Evans , G. and Taylor , S. 1995 . “ Deer management and tree regeneration in the RSPB reserve at Abernethy Forest ” . In Our Pinewood Heritage , Edited by: Aldhous , J.R. 186 – 195 . Farnham , , UK : Forestry Commission, RSPB, SNH .
- Buckland , S.T. , Anderson , D.R. , Burnham , K.P. and Laake , J.L. 1993 . Distance Sampling: Estimating Abundance of Biological Populations , London : Chapman & Hall .
- Buckland , S.T. , Anderson , D.R. , Burnham , K.P. , Laake , J.L. , Borchers , D.L. and Thomas , L. 2004 . Advanced Distance Sampling: Estimating Abundance of Biological Populations , Oxford , , UK : Oxford University Press .
- Catt , D.C. , Dugan , D. , Green , R.E. , Moncrieff , R. , Moss , R. , Picozzi , N. , Summers , R.W. and Tyler , G.A. 1994 . Collisions against fences by woodland grouse in Scotland . Forestry , 67 : 105 – 118 .
- Catt , D.C. , Baines , D. , Picozzi , N. , Moss , R. and Summers , R.W. 1998 . Abundance and distribution of Capercaillie Tetrao urogallus in Scotland 1992–1994 . Biol. Conserv. , 85 : 257 – 267 .
- Crawley , M.J. 1993 . GLIM for Ecologists , Oxford , , UK : Blackwell .
- Eaton , M.A. , Gregory , R.D. , Noble , D.G. , Robinson , J.A. , Hughes , J. , Procter , D. , Brown , A.F. and Gibbons , D.W. 2005 . Regional IUCN red listing: the process as applied to birds in the United Kingdom . Conserv. Biol. , 19 : 1557 – 1570 .
- Eaton , M.A. , Marshall , K.B. and Gregory , R.D. 2007 . Status of Capercaillie Tetrao urogallus in Scotland during winter 2003/04 . Bird Study , 54 : 145 – 153 .
- Efron , B. 1982 . The Jacknife, the Bootstrap and Other Resampling Plans , Philadelphia , PA : Society of Industrial and Applied Mathematics .
- Ellison , L.N. and Magnani , Y. 1984 . Changes in Black Grouse Tetrao tetrix densities in the French Alps . Int. Grouse Symp. , 3 : 434 – 460 .
- Grant , M.C. , Cowie , N. , Donald , C. , Dugan , D. , Johnstone , I. , Lindley , P. , Moncreiff , R. , Pearce‐Higgins , J.W. , Thorpe , R. and Tomes , D. 2009 . Black Grouse response to dedicated conservation management . Folia Zool , 58 : 195 – 206 .
- Hancock , M. , Baines , D. , Gibbons , D. , Etheridge , B. and Shepherd , M. 1999 . Status of male Black Grouse Tetrao tetrix in Britain in 1995–96 . Bird Study , 46 : 1 – 15 .
- Marcström , V. , Kenward , R.E. and Engren , E. 1988 . The impact of predation on boreal tetraonids during vole cycles: an experimental study . J. Anim. Ecol. , 57 : 859 – 872 .
- Moss , R. 1986 . Rain, breeding success and distribution of Capercaillie Tetrao urogallus and Black Grouse Tetrao tetrix in Scotland . Ibis , 128 : 65 – 72 .
- Moss , R. 1994 . Decline of Capercaillie (Tetrao urogallus) in Scotland . Gibier Faune Sauvage , 11 (special issue part 2) : 217 – 222 .
- Moss , R. and Oswald , J. 1985 . Population dynamics of Capercaillie in a north‐east Scottish glen . Ornis Scand , 16 : 229 – 238 .
- Moss , R. and Weir , D.N. 1987 . Demography of Capercaillie Tetrao urogallus in north‐east Scotland. III. Production and recruitment of young . Ornis Scand. , 18 : 141 – 145 .
- Moss , R. , Picozzi , N. , Summers , R.W. and Baines , D. 2000 . Capercaillie Tetrao urogallus in Scotland – demography of a declining population . Ibis , 142 : 259 – 267 .
- Moss , R. , Picozzi , N. and Catt , D.C. 2006 . Natal dispersal of Capercaillie Tetrao urogallus in northeast Scotland . Wildl. Biol. , 12 : 227 – 232 .
- Pulliam , H.R. 1988 . Sources, sinks, and population regulation . Am. Nat. , 132 : 652 – 661 .
- Rolstad , J. and Wegge , P. 1989 . Capercaillie habitat: a critical assessment of the role of old forest . Int. Grouse Symp. , 4 : 235 – 249 .
- Rothery , P. , Harris , M.P. , Wanless , S. and Shaw , D.N. 2002 . Colony size, adult survival rates, productivity and population projections of Black‐legged Kittiwakes Rissa tridactyla on Fair Isle . Atlantic Seabirds , 4 : 17 – 28 .
- SAS Institute . 2000 . SAS/STAT Users’ Guide, Version 8 , Cary , NC : SAS Institute .
- Sim , I.M.W. , Eaton , M.A. , Setchfield , R.P. , Warren , P.K. and Lindley , P. 2008 . Abundance of male Black Grouse Tetrao tetrix in Britain in 2005, and change since 1995–96 . Bird Study , 55 : 304 – 313 .
- Sjöberg , K. 1996 . “ Modern forestry and the Capercaillie ” . In Conservation of Faunal Diversity in Forested Landscapes , Edited by: DeGraaf , R.M. and Miller , R.I. 111 – 135 . London : Chapman & Hall .
- Slagsvold , T. and Grasaas , T. 1979 . Autumn population size of the Capercaillie Tetrao urogallus in relation to weather . Ornis Scand. , 10 : 37 – 41 .
- Storch , I. 1997 . Male territoriality, female range use and spatial organization of Capercaillie leks . Wildl. Biol. , 3 : 149 – 161 .
- Storch , I. 2001 . Tetrao urogallus Capercaillie . BWP Update , 3 (1) : 1 – 24 .
- Summers , R.W. and Dugan , D. 2001 . An assessment of methods used to mark fences to reduce bird collisions in pinewoods . Scottish Forestry , 55 : 23 – 29 .
- Summers , R.W. , Green , R.E. , Proctor , R. , Dugan , D. , Lambie , D. , Moncrieff , R. , Moss , R. and Baines , D. 2004 . An experimental study of the effects of predation on the breeding productivity of Capercaillie and Black Grouse . J. Appl. Ecol. , 41 : 513 – 525 .
- Summers , R.W. , Proctor , R. , Thornton , M. and Avey , G. 2004 . Habitat selection and diet of the Capercaillie Tetrao urogallus in Abernethy Forest, Strathspey, Scotland . Bird Study , 51 : 58 – 68 .
- Summers , R.W. , Willi , J. and Selvidge , J. 2009 . Capercaillie Tetrao urogallus nest loss and attendance at Abernethy Forest, Scotland . Wildl. Biol. , 15 : 319 – 327 .
- Thomas , L. , Laake , J.L. , Strindberg , S. , Marques , F.F.C. , Buckland , S.T. , Borchers , D.L. , Anderson , D.R. , Burnham , K.P. , Hedley , S.L. , Pollard , J.H. , Bishop , J.R.B. and Marques , T.A. 2005 . Distance 5.0. Release 1 , St Andrews , , UK : Research Unit for Wildlife Population Assessment, University of St Andrews .
- Wegge , P. and Kastdalen , L. 2007 . Pattern and causes of natural mortality of Capercaillie, Tetrao urogallus, chicks in a fragmented boreal forest . Ann. Zool. Fennici , 44 : 141 – 151 .
- Wegge , P. and Larsen , B.B. 1987 . Spacing of adult and subadult male Common Capercaillie during the breeding season . Auk , 104 : 481 – 490 .
- Wilkinson , N.I. , Langston , R.H.W. , Gregory , R.D. , Gibbons , D.W. and Marquiss , M. 2002 . Capercaillie Tetrao urogallus abundance and habitat use in Scotland, in winter 1998–99 . Bird Study , 49 : 177 – 185 .