Abstract
Capsule Clutch size and female body mass play major roles in duck incubation behaviour.
Aims To investigate the effects of incubation stage, clutch size, body size, body mass and weather on incubation attentiveness.
Methods Incubation behaviour was monitored with Temperature Data Loggers (TDLs). Nest temperature fluctuations were recorded both in the field in Common Pochards Aythya ferina and in aviaries in Mallards Anas platyrhynchos using TDLs deposited either in the nest (Pochards) or in a dummy egg placed in the clutch (Mallards). Both methods allowed data collection on incubation attentiveness.
Results Incubation attentiveness increased over the incubation period and with clutch size for both species. Body size appeared to affect incubation rhythms in Mallards only. Female Pochards were found to reload their nest with fresh material in response to precipitation events, presumably owing to the rise in water level.
Conclusions Clutch size and female body mass play major roles in duck incubation behaviour. Climatic events can also be prominent factors influencing incubation behaviour. We describe for the first time the nesting behaviour of Pochard females and show that they have the capacity to adjust nest topology in response to a rise in water level following rain.
Hatching success is a central element of bird breeding success (Deeming Citation2002). Nesting therefore plays a major role in bird population dynamics, and brood production is known to affect the size of breeding populations in subsequent years (Reynolds & Sauer Citation1991, Franklin et al. Citation2000, Hoekman et al. Citation2002, Sibly & Hone Citation2002). Breeding success (including nest success and survival of offspring until fledging) is perceived as an important parameter that directly affects the fitness of individuals and consequently the dynamics of a population (Drent & Daan Citation1980, Lessells Citation1991, Stearns Citation1992). Some evidence exists about the key role of incubation in determining nesting success both in altricial and precocial species (Aldrich & Raveling Citation1983, Afton & Paulus Citation1992, Yerkes Citation1998, Poussart et al. Citation2001, Deeming Citation2002, Møller Citation2005, Spaans et al. Citation2007). The incubation period is stressful in many bird species, mainly because nest attentiveness competes with maintenance activities of the adults, such as foraging (Tinbergen & Williams Citation2002). Female investment in laying and incubation is a prime determinant of offspring survival, especially in precocial species in which parental care is dramatically reduced compared to altricial species (but still exists, see Boos et al. Citation2007 for example). Nest predation is a key factor of reproductive success in most birds (Deeming Citation2002, West & Messmer Citation2004). Nevertheless, the nests of precocial species are almost always built on the ground and are therefore highly vulnerable to predation, especially from mammals (Sargeant & Raveling Citation1992, West & Messmer Citation2004).
Because nest success is a key component of reproduction in precocial birds, numerous studies focusing on this parameter have been conducted, especially in ducks (see for instance Aldrich & Raveling Citation1983, Afton & Paulus Citation1992, Yerkes Citation1998, Poussart et al. Citation2001, Spaans et al. Citation2007). Many studies were interested in monitoring nest attentiveness, from both fundamental and applied perspectives (see Deeming Citation2002 for a review). On the one hand, duck breeders are mainly interested in recording temperatures of clutches and monitoring incubation recesses, in order to determine the optimal mass that females should have at the onset of incubation to maximize hatching success (Decuypere & Michels Citation1992). On the other hand, wildlife managers tend to focus on both the consequences of nest attentiveness on nest success and also the causes and timing of nest predation (Afton & Paulus Citation1992, Hartman & Oring Citation2006). Nest attentiveness, which partly determines nesting success, has been related to female body mass or condition in various duck and geese species (Aldrich & Raveling Citation1983, Afton & Paulus Citation1992, Yerkes Citation1998, Poussart et al. Citation2001). Numerous studies have focused on the relationship between individual parameters (such as body mass or condition, clutch initiation date, incubation stage, and clutch size) and nest success and attendance in ducks and geese. The importance of body mass and condition and the use of nutrient reserves have been shown to play an important role in duck and goose reproductive success (Aldrich & Raveling Citation1983, Afton & Paulus Citation1992, Arnold et al. Citation1995, Blums et al. Citation1997, Citation2005, Blums & Clark Citation2004). Blums et al. Citation(1997) demonstrated the importance of body nutrients on nesting success for adult female diving ducks. Weather condition may also influence incubation rhythms (Hébert Citation2002). For example, during rainfall, females increase their nest attentiveness (Caldwell & Cornwell Citation1975), while ambient temperature can largely modulate nest attentiveness either for heating or cooling the eggs (Webb Citation1987).
Recently miniaturized Temperature Data Loggers (hereafter TDLs) have proven very effective tools, both for monitoring incubation behaviour (a drop in temperature usually indicates female recesses from the nest: Hoover et al. Citation2004, Cooper & Mills Citation2005) and for determining the exact time of nest abandonment or predation (Cooper & Mills Citation2005, Hartman & Oring Citation2006). We have used TDL devices to study the factors determining nest success in ducks.
First, we attempted to validate whether miniaturized TDL devices could be a reliable method for monitoring nest temperature in both captive and wild ducks. We aimed at deriving female incubating behaviour from temperature recordings, and determining the precise moment of nest predation or abandonment. For this purpose, we first used TDL devices placed in dummy eggs to monitor the nest attentiveness of incubating female Mallards Anas platyrhynchos held in outdoor aviaries in semi-captivity conditions.
Secondly, in order to assess the applicability of the TDL approach to wild nests under natural conditions, TDL devices were placed in natural nests of Common Pochards Aythya ferina monitored as part of a long-term population dynamic study initiated in 2003. We also aimed at investigating the effect of weather conditions (ambient temperature and precipitation) on incubation rhythms. In both diving and dabbling ducks, phenotypic traits might affect demographic parameters, such as survival, dispersal propensity, and breeding success (Blums & Clark Citation2004, Blums et al. Citation2003, Citation2005). Since energy allocated to incubation is thought to play an important role in breeding success (Afton & Paulus Citation1992), we examined the relationships between nest incubation parameters (nest attentiveness) and phenotypic correlates (clutch size, body size and/or condition).
In the present study, we address the following predictions: (1) because temperature variations can seriously reduce hatchability (Greenwood et al. Citation1995, Hébert Citation2002), we expect lower recess frequency and shorter recess duration during adverse weather conditions (low temperatures and rainfall); (2) clutch size is thought to affect incubation rhythms (Afton & Paulus Citation1992). Because of higher energy demands (Thomson et al. Citation1998), we expect a negative relationship between recess time (or recess frequency) and clutch size; (3) because being larger or in better condition provides greater reproductive outputs (Blums et al. Citation2005), we expect greater attentiveness in larger (better body condition) females. We also expect greater nesting success among larger (better body condition) females.
METHODS
Temperature data loggers
Previous studies of incubation behaviour have involved heavy and logistically complex apparatus (camera, photoelectric sensors, egg-implanted thermistors wired to an external recorder, or weighing devices placed under the nest, see Afton & Paulus Citation(1992) for a review on the equipment for the study of incubation in ducks). These systems have also been used to study other aspects of nesting ecology (e.g. body mass monitoring, information on predators, etc.). Because of their easy deployment, TDLs have been used successfully for the study of incubation behaviour and incubation temperature in many bird species (Hartman & Oring 2005, Arnold et al. Citation2006). To monitor nest temperature we used iButtons (Thermochron iButton DS1922L, Maxim Integrated Products, Sunnyvale, CA). They were 16 mm dia. × 6 mm stainless steel cylinders, 3 g, capable of logging data for up to 12 months (see http://www.maxim-ic.com/quick_view2.cfm/qv_pk/4088 for more information). When deploying these TDLs, incubating females were disturbed only once. Neither female behaviour nor nest survival seem to be affected by the presence of iButtons (Hartman & Oring Citation2006, pers. obs.).
Monitoring incubation of free-ranging female Pochards
Nest monitoring in the wild took place from 10 May to 10 June 2008 on Grand-Lieu Lake (northwestern France, 47°06′N/1°40′W), a large shallow lake (see Paillison et al. 2002 for a detailed description of the study site). Common Pochards are the dominant nesting duck at this site with up to 600 breeding pairs (Reeber Citation2009: 490 and 590 pairs in 2008 and 2009, respectively). Common Pochard nests were searched for by walking in suitable habitat type on the shoreline, mainly sedges Carex spp. and reedbeds (Phragmites, Phalaris). The nest-searching strategy employed in our study exploits the behaviour of incubating females which, when approached by a human observer while incubating, tend to leave the nest very conspicuously, thus giving clues about the location of that nest. Nests were both over water (mainly on sedge tussocks) and in ditches and reedbeds. The number of eggs and the incubation stage (estimated using the egg flotation method, see Westerkov Citation1950) were recorded and from this we estimated incubation initiation date. Twenty TDLs were employed. Each was placed into a latex pouch (a cut finger from a medical glove sealed with a knot) and this was attached by a wire to a small wood stick buried under the vegetation at the bottom of the nest. This ensured that the TDL stayed just under the eggs. Each TDL was set to record temperature (± 0.5°C) every 5 min allowing a 28-day monitoring period. Incubation in Pochards is approximately 25 days (Cramp & Simons Citation1977). Among the 20 nests monitored, 14 females were captured using Weller traps after 8–12 days of incubation. The following morphometric measurements were taken: body mass (± 5 g, Pesola spring balance), tarso-metatarsus length (± 0.1 mm, digital calliper), wing length (± 0.5 mm, stopped ruler). Potential modifications in female incubating behaviour induced by capture were taken into account by removing the first 24 h of recording after capture from data analyses. Based on nest examination we determined whether nest failure was due to nest depredation or desertion.
Abandoned nests were defined as those with cold eggs. Depredated nests were characterized by a reduction in egg numbers, signs of disturbance or broken shells, without any evidence that the female was still incubating. We concluded that some abandoned nests could have been secondarily predated and these were considered depredated according to our classification criteria.
Monitoring incubation of captive Mallards
Nest monitoring took place from 11 June to 22 June 2008 at the Centre d'Études Biologiques de Chizé (CEBC), western France (46°10′N, 0°20′W), using adult ducks which were descendents of wild-caught stock. Captive and wild Mallards usually breed until the end of July at this site. Recess frequency or duration is not influenced by laying date (Poussart et al. Citation2001).
Birds were kept at the CEBC for at least 3 months before the beginning of observations to ensure that they were habituated to their aviaries. Twenty pairs were housed individually in 8 m2 aviaries. Each aviary contained a nest-box of standardized size and shape, a 1.5 m2 pool, and a feeding patch supplied ad libitum with a mixture of crushed corn, wheat and commercial duck food. Each nesting female was monitored to determine clutch size and initiation of laying and incubation.
Nest temperature was recorded using TDLs placed within artificial eggs following Flint & MacCluskie Citation(1995). The artificial duck eggs (Ducatillon, France) were made with 4-mm thick plaster walls. TDLs were placed in the middle of each egg. All artificial eggs were deposited at the same date, with the result that the incubation stage was not necessarily the same for all nests. Artificial eggs containing the TDL device were placed and secured in the middle of the nest, using a 5-cm long metal stick pushed into the ground beneath the nest to ensure that the logger was as close as possible to the female brood patch.
TDLs recorded temperature (± 0.5°C) every 2 min. This allowed information storage for 11 consecutive days. As for Pochards, nests were not revisited until hatching. Ten days before clutch initiation, female mass (± 1 g, electronic balance), tarsus length, bill length and wing length were measured (± 0.5 mm).
Weather data
Data on weather conditions (daily and hourly average temperature, total rainfall mm) for the cities of Nantes and Niort were downloaded from http://www.meteociel.fr/. These data were collected < 20 km from the respective study sites.
Data analysis
All 20 TDLs deposited in Common Pochard nests could be retrieved, but one of the profiles revealed no incubation behaviour (only day/night temperature patterns). This was also the case for one female Mallard that did not incubate its nest. These latter two data sets were removed from the analyses. We used temperature time series to infer female nest attentiveness. A drop in temperature (off-bouts) indicated that the female was off the nest (see ). Recess duration was calculated as the difference between the time at which the temperature dropped first and the time when the temperature began to increase. Temperature off-bouts were recorded by hand by examining each time series thoroughly. We opted for this approach instead of the automation approach (Cooper & Mills Citation2005) because of the noise in the Pochard temperature recordings, which was probably due to the TDL location (i.e. under the eggs). For both Pochards and Mallards we recorded the time and duration of each off-bout. Because the TDL resolution was ± 0.5°C, we only examined decreases > 0.5°C. We computed one file per species that incorporated for each day and each individual the number of recesses (recess frequency) as well as their average duration. Changes in nest attentiveness over time were also examined. The covariate ‘Time’ refers to the days from clutch initiation date. Clutch initiation date was also included in the analyses. Habitat type (over water on tussocks or in the reedbed or ditch) was included in analyses as a dummy variable.
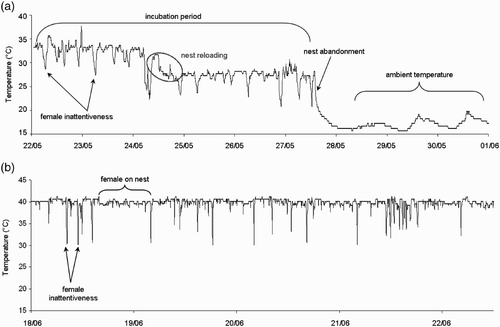
Figure 1. Two examples of temperature profiles for (a) Pochard nests in the wild and (b) Mallard eggs in captivity. A variety of information can be obtained from such profiles. Down peaks (i.e. rapid decrease followed by a later increase of nest or egg temperature) were interpreted as nest inattentiveness. Nest abandonment or depredation was detected when a day/night temperature (ambient) alternation occurred. A global temperature decrease indicates nest reloading with vegetation materials (see main text for more details).
We followed Peig & Green Citation(2009) to construct an index of mass. Peig & Green Citation(2009) recommend using one single body size variable that has the strongest correlation with body mass on a log-log scale, as this is likely to be the length that best explains that fraction of mass associated with structural size. In our case, wing length had a stronger correlation with body mass than tarsus length (Pearson r = 0.46 versus 0.13 and r = 0.49 versus 0.15 for Pochard and Mallard, respectively).
The effect of individual covariates such as body size, corrected body mass and clutch size on nest recesses was tested using Generalized Linear Mixed Models. Because biometric measurements were available for 14 female Pochards, we divided the analyses using, first, a restricted data set with the 14 females to test the effect of time, clutch size, and the phenotypic correlates (body size and corrected mass). Secondly, we used the whole data set if phenotypic covariates were not elicited. We considered individuals as a random effect, which resulted in giving the same weight to each individual and avoided pseudo-replication among individuals (Pinheiro & Bates Citation2000). Candidate models including all covariates, combinations of their possible interactions, and a null (intercept only) model were considered in the analyses. In all analyses, model selection was performed using Akaike's Information Criterion corrected for small sample size, AICc (Burnham & Anderson Citation1998). We based model comparisons on the difference between their respective AICc values (ΔAICc), with a difference lower than 2 indicating equal fit. When models presented equal fit (i.e. ΔAIC < 2), we selected the model with the lower number of parameters as a rule (Lebreton et al. Citation1992). Recess frequency and mean recess duration were log10-transformed to meet the assumptions of normality and homoscedasticity. Note that clutch size was not related to corrected body mass nor body size (all Pearson correlations < 0.13; all P > 0.4) for both Mallards and Pochards. All statistical analyses were performed using R 2.8.1.
RESULTS
Temperature profiles of Pochard nests in the wild and Mallard eggs in captivity showed different patterns with respect to the number of departures from the nest and the recess duration (, ). TDLs placed in dummy eggs under Mallards allowed us to measure absolute variation of incubation temperature (), while TDLs deposited in Common Pochard nests (which were not directly in contact with incubating females) only allowed measurements of variations in relative incubation temperature. Among the 19 Common Pochard nests monitored, eggs hatched in only 4. Nest desertion mainly occurred during daytime (n = 4/5 failures), while nest depredation mainly occurred at dusk (n = 8/9 failures). Only one nest depredation occurred at dawn. Nest depredation was particularly high in 2008. Most (29/31: 93.5%) of the nests discovered early in the season failed even before clutch completion (19/31). The 20 TDLs were deposited in nests with completed clutches. Clutch size was greater for monitored than for unmonitored nests (mean = 9.3 ± 2.6 se and 7.08 ± 1.6 se, respectively, t = –2.74; P = 0.01). Laying date was also later for monitored than for unmonitored nests (mean ordinal dates were 135.6 ± 1.2 se and 127.1 ± 1.6 se, respectively, t = –4.17; P < 0.01).
Table 1. Incubation rhythm components and egg temperature reported from TDLs in this study and from previous studies on close-related species. Mean values ± se are presented where available. The number of nests monitored is also given (n).
Effect of climatic condition on nest attentiveness
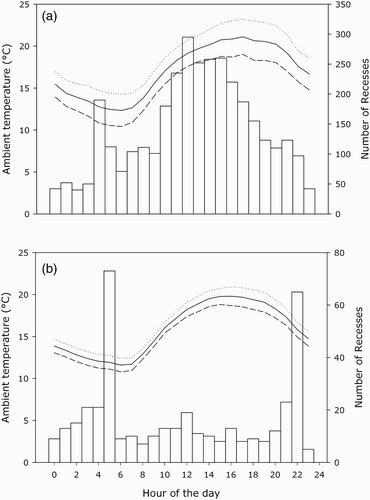
Incubation recesses did not occur during the same time of the day for the two species studied. In Mallards, most recesses occurred during daytime, while they mainly occurred at dawn and dusk for Pochards (). Moreover, nest recesses tended to be more frequent during the hottest time of the day in Mallards, but not in Pochards.
Figure 2. Periods of nest departure (open bars) for (a) Common Pochards in the wild and (b) Mallards in captivity. Mean ambient temperature is indicated by the solid line. The upper and lower limits of the 95% confidence interval are shown by the dotted and dashed lines, respectively.
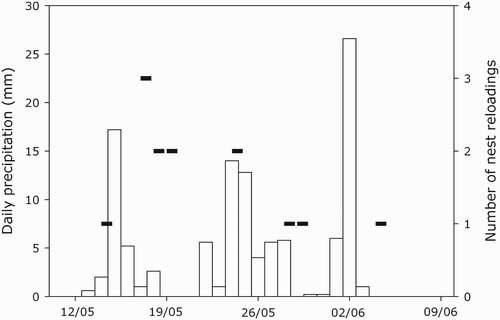
Twelve out of 19 temperature profiles of Pochard nests displayed a sharp decrease in temperature followed by stabilization, usually within less than 24 h. We hypothesize that this decrease in temperature could be due to the reloading of the nest with fresh vegetation (nest reloading in ). We tested this and performed logistic regressions with days reloading coded as 1 and days without reloading coded as 0. We tested the reloading probability with daily precipitation and precipitation that occurred, respectively, one, two, or three days prior. The reloading probability was positively related to precipitation that occurred two days prior (z = 2.02; P = 0.043), but not with other precipitation covariates (all z < 1.18; all P > 0.21). This finding supports the assumption that, in the case of heavy rainfall, female Common Pochards adjust their nest height with fresh vegetation in response to an increase in water level, which could otherwise result in the loss of their nest. For Pochards, we tested the effect of daily precipitation and minimum temperature on nest attentiveness. These two covariates were not retained in our model selection (see ). Furthermore, habitat type was not retained explaining Common Pochard nest attentiveness.
Table 2. Results of model selection from mixed linear models on daily recess frequency, mean recess duration, and total recess duration of female Pochards nesting on Grand-Lieu Lake (France). Nest identity was included as a random factor. Time (days since clutch initiation date), clutch initiation date, daily precipitation (Ppt), minimum temperature (Temp), clutch size (Clutch), nest fate factor (Fate), corrected body mass (Mass), and wing length were used as fixed explanatory variables. Morphometric measurements were available for 14 captured females. The model selection is based on these 14 females. The summary statistics of the most parsimonious models (shown in bold) are given below. Note that when body size or mass were not retained in the model selection (a and b), statistics are given for the whole dataset including the 19 monitored nests. The model selection for the whole dataset led to the same ranking models (data not shown).
Effect of time, clutch size and phenotypic correlates on nest attentiveness
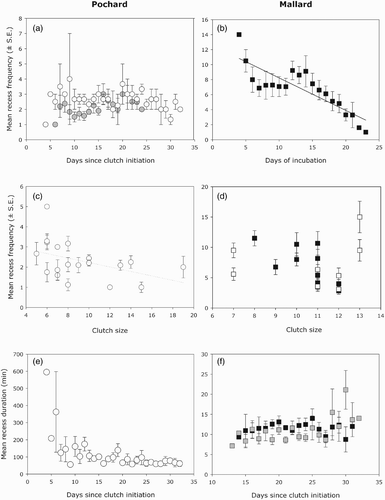
We explored the influence of time, clutch size, nest fate, body size and corrected body mass on recess frequency for Pochard nests. The results of model selection are presented in . For recess frequency, the most parsimonious models revealed an interaction between time and hatching success (; and negative relationship between recess frequency and clutch size (; for Pochards. Dugger & Blums Citation(2001) suggested a clutch size > 13 eggs would reveal nest parasitism in Common Pochards. Three out of the 20 monitored nests in our study met this criterion and might therefore have been parasitized. We also performed analyses without clutches > 13 eggs and the results were unchanged. Recess frequency increased over time for unsuccessful nests while a decrease was observed for the four successful nests (. The same pattern was observed in Mallards, for which an important decrease of the recesses occurred over time (see and . Clutch size (in interaction with wing length) also influenced recess frequency in Mallards. To investigate this interaction, we arbitrarily divided females into two groups, based on whether their wing length was above or below the median (. Larger females tend to decrease their recess frequency with larger clutches, while smaller ones do not present this pattern (even with a slight increase of recess frequency for clutches of 13 eggs). Concerning the mean recess duration, based on the models ranking, none of the models tested were better than the null model. However, when we used the whole data set, the model Time + Clutch was elicited with a ΔAICc > 2 compared to the null model. The statistics of this latter model are provided in . Mean recess times tend to decrease over time (, but tend to increase with clutch size. In Mallards, mean recess duration was explained by time in interaction with wing length. Smaller females increased their recess duration later in the season, while larger ones maintained constant recess duration throughout incubation (. Note that the time spent outside the nest by Mallards (for the whole incubation) was similar between body sizes (11.5 ± 0.1 min and 11.1 ± 0.3 min for larger and smaller females, respectively). However, corrected body mass did not explain attentiveness variation in Mallards and Pochards.
Table 3. Results of model selection from mixed linear models on daily recess frequency, mean recess duration, and total recess duration of female Mallards nesting in captivity. Nest identity was included as a random factor. Time (days of incubation), clutch size (Clutch), corrected body mass (Mass) and wing length were used as fixed explanatory variables. The summary statistics of the most parsimonious models (shown in bold) are given below.
Figure 4. Changes in mean recess frequency per day (± se) and (a) time since clutch initiation for successful (white circles) and failed (grey circles) nests; (b) days of incubation; (c, d) clutch size (large females Mallards are in black and small females are in white); (e, f) relationships between mean recess duration and days since clutch initiation for Common Pochards (open dots) and for large (black squares) and small (grey squares) Mallard females.
DISCUSSION
We have used two methods to monitor the nest attentiveness of female ducks. TDLs were either placed directly below the eggs at the bottom of the nest (wild Common Pochards) or into a dummy egg (captive Mallards). Each method has advantages and drawbacks. Both have proven to be adequate for monitoring nest attendance. However, the dummy egg had the advantage of measuring absolute incubation temperature as well as temperature change patterns. The nest method, which was employed in the wild, ensured that all TDLs could be retrieved, whereas dummy eggs could have been taken by predators. In our case, more than 66% of nest losses were due to predation (see also Greenwood et al. Citation1995).
Because the context (wild versus captive) and species effects were confounded, we cannot derive firm conclusions about the relative importance of species or habitat on nest attentiveness. Achieving this goal would require conducting experiments on the same species in different contexts and comparing species sharing the same habitat (Hébert Citation2002). We therefore divide this discussion into two sections dealing with captive Mallards and with Common Pochards.
Captive Mallards
Incubation rhythms recorded in Mallards are consistent with those from previous studies (). Incubation constancy and mean recess time found in our study were very similar to those found in Caldwell & Cornwell Citation(1975), which also recorded with loggers placed in dummy eggs. Considering that these two studies are separated by 38 years and were conducted on different continents, this strongly suggests that incubation behaviour and nest attentiveness in Mallards is highly stereotyped. The consistency with this previous study, which involved heavy logistic apparatus, indicates that TDLs are suitable in studying nest activity and nest temperature in ducks (Hartman & Oring 2005, Arnold et al. Citation2006). The temporal distribution of recesses was correlated with ambient temperature in captive Mallards. Wild Mallards exhibit different patterns by leaving their nest at dawn and dusk (Afton & Paulus Citation1992). This pattern was attributed to a predation avoidance strategy. As our outdoor enclosures prevent nest depredation, energy requirements were probably the main driving factor of nest attentiveness in captive Mallards. This is supported by the fact that most Mallard recesses occurred during daytime when ambient temperature was at its maximum. Indeed, when ambient temperature is highest, egg heat loss due to female departure is minimized. Over-heating was not a problem because eggs were deposited in nest boxes and therefore protected from the direct influence of solar radiation. Recess frequency dramatically decreased over time. Large Mallard females were able to increase nest attendance, while smaller ones tended to have more recesses. This may indicate that larger females are able to rely more on their body reserves than smaller ones, especially when the clutch is larger. Finally, we also found that, in Mallards, smaller females tended to increase their mean recess duration in late incubation. Our results on the effects of body size suggest that being larger allowed females to enhance nest attentiveness, especially at the end of incubation. In wild Mallards, body mass influences nesting success (Gloutney & Clark Citation1991): successful females were heavier than unsuccessful ones. Moreover, even if the heat production of the embryo is very small (Deeming Citation2002), it might also influence female attentiveness. Towards the end of incubation, heat energy generated by the embryos will reduce the rate of cooling and might allow females to take longer recesses than during early incubation. However, the reasons why only smaller females would benefit from heat production of embryos remain unclear.
Wild Common Pochards
Our results on Pochards are also consistent with previous studies of other diving duck species (see , and Afton & Paulus Citation1992, Baldassare & Bolen Citation2006 for reviews). To our knowledge, our study provides the first data on female incubation in Common Pochards.
The dawn and dusk recess pattern described above for Mallards also occurred in female Pochards. Concentrating recesses at dawn and dusk may function to minimize predation by predators which rely upon visual cues. Such pattern of nest recesses has also been found in Northern Shovelers Anas clypeata (Afton Citation1980) and in wild Mallards (Afton & Paulus Citation1992).
The effect of weather conditions
Adverse weather conditions can seriously affect nest survival (Greenwood et al. Citation1995). Weather is known to influence incubation rhythms in various bird species (see Hébert Citation2002 for a review on the effect of ambient temperature on incubation), especially for species living in extreme environments. However, the effects of weather on nest attentiveness differs between species. While female Mallards were found to reduce recess frequency during rainy days (Caldwell & Cornwell Citation1975), precipitation did not explain the amount of time spent on the nest in four duck species (Loos & Rohwer Citation2004). We found that female Pochards were likely to adjust the height of their nest during, or just after, intense precipitation events, and were probably triggered by increases in water level. This behaviour could have evolved to maximize egg survival by avoiding nest flooding. Other studies in diving ducks have also documented this kind of behaviour, where females add material during the nesting period in response to changes in water level (Low Citation1941, Citation1945). Low Citation(1941) stated that, ‘The capacity to add materials rapidly to the nests when the water level was rising often determined the success or failure of the clutch’. However, in our case, the effects of precipitation and temperature were not retained in our model selection process to explain nest attentiveness variation. Weather condition was not likely to affect nest recess frequency or duration, which is consistent with the study of Loos & Rohwer Citation(2004).
Nest failure
In our study, only 20% of Common Pochard nests hatched. Losses were due to predation (67%) or abandonment (33%). This high nest failure rate contrasts with that reported for the same species on Engure Lake (Latvia), where an average of 91–95% of nests hatched (Blums et al. Citation1997, Opermanis et al. Citation2001). However, Albrecht et al. Citation(2006) have reported high nest losses in fish ponds of the Czech Republic, where only 36% of nests hatched. The fact that all monitored nests were located on the boundaries of the lake may partly explain why such a high predation rate was observed. Indeed, Albrecht et al. Citation(2006) observed that nesting on terrestrial habitats seriously impacted nest survival in Common Pochards. This low breeding success was not typical for our breeding area (Caizergues, unpub. data). In 2008, water levels remained high late in the season and potentially reduced favourable areas of vegetation for nesting, which may have been easily detected by predators. Moreover, high numbers of wild boars were recorded in the same area in 2008. However, habitat type was not shown to affect nest attentiveness.
Clutch size
Clutch size was also found to influence recess frequency. This is in accordance with the study of Loos & Rohwer Citation(2004), where nest attendance increased with the number of eggs laid in four dabbling duck species, and with Deeming Citation(2002), who reviewed nest attentiveness in 354 avian species and found that attentiveness increased with clutch mass. Our results agree with the idea that egg number and clutch mass control incubation behaviour (Deeming Citation2002). The effect of clutch size on nest attentiveness agrees with our prediction: higher energy demand is associated with larger clutches (Thomson et al. Citation1998, Deeming Citation2002). Clutch size varied between 5 and 19 eggs (mean = 9.26; sd = 3.75). However, part of this variation can be attributed to intraspecific nest parasitism (Dugger & Blums Citation2001, Pöysä Citation2006, Baldassare & Bolen Citation2006). Over a period of five years of survey in Grand-Lieu Lake, intraspecific nest parasitism was observed for the first time in 2008. Reduced availability of nesting habitat due to flooding conditions could have been the indirect cause of this pattern. Canvasback Aythya valisineria eggs in nests parasitized by Redheads A. americana often have longer incubation periods and hatch asynchronously as a result of less-effective incubation due to the addition of parasitic eggs (Sayler Citation1996). Conversely, Dugger & Blums Citation(2001) did not find evidence that nest parasitism affected nest survival negatively. The fact that nest attentiveness increased for larger clutches, as in our study, may explain this result. Moreover, larger clutch sizes require increased incubation energy expenditure (Thomson et al. Citation1998).
Nest attentiveness over time
Nest attentiveness increased over time. Such a pattern has already been documented, though not in wild Common Pochards (see Afton & Paulus Citation1992). Aldrich & Raveling Citation(1983) found that recess frequency and duration increased throughout incubation, resulting in more foraging time for female Canada Geese Branta canadensis. This increase was due to the reduction of body reserves during incubation. Once a lower critical weight was reached, the duration of recess increased dramatically, probably because lipid reserves were depleted. Aldrich & Raveling Citation(1983) invoked both predation avoidance and physiological requirements (body reserves) to explain their results. While geese and Common Eider Ducks Somateria mollissima are capital breeders and depend narrowly on the body fuels stored before incubation (Criscuolo et al. Citation2002), the importance of body nutrient reserves on incubation rhythm and investment has also been documented in other waterfowl species, including Common Pochards (e.g. Gloutney et al. 1991, Shutler et al. Citation1998, Blums & Clark Citation2004, Spaans et al. Citation2007). However, corrected body mass at the beginning of incubation was not found to be a good predictor of attentiveness. Common Pochards are more likely to be income breeders and early mass may not be as crucial as for capital breeders such as geese. Ideally, nest attentiveness and body mass variation should be monitored throughout the incubation to ensure that body mass reduction induces changes in nest attentiveness (Aldrich & Raveling Citation1983, Criscuolo et al. Citation2002).
CONCLUSION
This article provides data on nest attentiveness of two common duck species using small TDLs deposited in the nest or inside dummy eggs. The two methodologies employed allowed monitoring nest attentiveness through the recording of incubation temperature, and also allowed the precise time of nest depredation to be determined. Many aspects of duck incubation have been investigated previously. However, other approaches such as cumbersome/expensive data loggers or visual observations do not allow the easy monitoring of a number of nests simultaneously. TDLs fill this gap by offering the possibility of monitoring numerous nests at the same time, with a high benefits/costs ratio. Nest attentiveness increased over time and clutch size (in association with body size in Mallards) played an important role in incubation behaviour. Having more eggs seemed to lead to a reduction in the number of incubation recesses, but incubation length increased. Understanding the effects of clutch, body size, climatic condition, and predation on Pochard nesting success would benefit from the comparison of successful versus failed nests.
ACKNOWLEDGEMENTS
This study benefited from the help of Stephanie Hennique, Adrien Henzel, Hervé Lelievre and Noël Guillon in the field. We are grateful to Cyril Eraud, Jean Marie Boutin and Vincent Bretagnolle for their support at various stages of this research. We certify that observations on live individuals were carried out in compliance with European legal requirements (European Convention ETS no. 123) and national permission delivered by the Museum National d'Histoire Naturelle (Centre de Recherches sur la Biologie des Populations d'Oiseaux). Matthieu Guillemain, Alexandre Villers and four anonymous referees provided helpful comments on an earlier draft of the manuscript. We thank Marie-Christine Cadieux for kindly improving the English of the original version of the manuscript.
Additional information
Notes on contributors
Mathieu Giraudeau
†Present address: School of Life Sciences, Arizona State University, Tempe, AZ 85287-4501, USA.Camille Duval
‡Present address: Centre for Ornithology, School of Biosciences, Birmingham University, Birmingham, UK.REFERENCES
- Afton , A. D. 1980 . Factors affecting incubation rhythms of Northern Shovelers . Condor , 82 : 132 – 137 .
- Afton , A. D. and Paulus , S. L. 1992 . “ Incubation and brood care ” . In Ecology and Management of Breeding Waterfowl , Edited by: Batt , B. D.J. , Afton , A. D. , Anderson , M. G. , Ankney , C. D. , Johnson , D. H. , Kadlec , J. A. and Krapu , G. L. 62 – 108 . Minneapolis , MN : University of Minnesota Press .
- Albrecht , T. , Horak , D. , Kreisinger , J. , Weidinger , K. , Klvana , P. and Michot , T. C. 2006 . Factors determining Pochard nest depredation along a wetland gradient . J. Wildl. Manage. , 70 : 784 – 791 .
- Aldrich , T. W. and Raveling , D. G. 1983 . Effects of experience and body weight on incubation behavior of Canada Geese . Auk , 100 : 670 – 679 .
- Arnold , J. M. , Sabom , D. , Nisbet , I. C.T. and Hatch , J. J. 2006 . Use of temperature sensor to monitor patterns of nocturnal desertion by incubating Common Terns . J. Field Ornithol. , 77 : 384 – 391 .
- Arnold , T. W. , Anderson , M. G. , Emery , R. B. , Sorenson , M. D. and de Sobrino , C. N. 1995 . The effects of late-incubation body mass on reproductive success and survival of Canvasacks and Redheads . Condor , 97 : 953 – 962 .
- Baldassare , G. A.R. and Bolen , E. G. 2006 . Waterfowl Ecology and Management , 2 , Malabar , FL : Krieger Publishing Co .
- Blums , P. and Clark , R. G. 2004 . Correlates of lifetime reproductive success in three species of European ducks . Oecologia , 140 : 61 – 67 .
- Blums , P. , Mednis , A. and Clark , R. G. 1997 . Effect of incubation body mass on reproductive success and survival of two European diving ducks: a test of the nutrient limitation hypothesis . Condor , 99 : 916 – 925 .
- Blums , P. , Nichols , J. D. , Hines , J. E. , Lindberg , M. S. and Mednis , A. 2003 . Factors affecting breeding dispersal of two European ducks on Engure Marsh, Latvia . J. Anim. Ecol. , 72 : 292 – 307 .
- Blums , P. , Nichols , J. D. , Hines , J. E. , Lindberg , M. S. and Mednis , A. 2005 . Individual quality, survival variation and patterns of phenotypic selection on body condition and timing of nesting in birds . Oecologia , 143 : 365 – 376 .
- Boos , M. , Zimmer , C. , Carriere , A. , Robin , J.-P. and Petit , O. 2007 . Post-hatching parental care behaviour and hormonal status in a precocial bird . Behav. Proc. , 76 : 206 – 214 .
- Burnham , K. P. and Anderson , D. R. 1998 . Model Selection and Inference , New York , NY : Springer-Verlag .
- Caldwell , P. J. and Cornwell , G. W. 1975 . Incubation behavior and temperatures of Mallard duck . Auk , 92 : 706 – 731 .
- Cooper , C. B. and Mills , H. 2005 . New software for quantifying incubation behavior from time-series recordings . J. Field Ornithol. , 76 : 352 – 356 .
- Cramp , S. and Simmons , K. E.L. 1977 . The Birds of the Western Palearctic. Vol 1: Ostrich to Ducks , Oxford : Oxford University Press .
- Criscuolo , F. , Gabrielsen , G. W. , Gendner , J. P. and Le Maho , Y. 2002 . Body mass regulation during incubation in female Common Eiders Somateria mollissima. J. Avian Biol . 33 : 83 – 88 .
- Decuypere , E. and Michels , H. 1992 . Incubation temperature as a management tool: a review . World's Poult. Sci. J. , 48 : 28 – 38 .
- Deeming , D. C. 2002 . Avian Incubation: Behaviour, Environment, and Evolution , Oxford : Oxford University Press .
- Drent , R. H. and Daan , S. 1980 . The prudent parent: energetic adjustments in avian breeding . Ardea , 68 : 225 – 252 .
- Dugger , B. D. and Blums , P. 2001 . Effect of conspecific brood parasitism on host fitness for Tufted Duck and Common Pochard . Auk , 118 : 717 – 726 .
- Flint , P. L. and MacCluskie , M. C. 1995 . A device for simultaneously measuring nest attendance and nest temperature in waterfowl . J. Field Ornithol. , 66 : 515 – 521 .
- Franklin , A. B. , Anderson , D. R. , Gutierrez , R. J. and Burnham , K. P. 2000 . Climate, habitat quality, and fitness in Northern Spotted Owl populations in northwestern California . Ecol. Monogr. , 70 : 539 – 590 .
- Gloutney , M. L. and Clark , R. G. 1991 . The significance of body mass to female dabbling ducks during late incubation . Condor , 93 : 811 – 816 .
- Greenwood , R. J. , Sargeant , A. B. , Johnson , D. H. , Cowardin , L. M. and Shaffer , T. L. 1995 . Factors associated with duck nest success in the prairie pothole region of Canada . Wild. Monogr. , 128 : 1 – 57 .
- Hartman , C. A. and Oring , L. W. 2006 . An inexpensive method for remotely monitoring nest activity . J. Field Ornithol. , 77 : 418 – 424 .
- Hébert , P. N. 2002 . “ Ecological factors affecting initiation of incubation behaviour ” . In Avian Incubation: Behaviour, Environment, and Evolution , Edited by: Deeming , D. C. 270 – 279 . Oxford : Oxford University Press .
- Hoekman , S. T. , Mills , L. S. , Howerter , D. W. , Devries , J. H. and Ball , I. J. 2002 . Sensitivity analyses of the life cycle of midcontinent Mallards . J. Wildl. Manage. , 66 : 883 – 900 .
- Hoover , A. K. , Rohwer , F. C. and Richkus , K. D. 2004 . From the field: evaluation of nest temperatures to assess female nest attendance and use of video cameras to monitor incubating waterfowl . Wildl. Soc. Bull. , 32 : 581 – 587 .
- Lebreton , J. D. , Burnham , K. P. , Clobert , J. and Anderson , D. R. 1992 . Modeling survival and testing biological hypotheses using marked animals – a unified approach with case-studies . Ecol. Monogr. , 62 : 67 – 118 .
- Lessells , C. M. 1991 . “ The evolution of life histories ” . In Behavioural Ecology , Edited by: Krebs , J. R. and Davies , N. B. 32 – 68 . Oxford : Blackwell Scientific Publications .
- Loos , E. R. and Rohwer , F. C. 2004 . Laying-stage nest attendance and onset of incubation in prairie nesting ducks . Auk , 121 : 587 – 599 .
- Low , J. B. 1941 . Nesting of the Ruddy Duck in Iowa . Auk , 58 : 506 – 517 .
- Low , J. B. 1945 . Ecology and management of the Redhead . Nyroca americana in Iowa. Ecol. Monogr , 15 : 35 – 69 .
- Møller , A. P. 2005 . Parasites, predators and the duration of developmental periods . Oikos , 111 : 291 – 301 .
- Opermanis , O. , Mednis , A. and Bauga , I. 2001 . Duck nests and predators: interaction, specialisation and possible management . Wildl. Biol. , 7 : 87 – 96 .
- Paillisson , J.-M. , Reeber , S. and Marion , L. 2002 . Bird assemblages as bio-indicators of water regime management and hunting disturbance in natural wet grasslands . Biol. Cons. , 106 : 115 – 127 .
- Peig , J. and Green , A. J. 2009 . New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method . Oikos , 118 : 1883 – 1891 .
- Pinheiro , J. C. and Bates , D. M. 2000 . Mixed-effects Models in S and S-PLUS , New York , NY : Springer .
- Poussart , C. , Gauthier , G. and Larochelle , J. 2001 . Incubation behaviour of Greater Snow Geese in relation to weather condition . Can. J. Zool. , 79 : 671 – 678 .
- Pöysä , H. 2006 . Public information and conspecific nest parasitism in goldeneyes: targeting safe nests by parasites . Behav. Ecol , 17 : 459 – 465 .
- Reeber, S. 2009. [Suivi Ornithologique du Lac de Grand-lieu en 2009.] Rapport SNPN (in French)
- Reynolds , R. E. and Sauer , J. R. 1991 . Changes in mallard breeding populations in relation to production and harvest rates . J. Wildl. Manage. , 55 : 483 – 487 .
- Sargeant , A. B. and Raveling , D. G. 1992 . “ Mortality during the breeding season ” . In Ecology and Management of Breeding Waterfowl , Edited by: Batt , B. D.J. , Afton , A. D. , Anderson , M. G. , Ankney , C. D. , Johnson , D. H. , Kadlec , J. A. and Krapu , G. L. 396 – 422 . Minneapolis , MN : University of Minnesota Press .
- Sayler , R. D. 1996 . Behavioral interactions among brood parasites with precocial young: Canvasbacks and Redheads on the Delta Marsh . Condor , 98 : 801 – 809 .
- Shutler , D. , Gloutney , M. L. and Clark , R. G. 1998 . Body mass, energetic constraints, and duck nesting ecology . Can. J. Zool. , 76 : 1805 – 1814 .
- Sibly , R. M. and Hone , J. 2002 . Population growth rate and its determinants: an overview . Phil. Trans. R. Soc. Lond. B. , 357 : 1153 – 1170 .
- Spaans , B. , van't Hoff , C. A. , van der Veer , W. and Ebbinge , B. S. 2007 . The significance of female body stores for egg laying and incubation in Dark-bellied Brent Geese Branta bernicla bernicla. Ardea . 95 : 3 – 15 .
- Stearns , S. C. 1992 . The Evolution of Life Histories , Oxford : Oxford University Press .
- Thomson , D. L. , Monaghan , P. and Furness , E. W. 1998 . The demands of incubation and avian clutch size . Biol. Rev. , 73 : 293 – 304 .
- Tinbergen , J. M. and Williams , J. B. 2002 . “ Energetics of incubation ” . In Avian Incubation: Behaviour, Environment, and Evolution , Edited by: Deeming , D. C. 299 – 313 . Oxford : Oxford University Press .
- Webb , D. R. 1987 . Thermal tolerance of avian embryos: a review . Condor , 89 : 874 – 898 .
- West , B. C. and Messmer , T. A. 2004 . Impacts and management of duck-nest predation: the managers' view . Wildl. Soc. Bull. , 32 : 772 – 781 .
- Westerkov , K. 1950 . Methods for determining the age of game bird eggs . J. Wildl. Manage. , 14 : 56 – 67 .
- Yerkes , T. 1998 . The influence of female age, body mass, and ambient condition on Redhead incubation constancy . Condor , 100 : 62 – 68 .