Abstract
Capsule The presence of Ferruginous Pygmy Owls Glaucidium brasilianum breeding in the xerophytic forest of Caldén Prosopis caldenia in central Argentina was slightly affected by forest maturity but neither by the structure of vegetation strata at the micro-habitat scale, nor by forest composition (mosaic of forest–grassland or shrubland) or proximity of water bodies at the macro-habitat scale.
Aims To assess the habitat characteristics selected by Ferruginous Pygmy Owls during the breeding season.
Methods Random transects were performed across a portion of the Luro Natural Reserve by broadcasting Ferruginous Pygmy Owl calls. Locations along these transects were used to carry out a presence/absence habitat analysis at micro- and macro-habitat levels.
Results Ferruginous Pygmy Owls were found in the majority of sampled locations. However, their presence was not associated with the vegetation structure and composition around sampled locations. Model outputs suggested similarity among presence and absence locations at both spatial scales, though owls showed a slight preference for areas with older trees and higher edge-density values.
Conclusion The Ferruginous Pygmy Owl was more abundant than previously thought in the Caldén xerophytic forest, emphasizing the habitat's conservation importance, particularly because of the land-use changes that the La Pampa region is experiencing (e.g. forest exploitation).
All animals occupy habitats, and their occupancy reflects a choice characterized by a variety of functional relationships with expected fitness (Morris et al. Citation2011). Consequently, habitat selection is a key element to understanding species distributions, spatial arrangements of populations and species conservation (Cody Citation1984, Whittingham et al. Citation2005, Campioni et al. Citation2010). Moreover, variation in time and space of such relationships introduces the concepts of ‘scale’ and ‘hierarchy’, which are key elements in habitat selection context (Johnson Citation1980). Though primary information on species habitat selection is accessible for many species, this information is lacking for others owing to logistical, economic, or cultural reasons (Henríques et al. Citation2003, Sergio et al. Citation2006).
A good example of such gaps in our knowledge is represented by the case of the Ferruginous Pygmy Owl Glaucidium brasilianum, a cavity-nesting raptor inhabiting a variety of ecosystems across the Neotropic ecozone (Proudfoot & Johnson Citation2000). While the ecological counterpart of this species in Europe, the Pygmy Owl Glaucidium passerinum, has been well studied with regards to its behaviour and ecological role at the community level (Rodriguez et al. Citation2007; Morosinotto et al. Citation2010; Lehikoinen et al. Citation2011), only a few studies are currently available on the habitat requirements, resource use, and distribution patterns of Ferruginous Pygmy Owls (Flesch Citation2003a,Citationb, Flesch & Steidl Citation2006, 2010). Moreover, the species has declined to endangered levels in the southwest USA owing to habitat loss (Johnson et al. Citation2003); in northwest Mexico it is still common in areas where woodlands occur in association with giant columnar Saguaro cacti Carnegiea gigantean. Moving southward, information on the owl's status decreases with latitude (Proudfoot & Johnson Citation2000). Since habitat fragmentation and loss are currently the main causes of environmental modification, the lack of information on species occurrence and abundance in Central America may represent a crucial weakness for potential conservation actions. Anthropogenic alteration of natural habitats poses particular threats to species which depend on resources such as nesting cavities (Solbrig Citation1996, Solbrig & Vera Citation1996, Fearnside Citation2001, Abraham Citation2002, Politi et al. Citation2009).
In the present study we evaluated habitat selection of Ferruginous Pygmy Owls during the breeding season in semiarid forest of central Argentina. For territorial raptors such as owls, the breeding season represents one of the most crucial periods of the biological cycle. Thus, habitat selection decisions are expected to influence the resources that are available for successful breeding (Orians & Wittenberger Citation1991). We first determined the presence of Ferruginous Pygmy Owls across the xerophytic forest of Caldén Prosopis caldenia. Second, we characterized, at local and home-range scales, habitat structure and composition of sampled locations. Finally, because the species is expected to be sensitive to breeding habitat manipulation (Proudfoot & Johnson Citation2000, and references therein), we discuss our results from a conservation perspective.
METHODS
Study area
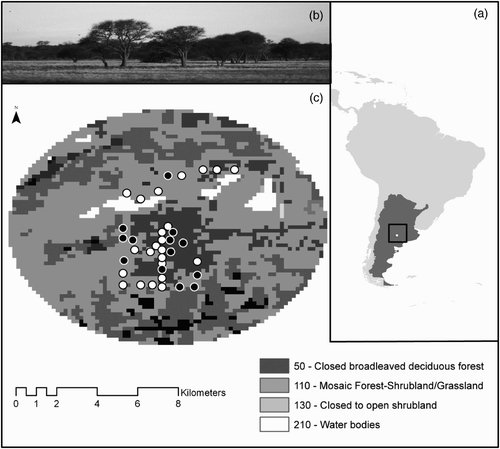
The study was carried out in the Parque Luro Natural Reserve (36°54′S, 64°15′W) located 35 km south of Santa Rosa City, in the central-east of La Pampa province, Argentina (). This 7600-ha natural reserve with a dominant landscape of thorny deciduous shrubland is placed on the phytogeographical district of the Espinal (Cabrera Citation1976) and is the only area devoted to protect Caldén forest. Topographically, the reserve is comprised of a valley which varies in altitude (up to 80 m). It has warm weather with temperatures oscillating between 35°C and –8°C (maximum summer–winter thermal amplitude) and moderate rains (600 mm yr−1) typically concentrated in spring and summer. Three main natural environments are represented in the reserve: woods with clearings covered by grassy savannahs, salt marsh and dunes with sammophilous vegetation and salty soils (Cano & Movia Citation1967; Anderson et al. Citation1970). The predominant tree species is the Caldén, forming more or less dense xerophytic forests. Other tree species found in the forest habitat are Sombra de Toro Jodina rhombifolia and Chañar Geoffoea decorticans. Piquillín Condalia microphylla, Llaollín Lycium chilense and Molle Schinus fasciculatus are the most common shrubs.
Figure 1. Maps of the study area. (a) location of Luro Natural Reserve within Argentina; (b) photo of the dominant habitat type in the reserve: the xerophytic wood of Caldén with clearings covered by grassy savannahs; (c) circular section of Luro Park enclosing the 36 sampled locations surveyed for the occurrence of Ferruginous Pygmy Owl during the breeding season 2010.
White points in (c) represent the locations where owl presence was recorded, black points represent absence; legend numbers correspond to the vegetation classification from the globcover land-cover maps of the world; vegetation represented in black (rain-fed croplands) was not surveyed.
Species data collection
Data collection was carried out in 2010 from the beginning of October until the end of December, which represents the breeding period for the species (J. H. Sarasola unpubl. data). During this period owls typically defend their territories against conspecific intrusion by performing vocal displays. We surveyed approximately 30 km of random transects along practicable tracks across a portion of the reserve trying to cover the larger types of vegetation formations that occurred within the study area. Along each transect we systematically broadcast territorial calls to elicit responses from Ferruginous Pygmy Owl along a series of calling stations spaced at least 550 m (range = 550–1000 m) apart. The minimum between-station distance corresponds to the broadcasting distance at which all responses can be assigned to different individuals (Proudfoot et al. Citation2002). However, if we detected an owl, we increased spacing of the next station up to a maximum of 1000 m to reduce the probability of detecting the same bird. A total of 36 calling stations where thus selected. At each station we followed a protocol recording vocalization and the location of owls responding to conspecific calls (Proudfoot et al. Citation2002). We first broadcast calls for two minutes, then recorded owls' responses during the next three minutes. In order to avoid bias in owl detection, we again broadcast the calls for two minutes if no owl responded to the first attempt. When no owls responded to two attempts, the point was identified as an absence location. When present, owls always approached the broadcasting station closely, becoming easily detectable. The specific location (i.e. the tree or branch) where the bird rested for the longest period while responding to the broadcasted calls was marked as the presence location. Subsequently, we performed a second survey only to the presence locations where the owls previously detected (by means of call broadcasting) were captured and banded, confirming the independence among sampled locations.
Habitat data collection at micro- and macro-habitat level
Habitat structure was characterized at each location (identified by global positioning system coordinates) at two different spatial scales. At the smaller spatial scale (hereafter ‘micro-habitat level’), we evaluated habitat complexity and structure by constructing a vertical vegetation profile of the surrounding habitat following the procedure employed by Sarasola et al. Citation(2005). We established a transect of 20 m in length in a random direction from the point and erected an 8-m vertical bar every metre (20 sampling points). The 8-m bar was marked at a constant interval of 50 cm until 4 m, then at 1-m intervals for the remaining 4 m. At each of these 20 sampling points, we counted the number of times and recorded the height at which vegetation contacted the vertical 8-m bar. We then calculated the percentage of vegetation cover by dividing the number of contacts at each height class by the total number of sampling points. Finally, for comparative purposes, we classified data into four vegetation strata: (1) grasses (height range = 0.25–1.00 m); (2) bushes (range = 1.25–2.50 m); (3) short trees or saplings (range = 2.75–3.75 m); and (4) tall and mature trees (range = 4 to > 8 m). In each location, we also recorded the tree density by counting all the trees located inside a plot of 20 × 4 m along the transect line. For each of those trees, we also measured dbh.
At the larger spatial scale (hereafter ‘macro-habitat level'), we demarcated a circular plot of radius 400 m (area = 50.4 ha) around each location. This approximately corresponds to the home-range size recorded for male Ferruginous Pygmy Owls in Mexico (home-range area ≤ 59 ha, 95% fixed kernel [Flesch & Steidl 2010]). The owls’ movement and territorial displays during calling allowed us to delineate the edges of the areas potentially used by them, allowing us to approximately locate the centre of the home-ranges. In a geographic information system software package (arcgis 9.3) we plotted and intersected our circular plots with a South America satellite image of land-cover (from globcover land-cover maps of the world using an automated processing chain from the 300-m envisat meris time series). Habitat in our sample location was classified into four habitat types corresponding to closed broadleaved deciduous forest (H1), mosaic forest–shrubland/grassland (H2), closed to open shrubland (H3), and water bodies (H4). At this spatial scale we also characterized landscape structure employing the following metrics: Shannon Diversity Index (SDI) as a proxy of habitat diversity; number of patches and edge density as a proxy of habitat heterogeneity (Donovan et al. Citation1995, Kie et al. Citation2002, Anderson et al. Citation2005) calculated by means of the patch analyst extension in arcview 3.2 (Elkie et al. Citation1999).
Statistical analysis
Habitat selection was modelled at two spatial scales employing data available for 36 presence/absence locations. At each spatial scale we performed a generalized linear model (glm) with binomial family distribution of errors where the dependent variable was the presence/absence of the species (presence = 1, absence = 0) at each location. In the first model, we analyzed habitat selection at the local scale; stand structure, dbh, and tree density represented the explanatory variables. In the second model, we analyzed habitat selection at the home-range scale; habitat composition (percentage of H1–H4 habitat types) and habitat structure (SDI, patch number and edge density) represented the explanatory variables. To reduce collinearity and the number of explanatory variables, pairs of strongly inter-correlated variables (r > 0.6) were considered as estimates of a single underlying factor. Only one of the two was retained for analysis, usually the one that we perceived as likely to be considered the more important by the study organism. Before carrying out the analysis at home-range scale, we first checked for spatial autocorrelation among habitat type percentage cover calculated for each location. To do that, we employed Moran's I test (spatial relationship = inverse; distance method = euclidean distance, Z = 1.4670, P = 0.14) (Cliff & Ord Citation1981). To select the best model, we used the aic adjusted for sample size (aicc ) as recommended by Burnham and Anderson (Citation2002). We retained the model with the lowest aicc score (i.e. the best compromise between accuracy and precision); we also provide Δaic and aic weights. In addition, we report explained deviance values, which provide the proportion of variance explained by the model. The analyses were performed in r (R Development Core Team Citation2009); the glm function in the stats package was employed for logistic regression models. Data are presented as means ± sd.
RESULTS
Ferruginous Pygmy Owls were recorded in 24 out of 36 locations (67% of sampled locations) and 20 individuals were captured in 18 of these 24 locations. Considering a circular area of 250-m radius around each broadcasting station (20 ha), and assuming that each of the locations where owls were recorded as present was occupied by a breeding pair (i.e. 24 territories or breeding pairs), the estimated density of owls for the surveyed area (36 points = 720 ha) was approximately 0.06 owls ha−1 (site density = 48 owls/720 ha). Such a density is similar to that recorded in other areas as in northern Mexico (Flesch & Steidl Citation2006).
Habitat structure profiles were similar in locations where owls were present and absent (; ). However, based on the mean vegetation profiles ( it is likely that there was a difference between habitats used and not used by owls in the higher vegetation stratum of between 4 and 7 m, corresponding to the tall and mature tree category. Locations where owls were found showed up to 80% of vegetation reaching 6 m in height (range: presence location = 0–80%; absence location = 0–55%). This result suggests that the top vegetation stratum may represent a substantial element of Ferruginous Pygmy Owl habitat in our study area. Moreover, model selection showed a moderate uncertainty about the most plausible model at micro-habitat level, with the dbh and the null model among the most supported models (). Owls seemed to positively select mature vegetation (higher dbh values) and negatively select habitat characterized by dense ground vegetation, bushes and small trees (see ).
Table 1. Habitat composition and structure profile of the presence (n = 24) and absence (n = 12) locations where Ferruginous Pygmy Owls were surveyed within the Luro Natural Reserve, Argentina.
Table 2. Summary of fitted parameters employed in model formulation to analyse habitat selection of Ferruginous Pygmy Owl at micro- and macro-habitat spatial levels. Selected models (Δaicc ≤ 2) with the relative (β ± se), aicc , Δaicc and aiccw values, percentage of explained deviance, are given.
Figure 2. Comparison between locations (n = 36) where Ferruginous Pygmy Owls were recorded as present (n = 24) or absent (n = 12) in Luro Natural Reserve, Argentina. (a) Mean habitat structure at local scale; (b) habitat composition comparison at home-range scale between absence and presence locations.
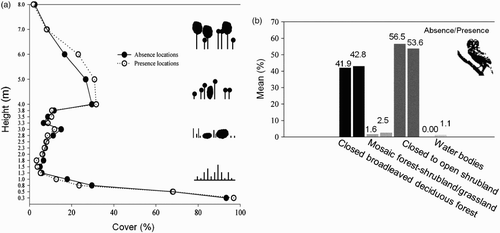
At the macro-habitat level, stations with and without owls were also characterized by similar percentages of vegetation types (; ). Indeed, model selection again, did not show a clear relationship between variables measured at this larger scale and Pygmy Owl presence (; ). However, there was a trend for owls to prefer heterogeneous habitat (higher values of edge density), which may provide owls with different types of resources.
DISCUSSION
The present study showed a high density of Ferruginous Pygmy Owls in the xerophytic forest of Caldén. We found no significant differences in habitat structure among sampled locations where pygmy owls were present and absent (). Two hypotheses may explain this apparent homogeneity. First, homogeneity might be because of restricted forest-area sampling based on a human point of view which does not always coincide with animal perceptual range. Second, homogeneity might also indicate a continuum of optimal habitat conditions across the xerophytic forest. If this is the case, locations without pygmy owls should represent points enclosed in owls' home-ranges and for that reason not necessarily defended by them during the playback sessions. Some evidence supports this latter explanation: (1) similarity in tree density and dbh values between presence and absence locations could indicate a continuum of optimal habitat conditions across the xerophytic forest; (2) the abundance of woodpeckers (avian excavators) in the reserve may also suggest that nesting cavities – a critical resource for secondary cavity-nesting species (Bonar Citation2000, Proudfoot & Johnson Citation2000) – are widely available in the study area (Cockle et al. Citation2008). The availability of nest locations across the study area was also corroborated by our observation that Ferruginous Pygmy Owls nested successfully just 5 times inside the 50 artificial nest boxes spread through the study area (J. H. Sarasola unpubl. data). Last but not least, we could not exclude food availability as a potential driver of Ferruginous Pygmy Owl spatial distribution, since it has frequently been recognised as a good predictor of the presence of territorial raptors (Newton Citation1979).
However, our results suggest a slight preference for habitat characterized by mature vegetation which may provide pygmy owls with suitable nesting cavities. In addition, older trees, which are also taller, may facilitate territorial tasks (e.g. territorial defence, and communication between pairs and neighbours [Flesch 2003b, Flesch and Steidl 2006]). Similarly, owls seem to use: (1) less dense forest areas, possibly to facilitate movement between nest- and feeding-sites; and (2) at the macro-habitat scale, heterogeneous habitat which may provide owls with different types of resources (e.g. cover, food, and perch- and nest-sites). These results corroborate with findings shown by previous habitat studies carried out in Arizona and Mexico, though in different ecosystems (Flesch 2003a, Flesch & Steidl Citation2006, Citation2010). Unfortunately, our reduced sample size is likely limiting the robustness of the habitat analysis, bringing uncertainty about the obtained results.
Though the relationship between Ferruginous Pygmy Owls and habitat selection was not completely clarified, it is worthwhile to discuss another aspect of our results. We believe that the relatively high abundance of owls in the xerophytic ecosystem (67% of our sampled locations) should be further investigated owing to the potential role of top predators as reliable indicators of biodiversity values. In fact, solid evidence exists of the association between apex predators that belong to higher trophic levels and habitat complexity, community richness and biodiversity levels (Sergio et al. Citation2004; Sergio et al. Citation2005, Sergio et al. Citation2006). Specifically, this latter relationship has been successfully tested for Glaucidium passerinum, the European counterpart of the Ferruginous Pygmy Owl, providing clear evidence of general patterns of association between charismatic top predators and biodiversity (Sergio et al. Citation2006). We suggest that the Ferruginous Pygmy Owl should be as valuable as its European ecological counterpart as a potential biodiversity indicator, but in a different ecological context. Two important ecological characteristics – the wide range of ecosystems it occupies (i.e. semiarid desert scrub, tropical, subtropical and cold temperate lowlands) and the diversity of its opportunistic diet (i.e. insects, reptiles, birds, amphibians, small mammals [Proudfoot & Beason Citation1997, Carrera et al. Citation2008]) – would seem to make the species a suitable candidate indicator of biodiversity, being potentially associated with richer communities in various ecosystems (Steenhof & Kochert Citation1988, Sergio et al. Citation2006).
As previously stated, Ferruginous Pygmy Owls declined to endangered levels in the southwestern USA owing to habitat loss and breeding habitat manipulation (Friedman et al. 1950, Fall Citation1973, Tewes Citation1995, Johnson et al. Citation2003). Such adverse circumstances at the northern boundary of the species' range may have followed the pattern described by Melles et al. Citation(2011), suggesting anthropogenic habitat loss as one of the contributory causes to the reduction in population size at range boundaries. Since Argentina represents the southern boundary of Ferruginous Pygmy Owl distribution and since no information is currently available on local and regional population status, concerns about its susceptibility to similar human-induced perturbations are fully justified. Specifically, the Pampas and Expinal ecoregions are subject to land-use changes where pastures, natural grasslands and forests historically used for cattle grazing have been converted to row crop production (Paruelo et al. Citation2005, Baldi & Paruelo Citation2008, Gavier-pizarroa et al. Citation2012). Moreover, domestic and international wood demand in Argentina has been increasing steadily and this has significantly increased the extent of forest lands under exploitation (Pacheco & Brown Citation2006). Recent studies in the Espinal region have observed that avian species richness decreased with decreasing size of woodland patches (Bucher et al. Citation2001 ). For both reasons, it is of extreme importance to conserve wooded ecosystems, such as xerophytic forest, which still persists despite anthropogenic pressure.
ACKNOWLEDGEMENTS
We are very grateful to V. Penteriani for helpful comments. The first draft of the manuscript has been greatly improved thanks to the comments of Will Cresswell and an anonymous referee. We appreciate the improvements in English usage made by Christina Riehl through the Association of Field Ornithologists' programme of editorial assistance. We are grateful to S. Liebana and C. Solaro for their help in the collection of field data and also to P. Lucas for his help with GIS analysis. During this work L.C. was supported by a doctoral grant (JAE pre-doc from the CSIC, SPAIN).
REFERENCES
- Abraham , E. M. 2002 . “ Lucha contra la desertificación en las tierras secas de Argentina. El caso de Mendoza ” . In El agua en Iberoamérica. De la escasez a la desertificación , Edited by: Cirelli , A. F. and Abraham , E. M. 23 – 35 . Buenos Aires : Cyted .
- Anderson , D. L. , Del Águila , J. A. and Bernardón , A. E. 1970 . Las formaciones vegetales de la Provincia de San Luis . Rev. Inv. Agropecuaria INTA, Serie 2, Biol. Produc. Vegetal , 7 : 83 – 153 .
- Anderson , D. P. , Forester , J. D. , Turner , M. G. , Frair , J. L. , Merrill , E. H. , Fortin , D. , Mao , J. S. and Boyce , M. S. 2005 . Factors influencing female home range sizes in elk (Cervus elaphus) in North American landscapes . Landsc. Ecol. , 20 : 257 – 271 . (doi:10.1007/s10980-005-0062-8)
- Baldi , G. and Paruelo , J. M. 2008 . Land-use and land cover dynamics in South American temperate grasslands . Ecol. Soc. , 13. http://www.ecologyandsociety.org/vol13/iss2/art6/
- Bonar , L. R. 2000 . Availability of pileated woodpecker cavities and use by other species . Wildl. Manage. , 64 : 52 – 59 . (doi:10.2307/3802974)
- Bucher, E.H., Costa Gorriz, B., Leynaud, G.C., 2001. Bird diversity and forest fragmentation in the semiarid espinal woodland of Cordoba, Argentina. Boletín de la Academia Nacional de Ciencias 66, 117–124.
- Burnham , K. P. 2002 . “ & Anderson D.R ” . In Model Selection and Multi-model Inference: A Practical Information-Theoretic Approach , 2 , New York : Springer-Verlag .
- Cabrera , A. L. 1976 . “ Regiones fitogeográficas argentinas ” . In Enciclopedia Argentina de jardinería, Vol. 2, Issue 1: 85 , Edited by: Kugler , W. F. Argentina : Acme Saci Buenos Aires .
- Campioni , L. , Delgado , M. M. and Penteriani , V. 2010 . Social status influences microhabitat selection: breeder and floater Eagle Owls Bubo bubo use different post sites . Ibis , 152 : 569 – 579 . (doi:10.1111/j.1474-919X.2010.01030.x)
- Cano , E. and Movia , C. 1967 . Utilidad de la fotointerpretación en la cartografía de comunidades vegetales del bosque de caldén (Prosopis caldenia) . Veg. Rep. Arg. , 8 : 1 – 44 .
- Carrera , J. D. , Fernandez , F. J. , Kacoliris , F. P. , Pagano , L. and Berkunsky , I. 2008 . Field notes on the breeding biology and diet of Ferruginous Pygmy-Owl (Glaucidium brasilianum) in the dry Chaco of Argentina . Ornitol Neotrop. , 19 : 315 – 319 .
- Cliff , A. D. and Ord , K. J. 1981 . Spatial Processes, Models and Applications , London : Pion .
- Cockle , K. , Martin , K. and Wiebe , K. 2008 . Availability of cavities for nesting birds in the atlantic forest, Argentina . Ornitol Neotrop. , 19 : 269 – 278 .
- Cody , M. L. 1984 . Habitat Selection in Birds , Orlando, FL : Academic Press .
- Donovan , T. M. , Thompson , F. R. , Faaborg , J. and Probst , J. R. 1995 . Reproductive success of migratory birds in habitat sources and sinks . Conserv. Biol. , 9 : 1380 – 1395 . (doi:10.1046/j.1523-1739.1995.09061380.x)
- Elkie , P. , Rempel , R. and Carr , A. 1999 . Patch Analyst User's Manual: A Tool for Quantifying Landscape Structure , Thunder Bay, ON : Ontario Ministry of Natural Resources, Northwest Science & Technology . http://www.cnfer.on.ca/SEP/patchanalyst/overview.htm
- Fall , B. A. 1973 . Noteworthy bird records from south Texas (Kenedy County) . Southwest Nat. , 18 : 244 – 247 . (doi:10.2307/3670425)
- Fearnside , P. M. 2001 . “ South American natural ecosystems, status of ” . In Encyclopedia of Biodiversity , Edited by: Levin , S. A. Vol. 5 , 345 – 359 . San Diego, CA : Academic Press .
- Flesch, A.D. 2003a. Ditribution, abundance, and habitat of cactus ferruginous pygmy-owl in Sonora, Mexico. MS Thesis, University of Arizona.
- Flesch , A. D. 2003b . Perch-site selection and spatial use by Cactus Ferruginous Pygmy-Owl in south-central Arizona . J. Rap. Res. , 37 : 151 – 157 .
- Flesch , A. D. and Steidl , R. J. 2006 . Population trends and implications for monitoring cactus ferruginous pygmy owls in northern Mexico . J. Wild. Manage. , 70 : 867 – 871 . (doi:10.2193/0022-541X(2006)70[867:PTAIFM]2.0.CO;2)
- Flesch , A. D. and Steidl , R. J. 2010 . Importance of environmental and spatial gradients on patterns and consequences of resource selection . Ecol. Appl. , 20 : 1021 – 1039 . (doi:10.1890/09-0076.1)
- Friedmann , H. L. , Griscom , L. and Moore , R. T. 1950 . Distributional check-list of the birds of Mexico. Part 1 . Pacific Coast Avifauna , 29 : 1 – 202 .
- Gavier-Pizarro , G. I. , Calamari , N. C. , Thompson , J. J. , Canavelli , S. B. , Solari , L. M. , Decarre , J. , Goijman , A. P. , Suarez , R. P. , Bernardos , J. N. and Zaccagnini , M. E. 2012 . Expansion and intensification of row crop agriculture in the Pampas and Espinal of Argentina can reduce ecosystem service provision by changing avian density . Agric. Ecos. Envir. , 154 : 44 – 55 . (doi:10.1016/j.agee.2011.08.013)
- Henríques , M. P.L. , Wunderle , J. M. JR and Willig , M. R. 2003 . Birds of the Tapajos National Forest, Brazilian Amazon: a preliminary assessment . Ornitol. Neotrop. , 14 : 307 – 338 .
- Johnson, D.H. 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61: 65–71.
- Johnson , R. R. , Cartron , J. L.E , Haight , L. T. , Duncan , R. B. and KIngsley , K. J. 2003 . Cactus ferruginous pygmy-owl in Arizona, 1872–1971 . Southwest. Nat. , 48 : 389 – 401 . (doi:10.1894/0038-4909(2003)048<0389:CFPIA>2.0.CO;2)
- Kie , J. G. , Bowyer , R. T. , Nicholson , M. C. , Boroski , B. B. and Loft , E. R. 2002 . Landscape heterogeneity at differing scales: effects on spatial distribution of mule deer . Ecology , 83 : 530 – 544 . (doi:10.1890/0012-9658(2002)083[0530:LHADSE]2.0.CO;2)
- Lehikoinen , A. , Ranta , E. , Pietiäinen , H. , Byholm , P. , Saurola , P. , Valkama , J. , Huitu , Henttonen , H. and Korpimäki , E. 2011 . The impact of climate and cyclic food abundance on the timing of breeding and brood size in four boreal owl species . Oecologia , 165 : 349 – 355 . (doi:10.1007/s00442-010-1730-1)
- Melles , S. J. , Fortin , M. J. , Lindsay , K. and Badzinski , D. 2011 . Expanding northward: influence of climate change, forest connectivity, and population processes on a threatened species' range shift . Global Change Biol. , 17 : 17 – 31 . (doi:10.1111/j.1365-2486.2010.02214.x)
- Morosinotto , C. , Thomson , R. L. and Korpimaki , E. 2010 . Habitat selection as an antipredator behaviour in a multi-predator landscape: all enemies are not equal . J. Anim. Ecol. , 79 : 327 – 333 . (doi:10.1111/j.1365-2656.2009.01638.x)
- Morris , D. W. , Moore , D. E. , Ale , S. B. and Dupuch , A. 2011 . Forecasting ecological and evolutionary strategies to global change: an example from habitat selection by lemmings . Global Change Biol. , 17 : 1266 – 1276 . (doi:10.1111/j.1365-2486.2010.02305.x)
- Newton , I. 1979 . Population Ecology of Raptors , London : Poyser .
- Orians , G. H. and Wittenberger , J. F. 1991 . Spatial and temporal scales in habitat selection . Am.Nat. , 137 : S29 – S49 . (doi:10.1086/285138)
- Pacheco , S. and Brown , A. D. 2006 . Ecología y producción de los Cedros (género Cedrela) en las Yungas de Argentina , Tucuman, Argentina : Subtropico .
- Paruelo , J. M. , Guerschman , J. P. and Veron , S. 2005 . Expansión agrícola y cambios en el uso del suelo . Ciencia Hoy , 15 : 14 – 23 .
- Politi , N. , Hunter , M. JR. and Rivera , L. 2009 . Nest selection by cavity-nesting birds in subtropical montane forests of the Andes: implications for sustainable forest management . Biotropica , 41 : 354 – 360 . (doi:10.1111/j.1744-7429.2008.00481.x)
- Proudfoot , G. A. and Beason , S. L. 1997 . Food habits of nesting ferruginous pygmy-owls in southern Texas . Wilson Bull. , 109 : 741 – 748 .
- Proudfoot , G. A. and Johnson , R. R. 2000 . “ Ferruginous pygmy-owl (Glaucidium brasilianum) ” . In The Birds of North America Online: Article 498 , Edited by: Poole , A. Ithica, NY : Cornell Lab of Ornithology . http://bna.birds.cornell.edu/bna/species/498doi:10.2173/bna.498
- Proudfoot , G. A.S , Beason , S. L. , Chavez-Ramirez , F. and Mays , J. L. 2002 . Responce distance of Ferruginous Pygmy-Owls to broadcasted conspecific calls . J. Rap. Res. , 36 : 170 – 175 .
- R Development Core Team 2009. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rodriguez , A. , Gunnar , J. and Henrik , A. 2007 . Composition of an avian guild in spatially structured habitats supports a competition–colonization trade-off . Proc. R. Soc. Biol. Sc. B , 274 : 1403 – 1411 . (doi:10.1098/rspb.2007.0104)
- Sarasola , J. H , Bragagnolo , L. A. and Sosa , R. A. 2005 . Changes in woody plant structure in fire-disturbed caldén forest of the Parque Luro Reserve, Argentina . Natural Areas J. , 25 : 374 – 380 .
- Sergio , F. , Marchesi , L. and Pedrini , P. 2004 . Integrating individual habitat choices and regional distribution of a biodiversity indicator and top predator . J. Biogeogr. , 31 : 619 – 628 . (doi:10.1046/j.1365-2699.2003.01002.x)
- Sergio , F. , Newton , I. and Marchesi , L. 2005 . Top predators and biodiversity . Nature , 436: 192
- Sergio , F , Newton , I , Marchesi , L. and Pedrini , P. 2006 . Ecologically justified charisma: preservation of top predators delivers biodiversity conservation . J. Appl. Ecol. , 43 : 1049 – 1055 . (doi:10.1111/j.1365-2664.2006.01218.x)
- Solbrig , O. T. 1996 . Towards a Sustainable Pampa Agriculture: Past Performance and Prospective Analysis , DRCLAS Working Paper on Latin America No. 96. David Rockefeller Center for Latin American Studies, Cambridge, MA
- Solbrig, O.T. and Vera, R.R. 1996. Impacto de la globalización en las llanuras del cono sur. In O.T. Solbrig (ed), III Foro del Ajusco, Globalización Económica y Desarrollo Sostenible en América Latina y el Caribe; Mesa: Impactos, Indicadores y Alternativas. UNEP and Colégio de México, 4-6 Septiembre 1996. México.
- Steenhof , K. and Kochert , M. N. 1988 . Dietary responses of three raptor species to changing prey densities in a natural environment . J. Anim. Ecol. , 57 : 37 – 48 . (doi:10.2307/4761)
- Tewes, M.E. 1995. Status of the ferruginous pygmy-owl in southern Texas and northeast Mexico. Project Report 2, Job 25. Texas Parks and Wildlife Department & Texas A&M University Kingsville, Kingsville, TX.
- Whittingham , M. J. , Swetnam , R. D. , Wilson , J. D. , Chamberlain , D. E. and Freckleton , R. P. 2005 . Habitat selection by yellowhammers Emberiza citrinella on lowland farmland at two spatial scales: implications for conservation management . J. Appl. Ecol. , 42 : 270 – 280 . (doi:10.1111/j.1365-2664.2005.01007.x)