Abstract
Capsule Vegetation structure and invertebrate abundance interact to influence both foraging sites and nestling provisioning rate; when invertebrate availability is low, adults may take greater risks to provide food for their young.
Aims To investigate nesting and foraging ecology in a declining farmland bird whose fledging success is influenced by the availability of invertebrate prey suitable for feeding to offspring, and where perceived predation risk during foraging can be mediated by vegetation structure.
Methods Provisioning rates of adult Yellowhammers feeding nestlings were measured at nests on arable farmland. Foraging sites were compared with control sites of both the same and different microhabitats; provisioning rate was related to habitat features of foraging‐sites.
Results Foraging sites had low vegetation density, probably enhancing detection of predators, or high invertebrate abundance at high vegetation density. Parental provisioning rate decreased with increasing vegetation cover at foraging sites with high invertebrate abundance; conversely, where invertebrate abundance was low, provisioning rate increased with increasing vegetation cover.
Conclusions Vegetation structure at foraging sites suggests that a trade‐off between predator detection and prey availability influences foraging site selection in Yellowhammers. Associations between parental provisioning rate and vegetation variables suggest that where invertebrate abundance is high birds increase time spent scanning for predators at higher vegetation densities; however, when prey are scarce, adults may take more risks to provide food for their young.
Populations of many avian species are in decline as birds have been unable to adapt to reductions in food availability (Both et al. Citation2006, Pearce‐Higgins et al. Citation2010) brought about by a combination of climate change and altered land management practices (Gaston et al. Citation2009, Hart et al. Citation2006). Where food availability in favoured foraging habitats is now insufficient, birds may be forced to forage in unfavourable habitats where food may be more abundant but predation risk may be increased (Butler et al. Citation2005, Whittingham et al. Citation2004), in order to feed themselves and their offspring adequately. However, empirical evidence for increased risk‐taking as a result of reduced food availability is scarce.
Populations of farmland birds have been declining in recent years owing to agricultural intensification (Chamberlain & Fuller Citation2000, Fuller et al. Citation1995). The majority of these declines began in the mid to late 1970s (Fuller et al. Citation1995), but Yellowhammers Emberiza citrinella apparently did not begin to decline until the late 1980s (Kyrkos et al. Citation1998). However, between then and 2007 they underwent an estimated population reduction of 54% (Eaton et al. Citation2008). Moreover, unlike many other farmland birds whose populations have now begun to stabilise, Yellowhammer populations are still declining (Eaton et al. Citation2008).
Yellowhammer populations are limited by nesting habitat (Kyrkos et al. Citation1998, Stoate & Szczur Citation2001, Whittingham et al. Citation2005) and adults construct nests both among herbaceous vegetation in ditches and within hedgerows (Bradbury et al. Citation2000). Nestling mortality in this species has been linked to weather variables such as cold temperatures and increased rainfall that decrease both numbers and activity levels of invertebrates (Bradbury et al. Citation2003, Stoate et al. Citation1998), and a reduction in the growth and body condition of chicks has been linked to the use of pesticides during the breeding season, through a decrease in invertebrate populations (Hart et al. Citation2006, Morris et al. Citation2005). Habitat type and structure can also influence the availability of invertebrates to foraging birds; grass margins, tractor tramlines and patches of bare ground are selected over cropped areas for foraging (Douglas et al. Citation2010, Perkins et al. Citation2002). Within cropped areas, broad‐leaved crops and bare ground are favoured, with cereal crops being utilised more often as non‐cropped habitats increase in vegetation density, reducing invertebrate accessibility in these favoured habitats (Douglas et al. Citation2009).
An interaction between food abundance and accessibility in predicting foraging site suitability for Yellowhammers, mediated by habitat structure, was proposed by Morris et al. (Citation2001). This was demonstrated by Douglas et al. (Citation2009), who found cut margins to be used more often than uncut margins by foraging Yellowhammers, indicating that accessibility of invertebrate prey plays a large part in determining the selection of foraging habitats. Predation risk to foraging adults may also play a part in habitat selection: Yellowhammers are sensitive to perceived predation risk (van der Veen Citation1999) and the choice of foraging habitat may be influenced by perceived predation risk mediated by habitat structure (Butler et al. Citation2005, Whittingham et al. Citation2004, Whittingham et al. Citation2006a, Whittingham & Evans Citation2004) as well as through food abundance and accessibility. When the relative density of prey in preferred foraging habitats – that may be more accessible or provide a greater visibility of predators (Whittingham et al. Citation2004) – falls below a certain threshold, foraging adults may be forced to switch foraging habitat to one which is less accessible or more risky in terms of predator detection (Butler et al. Citation2005). If foraging‐site selection influences provisioning rate, this may also impact upon chick demographics (Dunn et al. Citation2010), potentially with longer‐term population‐level implications (Metcalfe & Monaghan Citation2001).
Here, we examine the selection of both nest‐sites and foraging‐sites by Yellowhammers and relate foraging‐site selection to both food availability and predation risk to the foraging adult. First, we examine nesting ecology and nest‐site selection at the within‐hedgerow scale to determine whether specific microhabitats within a hedge are selected for nesting. Secondly, we investigate foraging ecology. Previous studies of Yellowhammer foraging‐site selection have mostly looked at site selection at the habitat scale (Morris et al. Citation2001, Perkins et al. Citation2002, Stoate et al. Citation1998). Yellowhammer foraging habits have been linked to bare ground and a short sward (Douglas et al. Citation2009, Stoate et al. Citation1998) and Yellowhammers are sensitive to predation risk (van der Veen Citation1999), which can be influenced by foraging habitat structure (Whittingham et al. Citation2004, Whittingham & Evans Citation2004). The present study compared foraging sites with randomly placed control sites, within the same microhabitat, for example tramlines within a crop, and within a different microhabitat. This was to help identify important features influencing habitat choice at the within‐field scale and to determine whether birds foraging in areas of low invertebrate abundance may take greater risks when foraging in order to provision their young adequately. Habitat features of foraging sites were also linked to parental provisioning rate. This was done to determine whether features of foraging sites may influence foraging success, which in turn is likely to have implications for chick provisioning.
METHODS
Study sites
Fieldwork was carried out between April and August in 2006 on three farms near Bramham, Yorkshire, and between May and July in 2007 and 2008 on 12 farms in Gloucestershire, Hampshire, Wiltshire and West Sussex. Land‐use consisted of a combination of arable crops (spring and winter wheat, spring and winter barley, oilseed rape, vining peas, potatoes, field beans, and sugar beet), grass grown for silage, set‐aside (grass‐sown and natural stubble re‐growth), agroforestry with arable set‐aside and pasture grazed by cattle, pigs or horses. Fields were bounded by ditches, hedgerows, tree‐lines, fences, grass margins or green lanes.
Nest location
Territorial pairs were located by repeated observations of singing males and foraging pairs. Once pairs had been located, observations allowed the approximate positioning of a nest to be detected; nests were then located by a systematic search of this region. The height of the nest above ground and vegetation type within which the nest was built were recorded, along with the height and width of the hedgerow at the nest‐site. Distance to the nearest songpost was also recorded; a songpost was defined as a piece of vegetation prominent above the rest of the hedgerow such as those used by male Yellowhammers; these tended to be tree branches, the top of Elder Sambucus nigra bushes, or long stems of Hawthorn Crataegus spp. bushes.
Fifty‐one nests were monitored across three breeding seasons between 2006 and 2008. To determine whether adult Yellowhammers exhibited selection for particular nest site features within hedgerows measurements were obtained from random sites within the hedgerow and within 25 m either side of the nest. Sites were selected through the use of random numbers marked along a 50‐m measuring tape; at each site hedge height, width and the distance to nearest songpost were recorded.
Foraging‐sites and nestling provisioning rates
Observations of adult foraging behaviour were carried out on between one and four occasions when chicks were between two and nine days old. The observer was positioned between 50 and 100 m from the nest to ensure the birds’ behaviour was not affected; a previous study observed foraging behaviour from a distance of 30 m with no noted effects on behaviour (Stoate et al. Citation1998). Adults were observed for one hour between 06:00 and 21:00 and food provisioning rate was calculated as the number of complete foraging trips per hour. The majority of provisioning watches were carried out in the morning; four watches were carried out in the afternoon and all but one of these nests also had a morning watch, minimising variation in data owing to diurnal variation in foraging. Watches were not carried out during heavy rain or strong winds.
During 2006, data on foraging sites were recorded during provisioning watches by a second observer. The distance of the site from the nest was measured to the nearest 1 m using a Bushnell Yardage Pro Sport Laser Rangefinder (Bushnell Performance Optics UK Ltd, Chessington; accuracy ± 1m). Each foraging site that could be accurately located (n = 34, 38% of trips) was paired with two control sites 5 m from the foraging site. The first control was within the same microhabitat (for example, in a crop tramline) and the second control was in a different microhabitat (for example, in the crop if the foraging site was within a tramline) in a randomly selected direction from the foraging site. For each foraging and control site, vegetation height (± 1 cm), vegetation density, measured by placing a measuring stick vertically on the ground and recording the lowest visible number (as per Douglas et al. Citation2010; ± 1 cm) and vegetation cover were recorded. Vegetation cover was assessed using a fisheye lens attached to a Nikon CoolPix p5000 digital camera (Nikon UK Ltd, Surrey, UK) placed on the ground facing upwards, using a delay shutter release timer to ensure the observer did not appear in the photograph. Photographs were taken at a time of day when the camera was not in direct sunlight, as this would confound the contrast between vegetation and sky. Photographs were subsequently analysed using Gap Light Analyser software (Frazer et al. Citation1999) to derive the percentage of sky visible in the image.
Invertebrate samples were collected from foraging and control sites using a leaf‐vacuum (Ryobi RGBV‐3100, Marlow, UK) modified by the use of a fine mesh to trap invertebrates and a 1‐cm wire mesh to keep vegetation out of the sample. Sampling followed the protocol of Douglas et al. (Citation2009), whereby each sampling site consisted of a 1 m square and 5 × 5‐second sucks (as per Hart et al. Citation2006) were taken from each corner and from the centre of the square. Samples were frozen and subsequently identified to order (Chinery Citation1993). Only invertebrates greater than 2 mm in length were included in analysis as invertebrates smaller than this are considered unlikely to form an important part of Yellowhammer nestling diet (Morris & Bradbury Citation2002).
Fledging success
Where first‐egg date was known, this and clutch size were used to predict hatch date; otherwise nests were visited at maximum intervals of three days during incubation in order to determine hatch date and monitor nest failures. Where nest failures occurred and the date was unknown, failure was assumed to have occurred at the mid‐point between the two final visits to the nest. Where nests were discovered at the chick stage and age was unknown comparisons were made with the feather tract development of chicks of known age (as per Bradbury et al. Citation2003). Nests were checked when chicks were 10 days old to determine fledging success; where a nest contained chicks at 7 days and the nest remained intact but was empty at 10 days (making predation of chicks immediately prior to fledging unlikely), the chicks were deemed to have fledged successfully.
Statistical analysis
Nest site selection
To determine whether features of nest sites differed from features of randomly selected sites within the same territory, a generalised linear mixed‐effect (lme) model with binomial error distributions was constructed using the lmer function within the lme4 package (Bates & Maechler Citation2009) in r (R Core Development Team Citation2006). Site ID (nest‐site or random site) was designated as the response variable with vegetation height, vegetation width, habitat and distance to the nearest songpost as predictor variables. Nest ID was designated as a random effect to control for differences between territories. Model selection methodology is detailed in the following subsection.
Foraging behaviour
To determine whether birds chose foraging‐sites based on vegetation height, density, cover or invertebrate abundance, two glmms with binomial error distributions were constructed in order to compare foraging‐sites to both control sites within the same microhabitat and control sites within a different microhabitat. Predictor variables were vegetation height, density, cover, the abundance of invertebrates >2 mm in length and two‐way interactions between invertebrate abundance and vegetation density, height and cover, as well as between vegetation height and density. To control for differences between site localities and between foraging adults, Site ID (designated for each pair of foraging and control sites) within nest ID were designated as random variables.
To determine whether foraging site selection was associated with parental provisioning rate, data from provisioning rates and foraging sites collected during the same provisioning watches were used to construct a lme model using the lme function within the nlme package (Pinheiro et al. Citation2009) in r. Provisioning rate was designated as the response variable and vegetation cover, height, density, total invertebrate abundance, abundance of invertebrates >2 mm in length, distance from nest and trip duration were designated as predictor variables. Chick age and brood size were also included in the model as larger broods and older chicks require higher provisioning rates. As vegetation structure may interact with food availability to influence provisioning rate, and this may be emphasised in older broods of nestlings when food demand is higher, we tested for the significance of three‐way interactions between vegetation cover, invertebrate abundance and chick age, and between vegetation height, invertebrate abundance and chick age. To control for differences in parental quality, nest ID was designated as a random factor.
For all models, comparisons using aic values were used to determine whether terms significantly improved the fit of the model; those that did not were removed in a stepwise fashion until only those terms that improved the fit of the model at P < 0.05 remained. Following model simplification, each term was re‐inserted into the minimum adequate model (MAM) in turn and compared with the MAM using aic comparisons to ensure lack of association with the response variable. Although model simplification by stepwise deletion has been criticised in the literature (Whittingham et al. Citation2006b), a recent study validated stepwise deletion as a method of model selection and established that it performed just as well as other methods of producing predictive models (Murtaugh Citation2009). Statistics are presented as mean ± se throughout.
RESULTS
Nesting ecology
The majority (65%) of nests were in hedgerows, mostly in Hawthorn; 15% were in Bramble Rubus spp. or herbaceous vegetation and 14% were in herbaceous vegetation associated with a wall or fence. The remaining 6% were on the ground among grasses. The height of nests above ground ranged from 0 to 210 cm with a mean nest height of 82.71 ± 7.71 cm.
Clutch size varied from two to five eggs (3.48 ± 0.14 eggs) and brood size ranged from one to four chicks (2.78 ± 0.14 chicks). From nests that successfully fledged at least one chick, the mean number of fledglings was 2.87 ± 0.20; however, across all nesting attempts that reached the egg stage, mean fledgling number was 1.375 ± 0.23 fledglings per nest.
When nest sites were compared with randomly selected points along the same boundary within 25 m of each nest during 2006, none of the features considered differed between nest sites and randomly selected sites (Table ), indicating that adults did not select specific features of a hedge when they selected nest sites. Results of a binomial glmm determining whether adult birds selected for features of a hedge when choosing a nest‐site; sites were either nest‐sites or randomly selected sites along the same field boundary within 30 m of the nest.
Foraging ecology
No differences were found between characteristics of foraging sites and control sites of similar microhabitat (Table ). However, both vegetation density and the interaction between invertebrate abundance and vegetation cover were found to influence whether a site of different microhabitat was used as a foraging site (Table ). Foraging‐sites had lower vegetation densities than control sites (Fig. ), and a positive correlation between invertebrate abundance and vegetation cover was found in control sites (t 25 = 2.59, P = 0.02) but not in foraging sites (t 26 = 0.97, P = 0.34) (Fig. ). Invertebrate abundance at foraging sites appeared higher than at control sites at low levels of vegetation cover although foraging sites tended to have lower levels of vegetation cover than control sites (Fig. ) with no visual evidence of overall differences in invertebrate abundance from the raw data (Fig. ).
Figure 1 Differences between Yellowhammer foraging sites and control sites within a different microhabitat were influenced by (a) vegetation density; and (b) an interaction between vegetation cover and invertebrate abundance; the line for control sites is predicted from the minimum adequate model (see Table ) with mean vegetation density (11.35 cm); dashed lines show ± se. Differences between foraging sites and control sites are presented for (c) vegetation cover and (d) invertebrate abundance for illustrative purposes; bars represent mean ± se.
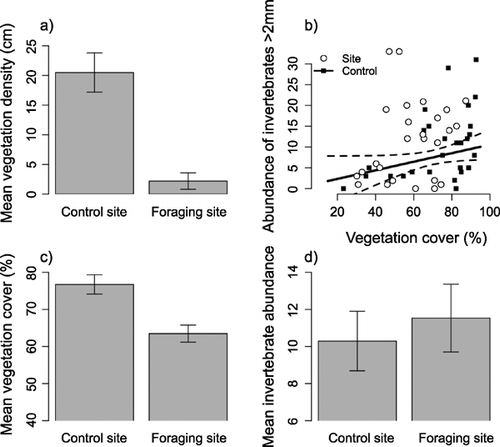
Figure 2 Parental provisioning rate was influenced by an interaction between vegetation cover, invertebrate abundance and chick age (Table ) (linear mixed‐effects model, F1,10 = 13.78, P < 0.01); surface is predicted from the minimum adequate model (see Table ) for mean trip duration (15.8 minutes), vegetation height (68.27 cm), vegetation density (2.20 cm) and brood size (three chicks), and for chicks at eight days of age.
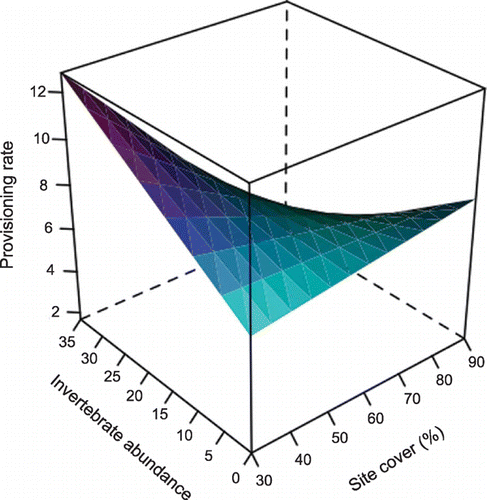
Table 2. Results of a gl mm comparing foraging sites with control sites of similar microhabitats 5 m away from the foraging site.
Table 3. Results of a gl mm comparing foraging sites with randomly selected control sites of different microhabitats 5 m away from the foraging site.
Parental provisioning rate was influenced by an interaction between vegetation cover, invertebrate abundance at foraging‐sites and chick age (Table ; Fig. ). With older chicks with high energetic demands, provisioning rate increased with increasing vegetation cover at low invertebrate abundance, whereas at high invertebrate abundance, provisioning rate decreased with increasing vegetation cover (Fig. ).
Table 4 Minimum adequate model (MAM) from a lmm determining which features of parental foraging sites are associated with provisioning rate.
DISCUSSION
Yellowhammer nesting habitat was similar to that found in other studies, with the majority of nests in hedgerows, followed by herbaceous vegetation (Bradbury et al. Citation2000, Kyrkos et al. Citation1998, Stoate & Szczur Citation2001). Preferences appeared to differ from a previous study (Stoate. et al. Citation1998), where nests were ‘located within herbaceous vegetation in field margins, rather than in the shrubby vegetation of the hedge itself’. This was not the case in the present study as all nests recorded as ‘in hedgerows’ were located within hedgerow vegetation, mostly hawthorn. The preference of this species for nesting in vegetated ditches (Bradbury et al. Citation2000) was not confirmed with this study; however, this is most likely because of differences between our study sites and those of Bradbury et al. (Citation2000), as well as a difference in the timing of the two studies. Our study sites had an abundance of hedgerows and a paucity of ditches, and our nest data were collected from June onwards when Yellowhammers are more likely to nest in hedgerows (Bradbury et al. Citation2000). Cornulier et al. (Citation2010) indicate a strong country‐wide correlation between Yellowhammer Common Birds Census index and hedgerow stock, suggesting our observation of association with hedgerows may be quite general.
The mean clutch size of 3.48 ± 0.14 found in this study compares favourably with the most recent study of Yellowhammer breeding success: Bradbury et al. (Citation2000) found a mean clutch size of 3.27 ± 0.03. Mean fledging success per nest was within the range of Bradbury et al. (Citation2000), and 45% of nests successfully fledged at least one chick, compared with 46.5% of nests recorded by Bradbury et al. (Citation2000). When compared with a figure of 45% fledging success in 1960 (Peakall Citation1960), there appears to have been little change in fledging success since the start of the Yellowhammer population decline.
Yellowhammers do not appear to select any of the hedgerow features examined when choosing a nest‐site within their territory as there was no apparent difference between nest sites and randomly selected points within the same territory in terms of habitat, hedgerow height or width, or distance to nearest songpost. As variation within the same length of hedgerow within the extent of a territory is likely to be relatively small, it is probable that the selection of a territory containing a length of high‐quality hedgerow is more important (Whittingham et al. Citation2005).
There was no difference between foraging‐sites selected by Yellowhammers and control sites of similar microhabitat; however, microhabitats used for foraging had lower vegetation density than control sites 5 m away in a different microhabitat, and higher invertebrate abundances at a higher percentage vegetation cover. This suggests that Yellowhammers initially select foraging microhabitats according to vegetation structure, but then according to food availability, with a lower vegetation cover providing a greater visibility of predators and thus a lower perceived predation risk (Whittingham et al. Citation2004, Whittingham & Evans Citation2004), and a lower vegetation density providing easier access to invertebrates despite their lower abundance. This also suggests that where Yellowhammers take higher risks by foraging in more dense vegetation, where perceived predation risk is higher (Butler & Gillings Citation2004, Whittingham et al. Citation2004), there is a pay‐off in terms of an increased invertebrate availability within the microhabitats selected for foraging (Butler et al. Citation2005). This concurs with suggestions made by Morris et al. (Citation2001) and studies by Perkins et al. (Citation2002) and Douglas et al. (Citation2009), suggesting that cutting patches within field margins would improve their value for birds by creating a mosaic of cut patches where accessibility is improved, adjacent to uncut patches where invertebrate abundance remains high (Douglas et al. Citation2009, Perkins et al. Citation2002). This also agrees with a recent study by Douglas et al. (Citation2010), which found Yellowhammer foraging‐sites to be characterised by lower vegetation height, lower vegetation density and a higher proportion of bare earth than control sites within cereal fields, indicating a higher degree of accessibility to foraging‐sites.
Parental provisioning rate increased with increasing vegetation cover in areas of low invertebrate abundance when chicks were relatively old, indicating that where food availability is low and energetic requirements of chicks are high, parents take more risks by foraging where cover (and invertebrate abundance) is higher in order to ensure sufficient food for their chicks. This may increase their own risk of predation by lowering their ability to detect predators (Whittingham et al. Citation2004). Conversely, when invertebrate abundance is high, provisioning rate decreases with increased vegetation cover, as expected when increased vegetation cover leads to an increased time spent scanning for predators and thus decreasing prey capture rate (Whittingham et al. Citation2004, Whittingham & Evans Citation2004), although the time spent searching for prey is likely to be decreased where prey is abundant (Whittingham et al. Citation2004). Provisioning rate impacts upon chick demographics in this species (Dunn et al. Citation2010), and conditions experienced when young can influence future survival and reproductive success (Metcalfe & Monaghan Citation2001, Taborsky Citation2006); thus, any factor influencing provisioning rate has the potential for longer‐term, population‐level implications.
Here we demonstrate that invertebrate abundance and vegetation cover interact to influence where birds forage and how efficiently they can provision their chicks. While increased vegetation cover leads to a higher perceived predation risk to the foraging bird, higher invertebrate abundances associated with increased cover can lead to birds selecting such sites for foraging, with resulting increases in provisioning rates to chicks in areas where invertebrate abundance tends to be low. In areas where invertebrate abundance is higher, provisioning rates to chicks are highest where vegetation cover is low as parents can forage adequately with minimum risk to themselves. This suggests that measures aimed at increasing the abundance of invertebrates in the farmland environment, for example the maintenance of uncropped habitats such as field margins and conservation headlands, can be improved by the establishment of sward heterogeneity within these habitats (Douglas et al. Citation2009, Perkins et al. Citation2002) in order to provide a mixture of microhabitats aimed at encouraging invertebrate populations and microhabitats enabling Yellowhammers to forage more efficiently.
ACKNOWLEDGEMENTS
Thanks are due to many landowners for permission to carry out fieldwork on their land. Sarah Butcher, Gemma Greenbank, Eleanor Foster and Romain Guerreiro helped with fieldwork during 2006. JCD was supported by BBSRC Studentship BBSSK200512132.
REFERENCES
- Bates , D. and Maechler , M. 2009 . lme4: linear mixed‐effects models using S4 classes R package version 0.999375‐32. Available at: http://CRAN.R-project.org/package=lme4 (accessed 24 June 2010)
- Both , C. , Bouwhuis , S. , Lessells , C.M. and Visser , M.E. 2006 . Climate change and population declines in a long‐distance migratory bird . Nature , 441 : 81 – 83 .
- Bradbury , R.B. , Kyrkos , A. , Morris , A.J. , Clark , S.C. , Perkins , A.J. and Wilson , J.D. 2000 . Habitat associations and breeding success of yellowhammers on lowland farmland . J. Appl. Ecol. , 37 : 789 – 805 .
- Bradbury , R.B. , Wilson , J.D. , Moorcroft , D. , Morris , A.J. and Perkins , A.J. 2003 . Habitat and weather are weak correlates of nestling condition and growth rates of four UK farmland passerines . Ibis , 145 : 295 – 306 .
- Butler , S.J. and Gillings , S. 2004 . Quantifying the effects of habitat structure on prey detectability and accessibility to farmland birds . Ibis , 146 : 123 – 130 .
- Butler , S.J. , Whittingham , M.J. , Quinn , J.L. and Cresswell , W. 2005 . Quantifying the interaction between food density and habitat structure in determining patch selection . Anim. Behav. , 69: : 337 – 343 .
- Chamberlain , D.E. and Fuller , R.J. 2000 . Local extinctions and changes in species richness of lowland farmland birds in England and Wales in relation to recent changes in agricultural land‐use . Agr. Ecosys. Environ. , 78 : 1 – 17 .
- Chinery , M. 1993 . Insects of Britain and Northern Europe , 3rd edn. , London : Harper Collins .
- Cornulier , T. , Robinson , R.A. , Benton , T.G. , Lambin , X. , Sutherland , W.J. and Elston , D.A. 2010 . Bayesian reconstitution of environmental change from disparate historical records: hedgerow loss and farmland bird declines . Meth. Ecol. Evol. , doi: 10.1111/j.2041-210X.2010.00054.x
- Douglas , D.J.T. , Vickery , J.A. and Benton , T.G. 2009 . Improving the value of field margins as a foraging habitat for farmland birds . J. Appl. Ecol. , 46: : 353 – 362 .
- Douglas , D.J.T. , Benton , T.G. and Vickery , J.A. 2010 . Contrasting patch selection of breeding Yellowhammers Emberiza citrinella in set‐aside and cereal crops . Bird Study , 57 : 69 – 74 .
- Dunn , J.C. , Hamer , K.C. and Benton , T.G. 2010 . Fear for the family has negative consequences: indirect effects of nest predators on chick growth in a farmland bird . J. Appl. Ecol. , 47 : 994 – 1002 .
- Eaton , M.A. , Balmer , D. , Burton , N. , Grice , P.V. , Musgrove , A.J. , Hearn , R. , Hilton , G. , Leech , D. , Noble , D.G. , Ratcliffe , N. , Rehfisch , M.M. , Whitehead , S. and Wotton , S. 2008 . The State of the UK’s birds 2007 RSPB, BTO, WWT, CCW, EHS, NE and SNH, Sandy, UK
- Frazer , G.W. , Canham , C.D. and Lertzman , K.P. 1999 . Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True‐colour Fisheye Photographs, Users Manual and Program Documentation , Millbrook , , New York : Simon Fraser University, Burnaby, British Columbia; Institute of Ecosystem Studies .
- Fuller , R.J. , Gregory , R.D. , Gibbons , D.W. , Marchant , J.H. , Wilson , J.D. , Baillie , S.R. and Carter , N. 1995 . Population declines and range contractions among lowland farmland birds in Britain . Cons. Biol. , 9 : 1423 – 1441 .
- Gaston , A.J. , Gilchrist , H.G. , Mallory , M.L. and Smith , P.A. 2009 . Changes in seasonal events, peak food availability, and consequent breeding adjustment in a marine bird: a case of progressive mismatching . Condor , 111 : 111 – 119 .
- Hart , J.D. , Milsom , T.P. , Fisher , G. , Wilkins , V. , Moreby , S.J. , Murray , A.W.A. and Robertson , P.A. 2006 . The relationship between Yellowhammer breeding performance, arthropod abundance and insecticide applications on arable farmland . J. Appl. Ecol. , 43 : 81 – 91 .
- Kyrkos , A. , Wilson , A. and Fuller , J.D. 1998 . Farmland habitat change and abundance of Yellowhammers Emberiza citrinella: an analysis of Common Birds Census data . Bird Study , 45 : 232 – 246 .
- Metcalfe , N.B. and Monaghan , P. 2001 . Compensation for a bad start: grow now, pay later? . Trends. Ecol. Evol. , 16 : 254 – 260 .
- Morris , A.J. and Bradbury , R.B. 2002 . Determinants of patch selection by Yellowhammers Emberiza citrinella foraging in cereal crops . Aspect. Appl. Biol. , 67 : 43 – 50 .
- Morris , A.J. , Whittingham , M.J. , Bradbury , R.B. , Wilson , J.D. , Kyrkos , A. , Buckingham , D.L. and Evans , A.D. 2001 . Foraging habitat selection by Yellowhammers (Emberiza citrinella) nesting in agriculturally contrasting regions in lowland England. . Biol. Cons. , 101 : 197 – 210 .
- Morris , A.J. , Wilson , J.D. , Whittingham , M.J. and Bradbury , R.B. 2005 . Indirect effects of pesticides on breeding Yellowhammer (Emberiza citrinella) . Agric. Ecosys. Environ. , 106 : 1 – 16 .
- Murtaugh , P.A. 2009 . Performance of several variable‐selection methods applied to real ecological data . Ecol. Lett. , 12 : 1061 – 1068 .
- Peakall , D.B. 1960 . Nest records of the Yellowhammer . Bird Study , 7 : 94 – 102 .
- Pearce‐Higgins , J.W. , Dennis , P. , Whittingham , M.J. and Yalden , D.W. 2010 . Impacts of climate on prey abundance account for fluctuations in a population of a northern wader at the southern edge of its range . Global Change Biol. , 16 : 12 – 23 .
- Perkins , A.J. , Whittingham , M.J. , Morris , A.J. and Bradbury , R.B. 2002 . Use of field margins by foraging Yellowhammers Emberiza citrinella . Agric. Ecosys. Environ. , 93 : 413 – 420 .
- Pinheiro , J. , Bates , D. , DebRoy , S. , Sarkar , D. and Core Team , R. 2009 . NLME: Linear and Nonlinear Mixed Effects Models, R package version 3.1‐96. Available at: http://cran.r-project.org/web/packages/nlme (accessed 24 June 2010)
- R Core Development Team . 2006 . R: A Language and Environment for Statistical Computing Available at: http://www.R-project.org (accessed 24 June 2010)
- Stoate , C. and Szczur , J. 2001 . Whitethroat Sylvia communis and Yellowhammer Emberiza citrinella nesting success and breeding distribution in relation to field boundary vegetation . Bird Study , 48 : 229 – 235 .
- Stoate , C. , Moreby , S.J. and Szczur , J. 1998 . Breeding ecology of farmland Yellowhammers Emberiza citrinella . Bird Study , 45 : 109 – 121 .
- Taborsky , B. 2006 . The influence of juvenile and adult environments on life‐history trajectories . Proc. Roy, Soc. Lond. B Biol. , 273 : 741 – 750 .
- van der Veen , I.T. 1999 . Effects of predation risk on diurnal mass dynamics and foraging routines of Yellowhammers (Emberiza citrinella). . Behav. Ecol. , 10 : 545 – 551 .
- Whittingham , M.J. and Evans , K.L. 2004 . The effects of habitat structure on predation risk of birds in agricultural landscapes . Ibis , 146 : 210 – 220 .
- Whittingham , M.J. , Butler , S.J. , Quinn , J.L. and Cresswell , W. 2004 . The effects of limited visibility on vigilance behaviour and speed of predator detection: implications for the conservation of granivorous passerines . Oikos , 106 : 377 – 385 .
- Whittingham , M.J. , Swetnam , R.D. , Wilson , J.D. , Chamberlain , D.E. and Freckleton , R.P. 2005 . Habitat selection by Yellowhammers Emberiza citrinella on lowland farmland at two spatial scales: implications for conservation management . J. Appl. Ecol. , 42 : 270 – 280 .
- Whittingham , M.J. , Devereux , C.L. , Evans , A.D. and Bradbury , R.B. 2006a . Altering perceived predation risk and food availability: management prescriptions to benefit farmland birds on stubble fields . J. Appl. Ecol. , 43 : 640 – 650 .
- Whittingham , M.J. , Stephens , P.A. , Bradbury , R.B. and Freckleton , R.P. 2006b . Why do we still use stepwise modelling in ecology and behaviour? . J. Anim. Ecol. , 75 : 1182 – 1189 .