Abstract
Capsule Herons responded to the lower abundance of a preferred fish by switching prey.
Aims To investigate how Grey Herons respond to the decline in abundance of a preferred fish, the Round Goby Neogobius melanostomus.
Methods Grey Herons Ardea cinerea breeding in the Gulf of Gdańsk area (Baltic Sea) were the study population. Regurgitated food and pellets were analysed. Biomass and abundance of fish was compared between 2000–02 (when the population of Round Goby was increasing) and 2008–09 (when the Round Goby population stabilized).
Results The abundance and biomass of Round Goby preyed upon by herons decreased from between 95 and 99% in 2000–02 to between 38 and 56% in 2008–09. Energy-rich salmonids, not recorded in 2000–02, were an important diet component in 2008–09 (42% of biomass). Small-sized fish abundance was higher in 2008–09 (37%) than in 2000–02 (9%).
Conclusions Grey Herons in 2008–09 adapted to the lower availability of Round Gobies by prey switching to other available fish – abundant but small-sized Three-spined Sticklebacks Gasterosteus aculeatus and less abundant but more profitable salmonids (easy to catch in the case of hatchery-reared, tagged individuals). Diet composition suggests that Grey Herons in 2008–09 exploited more diverse foraging areas (dry habitats, woods, garden ponds) than in 2000–02.
Changes in prey communities (e.g. an invasion of a new species) give an opportunity to study the dietary response of predators. Predators may have strong, fixed preferences for the most profitable prey type, and optimal foraging models predict that predators should always include the most profitable prey item in their diet regardless of its abundance (Charnov Citation1976). An alternative possible reaction, associated with generalist predators, is prey switching, in which preference is instantaneously altered when prey availabilities change. In this scenario, a predator's selection for a prey species increases when that species is abundant and decreases when it is scarce (Murdoch Citation1969, Murdoch et al. Citation1975, Murdoch & Oaten Citation1975).
The Gulf of Gdańsk (of the Baltic Sea) in Poland is an area where rapid changes in the fish community have occurred following the invasion of the Round Goby Neogobius melanostomus. In 1990, the first specimen of this fish was found in this region (Skóra & Stolarski Citation1993). The natural distribution of the Round Goby is the Ponto-Caspian basin and it was probably introduced into the Gulf of Gdańsk by transport of eggs and/or larvae in the ballast water of vessels sailing between the Caspian Sea or Black Sea and the Baltic (Skóra Citation1997). The Round Goby population expanded rapidly, becoming a dominant fish species in most of the shallow waters of the Gulf of Gdańsk (Sapota Citation2004) and leading to the decline in total biomass and population density of what was the most numerous fish in the Gulf of Gdańsk before the Round Goby invasion, the Three-spined Stickleback Gasterosteus aculeatus (Corkum et al. Citation2004). Fishing efficiency – catch per unit effort (CPUE) in kg/h – of the Round Goby in the southern Baltic increased in the period of 1993–2002, but it declined in 2003–04 (Grygiel Citation2007, Citation2008). This decline suggests that the invasion of the Round Goby reached the saturation level (Psuty & Wilkońska Citation2009). Data from the following years (2006–10) suggest further decrease in CPUE of the Round Goby in the southern Baltic and the Gulf of Gdańsk (Dr. Grygiel W., Sea Fisheries Institute in Gdynia, Poland, personal communication).
The Round Goby, as a small (length up to 25 cm), fat, and relatively poorly swimming fish spawning in shallow coastal waters, is an ideal prey for piscivorous birds (Skóra Citation1997). Indeed, the Round Goby has become an important food component of Great Cormorants Phalacrocorax carbo (Bzoma Citation1998) and Grey Herons Ardea cinerea (Jakubas Citation2004, Jakubas & Mioduszewska Citation2005) feeding in the Gulf of Gdańsk. Grey Herons are wading birds which are highly opportunistic in prey selection. Their diet varies widely with habitat and season and may be dominated, depending on location, by fish, crustaceans, or mammals (Cramp Citation1998, Kushlan & Hancock Citation2005). Studies from western Europe show that the rapid changes in prey availability following the introduction of a new prey species, produce large qualitative changes in the diet of Grey Herons (Peris et al. Citation1994, Adams & Mitchell Citation1995). Such a change in diet may be caused by decline in the native prey species population, or represent a functional shift in prey choice to a more abundant, more easily caught species (Peris et al. 1993, Adams & Mitchell Citation1995).
The aim of this study was to investigate whether the observed decline in fishery catches of Round Gobies (reflecting decline in its availability for predators) affected diet composition of Grey Herons breeding on the Gulf of Gdańsk coast. Data on the Grey Heron diet composition were compared between two periods: 2000–02 (the phase of a rapid expansion of the Round Goby population) and 2008–09 (the phase of saturation and decline in fish catches).
METHODS
Study area
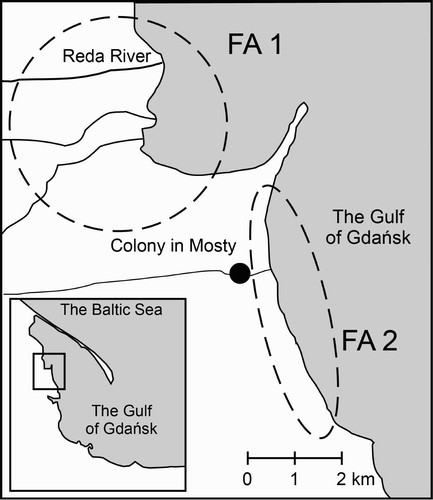
The study was conducted in the breeding colony of Grey Herons at Mosty (54°37′N, 18°29′E) located in the Reda River Valley, 0.7 km from the Gulf of Gdańsk coast (the Baltic Sea, north Poland) (). The herons nest on Common Alders Alnus glutinosa in a small wood. The Grey Herons' feeding grounds are situated within an 8-km radius of the colony – in pastures, meadows, swamps, reed beds, canal banks, sandbanks in the mouth of the Reda River, and in the shallow coastal waters of the Gulf of Gdańsk (; Jakubas Citation2004). There were 363 and 369 Grey Heron nests at the colony in 2006 and 2008 respectively (Manikowska Citation2010, Żółkoś et al. Citation2010).
Figure 1. Study area with the colony in Mosty (black point) and the main foraging areas (FAs) of Grey Herons Ardea cinerea (broken line ovals; FA1, Reda River Valley including wet meadows, canals and the river mouth; FA2, the Gulf of Gdańsk coastal area including shallow coastal water and wet meadows).
Data collection and analyses
Grey Heron diet composition was determined using regurgitated food and pellets collected in the colony in 2008–09. The colony area was searched for pellets and regurgitated prey during weekly visits throughout the chick-rearing period (May–July) in 2008 and 2009.
All collected regurgitated items were classified as partly digested (mostly fish with digested heads) or undigested. They were identified using the following literature: fish, Miller & Loates Citation(1997) and Brylińska Citation(2000); mammals, Kowalski Citation(1964) and Pucek Citation(1984); crustaceans, Żmudziński Citation(1974). All intact fish were measured from the tip of head to the fork of tail with 0.5 cm accuracy.
In total, 90 regurgitated fish (59 undigested and 31 partly digested; 42 in 2008 and 48 in 2009, respectively), three mammals (two in 2008 and one in 2009) and 30 prawns (all in 2009) were found.
The collected pellets were stored in a plastic bag and dried for three to four days at room temperature before analysis. All mammal bones, invertebrate remains, feathers and other solid parts were removed from a pellet to permit better species identification. Mammal skulls were identified following Pucek Citation(1984). From each pellet collected, ten hairs were randomly sampled for microscopic analysis. Scale pattern of the hair cuticula, medullary patterns and pattern of sectioned hair were used for species identification, using the keys of Dziurdziuk Citation(1973) and Teerink Citation(2003). Additionally, hairs taken from the collected pellets were compared with hairs from a collection of known species, stuffed mammals. Hairs were prepared for identification by the methods proposed by Dziurdziuk Citation(1973). In five pellets collected in 2009, apart from hairs and bones, celluloid ichthyological tags were found. The information about the species and body length of the tagged fish was obtained from the Inland Fisheries Institute, Department of Migratory Fish in Gdańsk, Poland. Insects in the pellets were not analyzed in the present study, since they are considered unimportant in the energy budget of large herons (Draulans et al. Citation1987). In total, 35 pellets (16 in 2008 and 19 in 2009) were collected and analysed. Herons have an extremely efficient digestive system (Vinokurov Citation1960), and the majority of fish bones, scales and otoliths are digested completely (Draulans et al. Citation1987). Thus, these types of remains were not found in pellets.
To compare the changing diet composition and fish length distribution in 2008–09 with the period of rapid expansion of Round Goby, we compared our results with data collected in the same manner in 2000–02 and reported by Jakubas Citation(2004) and Jakubas & Mioduszewska Citation(2005). The length of fish was reported as median and the first and third quartiles (hereafter Q1–Q3). The biomass of the regurgitated, undigested fish was calculated for the majority of species (10 of 13 in 2008–09 and 3 of 3 in 2000–02) based on regression equations given in the literature (Reimchen Citation1990, Dirksen et al. Citation1995, Altindağ et al. Citation1998, Wandzel Citation2003, Arslan et al. Citation2004). Differences in frequency of prey occurrence, abundance and biomass of fish between the studied periods: 2000–02 and 2008–09 were analysed using 2 × 2 χ2 tests (with Yates' correction in the case of small sample size). All statistical analyses were performed in statistica 8.0 (StatSoft, Inc., Tulsa, Oklahoma).
RESULTS
Regurgitated food
Fish constituted the majority of regurgitated prey both in 2008–09 (73.2% of abundance, n = 123) and 2000–02 (99.4% of abundance, n = 177). In 2008–09, Baltic Prawns Palaemon adspersus (30 individuals) and mammals made up 24.4% and 2.4% of collected prey items, respectively. In 2000–02, mammals constituted 0.6% of abundance. The proportion of fish to other regurgitated prey (1 : 0.27) was significantly lower than in 2000–02 (1 : 0.01; χ 2 1 = 36.3, P < 0.0001) when Baltic Prawns were not recorded. Different species of regurgitated mammals were found in 2008–09 (two European Water Voles, one European Mole) and 2000–02 (one Eurasian Water Shrew Neomys fodiens).
The number of species/taxa of regurgitated fish found in 2008–09 was 14, or 3.5 times higher than was found in 2000–02 samples. All species found in 2000–02 (Round Goby, Three-spined Stickleback, Perch Perca fluviatilis and European Plaice Platessa platessa) were also recorded in 2008–09. The relative abundance and biomass of the Round Goby in 2008–09, while still the most common species found among regurgitated fish, was significantly lower (abundance 1.7 times and biomass 2.6 times) in 2008–09 than it was in 2000–02 samples (). The proportion of the Round Goby to other species of regurgitated fish was significantly lower in 2008–09 (1 : 0.8 by abundance and 1 : 0.65 by biomass) compared with 2000–02 sample results (1 : 0.1 by abundance, χ 2 1 = 61.3, P < 0.001; 1 : 0.005 by biomass, χ 2 1 = 1873.2, P < 0.0001). The relative abundance and biomass of Three-spined Sticklebacks in 2008–09 was significantly higher than in 2000–02 (abundance six times and biomass eight times; ). Also the relative abundance and biomass of other species of fish collected in 2008–09 was significantly higher than in 2000–02 samples reported (abundance 19 times and biomass 407 times; ). In 2008–09, among other species of regurgitated fish, the Crucian Carp Carassius carassius and salmon/trout Salmonidae (species not identified due to advanced digestion) constituted 3% of abundance each. Perch, Goldfish Carassius auratus auratus and Sea Trout made up 2% of abundance each. Tench Tinca tinca, Roach Rutilus rutilus, Brown Trout Salmo trutta morpha fario, European Plaice, European Flounder Platichthys flesus, Bleak Alburnus alburnus and Rudd Scardinius erythrophthalmus made up 1% of abundance each. In that group salmonids (Sea Trout, Brown Trout and salmon/trout) constituted 6.7% of abundance and made up as much as 42.0% of biomass of regurgitated fish. Among other fish species, Perch and European Plaice were found in 2000–02 and 2008–09 constituting less than 3% of abundance in both periods studied.
Table 1. Relative abundance (undigested and partly digested fish combined) and biomass (only undigested fish) of regurgitated fish collected in the Grey Heron Ardea cinere a colony in Mosty in 2000–02 and 2008–09.
The length of regurgitated, undigested fish found in the colony in 2008–09 was significantly higher than in 2000–02 (). The distribution of length of the fish differed between the periods studied (Kolmogorov–Smirnov test, P < 0.001) as fish smaller than 81 mm made up 39% in 2008–09 and 9% in 2000–02. Those longer than 140 mm constituted 15% in 2008–09 and 8% in 2000–02 (). The length of regurgitated, partly digested fish was similar in both studied periods (). The biomass of regurgitated, undigested fish in 2008–09 was significantly lower than in 2000–02 (). Both the length and the biomass of undigested Round Gobies did not differ significantly between 2000–02 and 2008–09 ().
Figure 2. Body length classes of fish (all species combined) found in the colony of Grey Herons Ardea cinerea in Mosty in 2000–02 and 2008–09 (undigested regurgitated) and 2009 (Sea Trout tagged in 2009; length measured when they were tagged).
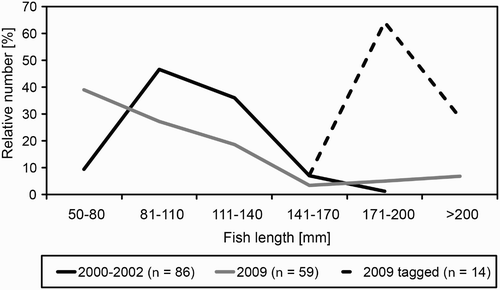
Table 2. Body length and biomass of regurgitated, undigested and partly digested fish found in the colony of Grey Herons Ardea cinerea in Mosty in 2000–02 and 2008–09 (undigested regurgitated).
Pellet composition
Of 118 pellets collected in the colony in 2008–09, 98% contained mammal hairs, as did 100% of the 35 pellets collected in 2000–02. Other items, such as mammal bones, bird feathers and ichthyological tags were found in less than one quarter of pellets. No differences were found in frequency of occurrence of hairs (χ2 1 = 0.01, P = 0.94), mammal bones (24% versus 20%, χ2 1 = 0.02, P = 0.89), feathers (4% versus 3%, χ2 1 = 0.27, P = 0.60) between 2000–02 and 2008–09. Ichthyological tags were recorded only in 2008–09 (14%), a shell of the Ramshorn Snail Planorbis planorbis only in 2000–02 (1%).
The analysis of mammal bones showed that the most common mammal species were Microtus sp. (2008–09) and the European Water Vole Arvicola terrestris (2000–02). Significant differences between 2008–09 and 2000–02 were found in the frequency of occurrence of European Water Voles Microtus sp., and European Moles Talpa europea ().
Table 3. Frequency of pellets of Grey Herons Ardea cinerea containing different mammalian taxa (identified by bone remains/hair) in 2000–02 (Jakubas & Mioduszewska Citation2005) and 2008–09.
Analysis of hair revealed that Microtus sp. was the most frequent mammal prey of Grey Herons in 2008–09 and 2000–02, found in over 60% of pellets, resp-ectively (). Its frequency was similar in both studied periods. The frequencies of other numerous prey, European Water Voles (60% in 2008–09, 42% in 2000–02) were also similar. European Moles, whose hair was found in 47% of pellets collected in 2000–02, occurred rarely in 2008–09 (in 6% of pellets). Some mammals (mice from genus Apodemus, House Mouse Mus musculus, Edible Dormouse Glis glis) were recorded only in 2008–09 ().
All 20 ichthyological tags found in five pellets collected in 2009 had been attached to the Sea Trout Salmo trutta morpha trutta. All those trout were hatchery-reared. They were tagged and released as 2–3-year-old (age category 2+) smolts into the Reda River in Mrzezino (5–6 km from the colony at Mosty). Five Sea Trout were tagged a year before finding the labels in the pellets. The estimated length of the Sea Trout from the Reda River after one year from tagging is 519 mm (Bartel Citation2000). The tags of the remaining 14 trout were found in the Grey Heron pellets 15–30 days after the tag attachment. For those fish, body length during the tagging ranged from 160 to 235 mm (median = 190 mm, Q1–Q3 = 180–205 mm; ). Calculated biomass of those trout ranged from 53.1 to 166.4 g (median = 95.8 g, Q1–Q3 = 75.4–110.9 g).
DISCUSSION
The present study demonstrated a change in the composition of the diet of Grey Herons breeding in Mosty, Poland. The abundance and biomass of the most important prey species in 2000–02 – the Round Goby – declined in dietary abundance by a factor of 1.7, while biomass declined by a factor of 2.6 between 2000–02 and 2008–09. Data on fishing efficiency (CPUE) from 2003–04 (Grygiel Citation2007, Citation2008) and the following years strongly suggest a decline in the Round Goby population in the southern Baltic and the Gulf of Gdańsk during this time period. Lower abundance of the Round Goby in the diet in 2008–09 suggests that Grey Herons adapted to the lower availability of this introduced fish in the foraging area by prey switching to other fish species – abundant but small-sized Three-spined Sticklebacks (the most abundant fish in the Gulf of Gdańsk in the 1990s [Corkum et al. 2004]) and less abundant but large-sized and more profitable salmonids.
Salmonids (mainly Sea Trout), not recorded in 2000–02, constituted an important component of sampled heron diets in 2008–09, especially considering their size (>200 mm, regurgitated; >151 mm, tagged), biomass (42% considering only regurgitated prey) and mean energy content of the whole fish (6.3 times higher – 900 kJ in anadromous Brown Trout from north Europe, age group 2+ [Jonsson & Jonsson Citation1998] – than in Round Goby – 142 kJ in average-sized individual from the Gulf of Gdańsk [Sapota Citation2005]). Grey Herons are known for showing preference for selecting larger fish and neglecting smaller ones (Moser Citation1986, Feunteun & Marion Citation1994, Gwiazda & Amirowicz Citation2006). Studies from south Poland revealed that Grey Herons foraged mainly in the worst available habitat (low fish abundance and high water turbidity) but with the largest prey size (Gwiazda & Amirowicz Citation2006). However, it is unlikely that Grey Herons from Mosty had a fixed preference for salmonids as this type of prey were not recorded in the diet in 2000–02 samples, despite common occurrence in the Reda River at that time (Radtke et al. Citation2007). Grey Herons might have taken advantage of the naivety to predators of hatchery-reared, tagged salmonids. Hatchery fish may not develop the full potential for antipredatory behaviour. They are accustomed to existing at high densities and foraging under conditions that require behaviour different from that which is adaptive under natural conditions. They tend to linger in the release area rather than dispersing and also position themselves in shallows close to the bank and nearer the water surface, all of which makes them more vulnerable to heron predation. Stress and the disorientation associated with tagging and transfer to a natural but novel habitat may further erode predator vigilance and recognition, and the ability to respond behaviourally to threat (Olla et al. Citation1998). Moreover, vivid colours of the tags (majority of them were red) may have made spotting the fish easier for the herons. Grey Herons have been reported to forage on easily caught prey such as tagged smolts, or caged fish that were blind or in poor body condition (Carss Citation1993, Jepsen et al. Citation1998, Koed et al. Citation2002).
Between 2000–02 and 2008–09, Grey Herons changed the main foraging areas used during the chick-rearing periods. Analysis of the flight directions of Grey Herons departing from the colony in Mosty in 1999–2000 revealed that the majority of them flew to forage in the area FA2 (), along the shallow coastal waters (38% of birds), where they preyed mainly on Round Gobies (Jakubas Citation2004). In 2009, only 14% of observed birds were recorded feeding in the area FA2 (Manikowska Citation2010). More than half of all feeding birds (57%) observed in 2009 were in the vicinity of the Reda River Mouth (part of foraging area FA1). In 1999–2000, only 14% of Grey Herons flew towards FA1 (Jakubas Citation2004). Such a pattern suggests habitat switching from shallow coastal waters (FA2) abundant in Round Gobies in 2000–02 to alternative foraging areas such as FA1 (where herons probably mainly foraged on trout), reflecting changes in food availability. Moreover, species composition of prey in 2008–09 suggests exploiting more diversified foraging areas compared with 2000–02. Occurrence of Goldfish in regurgitated food and direct observation of foraging herons indicate exploitation of garden ponds in surrounding villages for the latter time period. Species of mammals identified in pellets (Striped Field Mouse Apodemus agrarius, Wood Mouse Apodemus sylvaticus, other mice from genus Apodemus and Edible Dormouse) suggest that herons were exploiting dry habitats like grasslands, cultivated fields, forests and gardens (Kowalski Citation1964). Areas where those species should be available are located within a 5–10-km radius from the colony.
When investigating temporal changes in diet it is important to consider several additional factors such as environmental changes in the colony (e.g. changes in vegetation under the colony or in the population of scavengers altering the detection of small regurgitated prey) and/or foraging grounds (e.g. weather conditions, changes in human disturbance level altering exploitation of particular areas by herons). Frequent exploitation of the foraging area in the Reda River Mouth by herons (part of FA1, where they probably hunted for trout) in 2009 might have been possible due to a total ban on fish angling, issued the first time in that year. Undisturbed herons might have been more effective in hunting for tagged trouts then compared with 2000–02 when hatchery-reared trouts were also tagged and released but the Reda River Mouth was open for anglers (Manikowska Citation2010). It seems that apart from that mentioned change, nesting habitat and foraging areas did not change considerably between 2000–02 and 2008–09. This suggests that the observed changes in Grey Heron diet composition were caused mainly by lower availability of Round Gobies.
The effect of changes in diet and prey community on the Grey Heron population dynamics is not clear. Although the size of the colony in Mosty decreased from 412–493 nests in 2002–2003 (Jakubas Citation2004) to 363–369 nests in 2006–2008 (Manikowska Citation2010, Żółkoś et al. Citation2010), decline in nest numbers has also been reported from the other large heronries in northern Poland in the last 15 years (Żółkoś et al. Citation2010). Thus, concurrent decline in the Round Goby population and size of the heronry in Mosty might have been coincidental. The Grey Heron population size may respond to factors other than availability of food (Adams & Mitchell Citation1995).
In conclusion, we found that the diet of Grey Herons breeding in Mosty changed between 2000–02 and 2008–09. The abundance and biomass of Round Gobies consumed by Grey Herons declined, possibly reflecting stabilization and/or decrease in population of this invasive species in the decade since its early expansions following introduction. This suggests that Grey Herons switched from foraging on the declining Round Goby to more abundant but small-sized fish (like Three-spined Sticklebacks) and less abundant but more energetically profitable salmonids (easy to catch in case of hatchery-reared, tagged individuals).
ACKNOWLEDGEMENTS
We thank Krzysztof Ślepowroński for help in the field. We would like to thank Dr. Włodzimierz Grygiel for information about CPUE of the Round Goby in 2006–2010, Dr. Michał Skóra for information about tagged Sea Trout, Dr. Katarzyna Wojczulanis-Jakubas, Dr. Simon Gillings and an anonymous reviewer for critical comments on the first version of the manuscript. We appreciate the improvements in English usage made by Phil Whitford through the Association of Field Ornithologists' program of editorial assistance.
Additional information
Notes on contributors
Brygida Manikowska
Present address: University of Gdańsk, Bird Migration Research Station, al. Marsz. Piłsudskiego 46, 81-378 Gdynia, Poland.REFERENCES
- Adams , C. E. and Mitchell , J. 1995 . The response of a Grey Heron Ardea cinerea breeding colony to rapid change in prey species . Bird Study , 42 : 44 – 49 .
- Altindağ , A. , Yiğit , S. , Ahiska , S. and Özkurt , Ş. 1998 . The growth features of tench (Tinca tinca L., 1758) in the Kesikköprü dam lake . Turk. J. Zool. , 22 : 311 – 318 .
- Arslan , M. , Yıldırım , A. and Bektaş , S. 2004 . Length–weight relationship of brown trout, Salmo trutta L., inhabiting Kan Stream, Çoruh Basin, north-eastern Turkey . Turk. J. Fish. Aquat. Sci. , 4 : 45 – 48 .
- Bartel , R. 2000 . Effectiveness of stocking tagged sea trout smolts into Reda River and Puck Bay . Archiv. Polish Fish. , 8 : 149 – 159 .
- Brylińska , M. 2000 . Ryby słodkowodne Polski , Warsaw , , Poland : PWN .
- Bzoma , S. 1998 . The contribution of round goby (Neogobius melanostomus Pallas, 1811) to the food supply of cormorants (Phalacrocorax carbo Linnaeus, 1758) feeding in the Puck Bay . Bull. Sea Fish. Inst. , 2 ( 144 ) : 39 – 47 .
- Carss , D. N. 1993 . Grey heron, Ardea cinerea L., predation at cage fish farms in Argyll, western Scotland . Aquaculture Res. , 24 : 29 – 45 .
- Charnov , E. L. 1976 . Optimal foraging: attack strategy of a mantid . Am. Nat. , 110 : 141 – 151 .
- Corkum , L. D. , Sapota , M. R. and Skóra , K. E. 2004 . The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean . Biol. Invasions , 6 : 173 – 181 .
- Cramp , S. 1998 . The Complete Birds of the Western Palearctic CD-ROM. Oxford University Press, Oxford, UK
- Dirksen , S. , Boudewijn , T. J. , Noordhuis , R. and Marteijn , E. C.L. 1995 . Cormorants Phalacrocorax carbo sinensis in shallow eutrophic freshwater lakes: prey choice and fish consumption in the non-breeding period and effects of large-scale fish removal . Ardea , 83 : 167 – 184 .
- Draulans , D. , Perremans , K. , van Vessem , J. and Pollet , M. 1987 . Analysis of pellets of the grey heron Ardea cinerea, from colonies in Belgium . J. Zool. Soc. Lond. , 211 : 659 – 708 .
- Dziurdziuk , B. 1973 . Klucz do oznaczania włosów ssaków Polski . Acta Zool. Cracov. , 18 : 73 – 92 .
- Feunteun , E. and Marion , L. 1994 . Assessment of grey heron predation on fish communities: the case of the largest European colony . Hydrobiologia , 279–280 : 327 – 344 .
- Grygiel , W. 2007 . “ Round goby (Neogobius melanostomus Pallas, 1811) ‘semi-domestic’ species in the Gulf of Gdansk (the southern Baltic; 1993–2004) ” . Poster presented at the ICES Annual Scientific Conference, Helsinki
- Grygiel , W. 2008 . Gatunki inwazyjne w Morzu Bałtyckim, ze szczególnym uwzględnieniem babki byczej . Wiad. Ryb. , 164 : 18 – 22 .
- Gwiazda , R. and Amirowicz , A. 2006 . Selective foraging of Grey Heron (Ardea cinerea) in relation to density and composition of the littoral fish community in a submontane dam reservoir . Waterbirds , 29 : 226 – 232 .
- Jakubas , D. 2004 . The response of the Grey Heron to a rapid increase of the Round Goby . Waterbirds , 27 : 304 – 307 .
- Jakubas , D. and Mioduszewska , A. 2005 . Diet composition and food consumption of the grey heron (Ardea cinerea) from breeding colonies in northern Poland . Eur. J. Wildl. Res. , 51 : 191 – 198 .
- Jepsen , N. , Aarestrup , K. , Økland , F. and Rasmussen , G. 1998 . Survival of radiotagged Atlantic salmon (Salmo salar L.) and trout (Salmo trutta L.) smolts passing a reservoir during seaward migration . Hydrobiologia , 371–372 : 347 – 353 .
- Jonsson , N. and Jonsson , B. 1998 . Body composition and energy allocation in life-history stages of brown trout . J. Fish Biol. , 53 : 1306 – 1316 .
- Koed , A. , Jepsen , N. , Aarestrup , K. and Nielsen , C. 2002 . Initial mortality of radio-tagged Atlantic salmon (Salmo salar L.) smolts following release downstream of a hydropower station . Hydrobiologia , 483 : 31 – 37 .
- Kowalski , K. 1964 . Klucze do oznaczania kręgowców Polski. Część V. Ssaki – Mammalia , Edited by: Kowalski , K. Warsaw , , Poland : PWN .
- Kushlan , J. A. and Hancock , J. A. 2005 . The Herons. Bird Families of the World , Oxford , , UK : Oxford University Press .
- Manikowska , B. 2010 . “ Wybrane aspekty ekologii rozrodu czapli siwej (Ardea cinerea) w kolonii lęgowej w Mostach ” . Poland : University of Gdańsk . MSc thesis
- Miller , P. J. and Loates , M. J. 1997 . Fish of Britain and Europe. Collins Pocket Guide , London : Harper Collins Publishers .
- Moser , M. E. 1986 . Prey profitability for adult Grey Herons Ardea cinerea and the constraints on prey size when feeding young nestlings . Ibis , 128 : 392 – 405 .
- Murdoch , W. W. 1969 . Switching in general predators: experiments on predator specificity and stability of prey populations . Ecol. Monogr. , 39 : 335 – 354 .
- Murdoch , W. W. and Oaten , A. 1975 . Predation and population stability . Adv. Ecol. Res. , 9 : 2 – 131 .
- Murdoch , W. W. , Avery , S. and Smyth , M. E.B. 1975 . Switching in predatory fish . Ecology , 56 : 1094 – 1105 .
- Olla , B. L. , Davis , M. W. and Ryer , C. H. 1998 . Understanding how the hatchery environment represses or promotes the development of behavioral survival skills . Bull. Mar. Sci. , 62 : 531 – 550 .
- Peris , S. J. , Briz , F. J. and Campos , F. 1994 . Recent changes in the food of the Grey Heron Ardea cinerea in central-west Spain . Ibis , 136 : 488 – 489 .
- Pucek , Z. 1984 . Klucz do oznaczania ssaków Polski , Warszawa : PWN .
- Psuty , I. and Wilkońska , H. 2009 . The stability of fish assemblages under unstable conditions: a ten year series from the Polish part of the Vistula Lagoon . Archiv. Polish Fish. , 17 : 65 – 76 .
- Radtke , G. , Grochowski , A. and Dębowski , P. 2007 . Ichtiofauna dorzecza Redy oraz pozostałych małych cieków wpadających do Zatoki Gdańskiej . Sci. Annu. Polish Angling Assoc. , 20 : 83 – 112 .
- Reimchen , T. E. 1990 . Size–structured mortality in a threespined stickleback (Gasterosteus aculeatus)–cutthroat trout (Oncorhynchus clarki) community . Can. J. Fish Aquat. Sci. , 47 : 1194 – 1205 .
- Sapota , M. R. 2004 . The round goby (Neogobius melanostomus) in the Gulf of Gdańsk – a species introduction into the Baltic Sea . Hydrobiologia , 514 : 219 – 224 .
- Sapota , M. R. 2005 . Biologia i ekologia babki byczej Neogobius melanostomus (Pallas 1811) gatunku inwazyjnego w Zatoce Gdańskiej University of Gdańsk Press, Gdańsk, Poland
- Skóra , K. E. 1997 . “ Neogobius melanostomus ” . In Baltic Sea Alien Species Database Edited by: Olenin , S. and Daunys , D. http://www.corpi.ku.lt/nemo/neogob.html (accessed 31 January 2011)
- Skóra , K. E. and Stolarski , J. 1993 . New fish species in the Gulf of Gdańsk Neogobius sp . Bull. Sea Fish. Inst. , 1 ( 128 ) : 83 – 84 .
- Teerink , B. J. 2003 . Hair of West-European Mammals , Cambridge , , UK : Cambridge University Press .
- Vinokurov , A. A. 1960 . “ [On the food digestion role in heron Ardea purpurea] ” . In Bull. Mosk. Obshchestva Ispytatelei Prirody Otdel Biol. Moskva 65: 10 (in Russian)
- Wandzel , T. 2003 . Babka okrągła Neogobius melanostomus (Pallas, 1811) – nowy komponent ichtiocenozy południowego Bałtyku. Sea Fisheries Institute, Gdynia, Poland
- Żmudziński , L. 1974 . Świat zwierzęcy Bałtyku , Warsaw , , Poland : WSiP .
- Żółkoś , K. , Meissner , W. , Kalasiński , M. , Górska , E. , Mellin , M. , Ibron , I. and Wysocki , D. 2010 . Liczebność i rozmieszczenie kolonii czapli siwej Ardea cinerea w północnej Polsce . Ornis Polonica , 51 : 30 – 42 .