Abstract
Capsule Body mass of Whiskered Tern chicks from the central parts of subcolonies grew at a higher rate in comparison to chicks hatched in the peripheral zones. Growth rates of both body mass and head length correlated positively with nest density. We suggest that spatial distribution of pair quality within the colony of Whiskered Terns follows a central–periphery gradient.
Chick growth is generally known as a highly variable trait, which depends on both environmental factors as well as intrinsic characteristics of adults (Stienen & Brenninkmeijer Citation2002). Parental quality, expressed by age, experience or physical condition, may directly affect chick growth by several means. According to life-history theory, it is expected that older birds increase parental investment in order to maximize their fitness (Trivers Citation1974). Greater parental investment is also exhibited by individuals of higher physical condition (e.g. Erikstad et al. Citation1997). Furthermore, chick growth rates depend on the provisioning rates and the quality of delivered prey, which are closely related to the experience of parents (van de Pol et al. Citation2006). The effect of prey quality on chick growth rates has been demonstrated in several waterbird species (e.g. Golet et al. Citation2000). On the other hand, parental quality may indirectly influence growth of chicks, as high-quality breeding pairs are known to produce larger and more nutritious eggs (Amundsen et al. Citation1996), which support rapid growth in the earliest stages of post-hatching development.
Breeders of different quality are not distributed randomly within waterbird colonies (Coulson Citation1968) and we expect that the spatial patterns of chick growth would correspond to the spatial distribution of pair quality. As many other reproductive parameters, such as hatching and fledging success or chick survival, may be additionally affected by the level of predation, chick growth rates are considered more adequate proxies of pair quality. The relationship between chick growth and parental quality was demonstrated in several tern species, such as Roseate Terns Sterna dougallii (Nisbet et al. Citation1998) and Common Terns Sterna hirundo (Arnold et al. Citation2004).
We investigated spatial patterns of chick growth in Whiskered Tern Chlidonias hybrida, a species of unfavourable pan-European conservation status and a conservation priority of the European Union Wild Birds Directive (BirdLife International Citation2004). The study took place in 2011 in the Whiskered Tern colony at the Jeziorsko reservoir (51°44′N, 18°38′E), central Poland. The nests in the colony were located at the floating beds of Amphibious Bistort Polygonum amphibium, but the distribution of vegetation was uneven. There was a deep water channel (about 80 m wide) running through the central part of the colony, which separated the nesting area into two distinct subcolonies. We recorded 74 nests with clutches in the colony and there were, respectively, 32 and 42 nests in each of the subcolonies. Fifteen of the clutches were lost during the incubation period.
From the onset of hatching (8 July) the colony was visited in 5-day intervals until all the chicks in the colony had fledged. The last chicks hatched on 13 August and the last visit to the colony took place on 28 August, when only one fully grown chick was found. All the nests were individually marked with plastic tags. On each occasion we tried to recapture all living chicks in the colony. All unmarked chicks were ringed and assigned to their natal nests. Distances between particular nests were moderately high (mean nearest neighbour distance 6.19 ± 1.44 m) and there was a considerable between-brood variation in hatching date, which enabled assignment of all chicks to their natal nests without application of nest enclosures. On the day of ringing, age of each chick was estimated on the basis of growth curves given by Paillisson et al. Citation(2008), which also allowed calculations of exact hatching dates. Because hatching of Whiskered Tern chicks within a brood is synchronous (Paillisson et al. Citation2008), we did not determine hatching positions. In total, 140 chicks from 59 broods were marked. During each visit we measured total head length of each chick with calipers to the nearest 0.1 mm. All the chicks were also weighed with electronic scale to the nearest 0.1 g. We fitted logistic curves of the form y = A/[1 + B*exp(–KT)] to the collected measurements of head length and body mass of all chicks from each brood altogether, where y refers to the body measurement at age T, A is an asymptotic value, B is a constant of integration, and K is the growth rate constant (Richner Citation1989). We could not fit the curves separately for each chick, as the number of gathered measurements was too low. This approach also allowed us to include measurements from chicks which did not survive until fledging. We used parameter K from the fitted curves as an indicator of brood growth rates. The growth curves were fitted only for the broods in which no less than five individual measurements were obtained and at least one chick was recaptured and measured after the age of 15 days, when morphological parameters of Whiskered Tern reach asymptotic values (Paillisson et al. Citation2008). These conditions were satisfied only for 25 broods, due to the high rate of chick mortality at the early stages of post-hatching development and high rate of emigration from the colony shortly before fledging. Analysed broods were randomly distributed within the colony, as there were no differences in the coordinates of broods included and excluded from the analysis (Wilks: F 2, 71 = 0.48, P = 0.62). In the large majority of analysed broods we recorded three hatchlings (n = 22). There were only three broods in which two chicks hatched.
To obtain nest-location characteristics, we mapped all the nests within the colony with a hand-held global positioning system (GPS) unit (Garmin GpsMap 60Cx, Olathe, KS, USA) with European Geostationary Navigation Overlay Service (EGNOS) ensuring accuracy of 1–1.5 m. On the basis of collected coordinates a distance matrix was constructed for all the nests in the colony. Distances between nests were used to calculate nest density, which was defined as the number of other active nests within a radius of 15 m from each particular nest. For each nest we also calculated distance to the centre of the subcolony. The centre was defined as the mean coordinates of all the nests within the subcolony. The distance between the centres of the subcolonies was 225 m. There were no differences in nest-location characteristics between both subcolonies (nest density: 6.29 ± 0.71 nests/15 m radius versus 6.63 ± 0.82 nests/15 m radius, t 42, 32 = 0.31, P = 0.75; distance to the centre of the subcolony: 31.14 ± 4.84 m versus 31.60 ±5.37 m, t 42, 32 = 0.76, P = 0.45; all values presented as means ± se), so all data were treated jointly. There was a strong negative correlation between nest density and distance to the centre of the subcolony (r = –0.75, n = 74, P < 0.001). Nest-location characteristics were calculated for all the nests in the colony, as there was no time gap between the breeding activities of the earliest and the latest pairs (i.e. all the clutches in the colony were laid before the first chicks fledged).
We used general linear models (GLMs) to test the effects of different independent variables on the chick growth rates. In order to avoid multicollinearity, separate GLMs were used to test the effects of nest density and distance to the centre of subcolony. The magnitude and directions of significant relationships were described with beta coefficients (β) of regression analysis (mean ± se). The percent of variance in chick growth explained by particular independent variables was estimated with partial eta-squared. All statistics followed Zar Citation(1996).
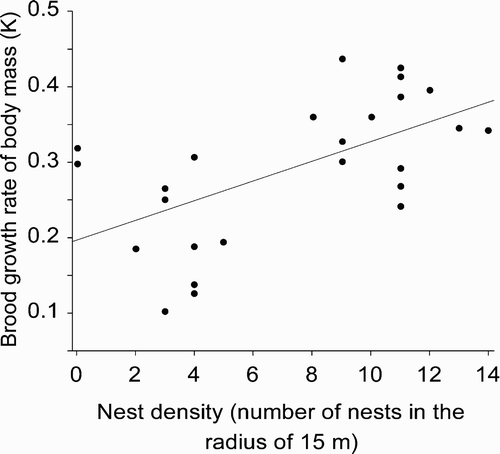
Brood growth rate of body mass did not depend on brood size (F 1, 22 = 0.60, P = 0.44), but it was significantly related to date of hatching (F 1, 23 = 4.66, P = 0.042). The relationship of growth rate with hatching date was negative, as body mass increase of chicks hatched later in the season was slower (β = –0.018 ± 0.008). We found a significant relationship of brood growth rate of body mass with distance to the centre of the subcolony (F 1, 23 = 8.51, P = 0.008; ). The relationship was negative, which indicated that chicks raised in the centres of subcolonies grew at a higher rate (β = –0.002 ± 0.001). There was also a positive relationship between brood growth rate of body mass and nest density (F 1, 23 = 12.23, P = 0.002, β = 0.013 ± 0.004; ). Distance to the centre of the subcolony and nest density explained, respectively, 27.0 % and 34.7 % of variance in growth rate of body mass. After accounting for the variation in hatching date, both relationships remained significant (distance to the centre of the subcolony: F 1, 22 = 5.61, P = 0.027; nest density: F 1, 22 = 7.79, P = 0.011).
Figure 1. Relationship between brood growth rate of body mass expressed by the parameter K of the fitted logistic curve and distance to the centre of the subcolony in the Whiskered Tern chicks at Jeziorsko reservoir.
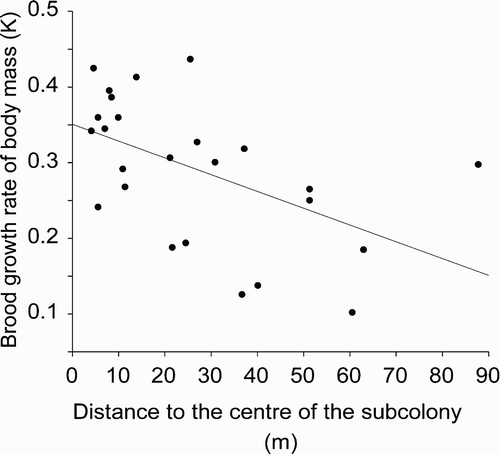
Figure 2. Relationship between brood growth rate of body mass expressed by the parameter K of the fitted logistic curve and nest density in the Whiskered Tern chicks at Jeziorsko reservoir.
Brood growth rate of head length did not depend on brood size (F 1, 22 = 0.09, P = 0.77) or hatching date (F 1, 23 = 3.90, P = 0.06). There was a significant relationship between growth rate of head length and nest density (F 1, 23 = 4.44, P = 0.046). We found that chicks from nests located in the areas of high nest density grew at a higher rate than chicks from areas of low nest densities (β = 0.004 ± 0.002). Nest density explained 16.2 % of variance in growth rate of head length. There was no significant relationship between brood growth rate of head length and distance to the centre of the subcolony (F 1, 23 = 2.50, P = 0.13).
Breeding habitat of Whiskered Tern is highly homogeneous, so little variation in the physical quality of nest sites is expected. For this reason, a central–periphery gradient of pair quality is likely to occur in this species. Breeding in the central parts of the colony is beneficial in terms of fitness, as the antipredator safety is higher due to more effective group defence in comparison to the peripheral zones of the colony (Yorio & Quintana Citation1997). Therefore, good-quality early breeding pairs tend to occupy the most attractive central sites and relegate poor-quality individuals to the less favourable edge sites. Higher fledging success of individuals breeding in higher densities or in the centres of colonies have been recorded in several larid species, including Black-headed Gulls Chroicocephalus ridibundus (Patterson Citation1965), Black-legged Kittiwakes Rissa tridactyla (Coulson Citation1968), Ring-billed Gulls Larus delawarensis (Dexheimer & Southern Citation1974), and Caspian Terns Hydroprogne caspia (Antolos et al. Citation2006). Higher survival of chicks hatched in the central parts of the colonies was demonstrated for Common Terns (Becker Citation1995) and European Herring Gulls Larus argentatus (Savoca et al. Citation2011). It was also found that central pairs of Kittiwake have higher survival rates and lifetime reproductive success in comparison to edge breeders which confirmed their higher intrinsic fitness (Aebischer & Coulson Citation1990), although the differences in quality may not be appreciable at the time of recruitment and were suggested to develop over successive breeding seasons (Coulson Citation2011).
In this study we found that Whiskered Tern chicks hatched in the central parts of the subcolonies, characterized by high nest densities, grew at a higher rate in comparison to chicks hatched in the peripheral zones, where nests where much more loosely aggregated. It is widely recognized that breeding Chlidonias terns are highly sensitive to food supply and their reproductive effort may be strongly affected by their access to food resources (Paillisson et al. Citation2007). According to life-history theory, parents should balance their investment in offspring production against their future survival and reproduction (Stearns Citation1992). As in long-lived species of birds it is the priority to invest energy in self-maintenance to prolong the potential for future reproduction, lower-quality individuals of poor physical condition should limit their investment in the present brood (Burger & Gochfeld Citation1991), even though it is likely to decrease post-fledging survival and recruitment of the offspring (Gebhardt-Henrich & Richner Citation1998). In birds not only investment but also rearing skills and foraging efficiency are known to increase with age (Curio Citation1983, Reid Citation1988). Therefore, poor-quality breeders should exhibit lower chick feeding rates resulting in slower growth of nestlings, which suggests that chick growth rates may be treated as a reliable proxy of parental quality. The effect of nest location on chick growth rates was much more strongly pronounced for body mass than for structural size expressed by head length. These results are consistent with data from other waterbird species, indicating lower susceptibility of skeletal growth to environmental factors or food delivery rates (e.g. Pinaud et al. Citation2005).
Assuming reliability of chick growth rates as predictors of parental quality, our results confirm the central–periphery distribution of pairs in the studied colony of Whiskered Terns. In our study individual quality is understood in a broad sense which includes all characteristics of parents affecting their reproductive output, i.e. age, experience and physical condition (Arnold et al. Citation2006). The central–periphery gradient of pair quality was already suggested for Whiskered Tern on the basis of clutch size analysis (Minias et al. Citation2011). Similarly to the spatial patterns of chick growth rates, the central–periphery gradient was found for separate subcolonies, rather than for the whole colony.
ACKNOWLEDGEMENTS
We thank all participants of the fieldwork, especially Emilia Lesner, Anna Piasecka and Przemysław Wylegała. We also thank two anonymous reviewers for their helpful comments on the earlier drafts of the manuscript.
REFERENCES
- Aebischer , N. J. and Coulson , J. C. 1990 . Survival of the kittiwake in relation to sex, year, breeding experience and position in the colony . J. Anim. Ecol. , 59 : 1063 – 1071 .
- Amundsen , T. , Lorentsen , S.-H. and Tveraa , T. 1996 . Effects of egg size and parental quality on early nestling growth: an experiment with the Antarctic petrel . J. Anim. Ecol. , 65 : 545 – 555 .
- Antolos , M. , Roby , D. D. , Lyons , D. E. , Anderson , S. K. and Collins , K. 2006 . Effects of nest density, location and timing of breeding success of Caspian Terns . Waterbirds , 29 : 465 – 472 .
- Arnold , J. M. , Hatch , J. J. and Nisbet , I. C.T. 2004 . Seasonal declines in the reproductive success of the common Tern Sterna hirundo: timing or parental quality . J. Avian Biol. , 35 : 33 – 45 .
- Arnold , J. M. , Hatch , J. J. and Nisbet , I. C.T. 2006 . Effects of egg size, parental quality and hatch-date on growth and survival of Common Tern Sterna hirundo chicks . Ibis , 148 : 98 – 105 .
- Becker , P. H. 1995 . Effects of coloniality on gull predation on Common Tern (Sterna hirundo) chicks . Colonial Waterbirds , 18 : 11 – 22 .
- BirdLife International . 2004 . Birds in Europe: Population Estimates, Trends and Conservation Status , BirdLife International, Cambridge .
- Burger , J. and Gochfeld , M. 1991 . Reproductive vulnerability: parental attendance around hatching in Roseate (Sterna dougallii) and Common (S. hirundo) Terns . Condor , 93 : 125 – 129 .
- Coulson , J. C. 1968 . Differences in the quality of birds nesting in the centre and on the edges of the colony . Nature , 217 : 478 – 479 .
- Coulson , J. C. 2011 . The Kittiwake , London : T & AD Poyser .
- Curio , E. 1983 . Why do young birds reproduce less well? . Ibis , 125 : 400 – 404 .
- Dexheimer , M. and Southern , W. E. 1974 . Breeding-success relative to nest location and density in Ring-billed Gull colonies . Wilson Bull , 86 : 288 – 290 .
- Erikstad , K. E. , Asheim , M. , Fauchald , P. , Dahlhaug , L. and Tveraa , T. 1997 . Adjustment of parental effort in the puffin – the roles of adult body condition and chick size . Behav. Ecol. Sociobiol. , 40 : 95 – 100 .
- Gebhardt-Henrich , S. and Richner , H. 1998 . “ Causes of growth variation and its consequences for fitness ” . In Avian Growth and Development , Edited by: Starck , J. M. and Ricklefs , R. E. 324 – 339 . Oxford : Oxford University Press .
- Golet , G. H. , Kuletz , K. J. , Roby , D. D. and Irons , D. B. 2000 . Adult prey choice affects chick growth and reproductive success in Pigeon Guillemots . Auk , 117 : 82 – 91 .
- Minias , P. , Kaczmarek , K. , Janiszewski , T. and Wojciechowski , Z. 2011 . Spatial variation in clutch size and egg size within the colony of Whiskered Terns (Chlidonias hybrida) . Wilson J. Ornithol. , 123 : 486 – 491 .
- Nisbet , I. C.T. , Spendelow , J. A. , Hatfield , J. S. , Zingo , J. M. and Gough , G. A. 1998 . Variations in growth of Roseate Tern chicks: II. Early growth as an index of parental quality . Condor , 100 : 305 – 315 .
- Paillisson , J.-M. , Reeber , S. , Carpentier , A. and Marion , L. 2007 . Reproductive parameters in relation to food supply in the Whiskered Tern (Chlidonias hybrida) . J. Ornithol. , 148 : 69 – 77 .
- Paillisson , J.-M. , Latraube , F. and Reeber , S. 2008 . Assessing growth and age of Whiskered Tern Chlidonias hybrida chicks using biometrics . Ardea , 96 : 271 – 277 .
- Patterson , I. J. 1965 . Timing and spacing of broods in the Black-headed Gull Larus ridibundus . Ibis , 107 : 433 – 459 .
- Pinaud , D. , Cherel , Y. and Weimerskirch , H. 2005 . Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in yellow-nosed albatrosses . Mar. Ecol. Prog. Ser. , 298 : 295 – 304 .
- Reid , W. V. 1988 . Age specific patterns of reproduction in the Glaucous-winged Gull: increased effort with age? . Ecology , 69 : 1454 – 1465 .
- Richner , H. 1989 . Habitat-specific growth and fitness in Carrion Crows (Corvus corone corone) . J. Anim. Ecol. , 58 : 427 – 440 .
- Savoca , M. S. , Bonter , D. N. , Zuckerberg , B. , Dickinson , J. L. and Ellis , J. C. 2011 . Nesting density is an important factor affecting chick growth and survival in the Herring Gull . Condor , 113 : 565 – 571 .
- Stearns , S. C. 1992 . The Evolution of Life Histories , Oxford : Oxford University Press .
- Stienen , E. W.M. and Brenninkmeijer , A. 2002 . Variation in growth in Sandwich Tern chicks Sterna sandvicensis and the consequences for pre- and post-fledging mortality . Ibis , 144 : 567 – 576 .
- Trivers , R. L. 1974 . Parent–offspring conflict . Am. Zool. , 14 : 249 – 264 .
- van de Pol , M. , Bakker , T. , Saaltink , D.-J. and Verhulst , S. 2006 . Rearing conditions determine offspring survival independent of egg quality: a cross-foster experiment with Oystercatchers Haematopus ostralegus . Ibis , 148 : 203 – 210 .
- Yorio , P. and Quintana , F. 1997 . Predation by Kelp Gulls Larus dominicanus at a mixed-species colony of Royal Terns Sterna maxima and Cayenne Terns Sterna eurygnatha in Patagonia . Ibis , 139 : 536 – 541 .
- Zar , J. H. 1996 . Biostatistical Analysis , London : Prentice-Hall .