Abstract
Capsule The total area encapsulated by the hunting ranges of breeding Short-eared Owls Asio flammeus was broadly similar between day and night and also through the season, but activity was concentrated more on lower altitude graminoid-dominated areas during daylight and early in the breeding season.
Aims To assess the representativeness of diurnal observations alone for determining ranging behaviour and habitat-use of breeding Short-eared Owls.
Methods The diurnal and nocturnal ranging behaviours of four radiotagged Short-eared Owls were compared. Further diurnal field observations were used to assess the representativeness of the behaviour of the radiotagged birds.
Results Short-eared Owls tended to hunt more over higher ground (where there was a greater ericaceous component within the moorland vegetation) at night and also later in the breeding season; the area of the total hunting range remained similar through the season (mean about 200 ha). Nocturnal activity rates closely matched those during peak diurnal activity periods.
Conclusion Diurnal observations of breeding Short-eared Owls could provide representative data to inform breeding population densities and distribution but they may not be representative of overall habitat-use or ranging behaviour within home-ranges. Assessments of the risks to breeding Short-eared Owls of developments on moorland (e.g. forestry, wind turbine construction, grazing changes) based on diurnal observations alone may not reflect the true scale of potential impacts, which would also need to consider nocturnal behaviour.
As a species, the Short-eared Owl Asio flammeus lies towards the irruptive end of the continuum of migration strategies (Newton Citation2006), with an ability to shift its distribution and establish breeding territories within suitable habitats wherever there is a sufficient abundance of small mammal prey, particularly voles (Village Citation1987, Korpimäki & Norrdahl Citation1991, Korpimäki Citation1994, Poulin et al. Citation2001). Accordingly, breeding densities and territory sizes can vary, also dependent on local prey abundance (Mikkola Citation1983, Village Citation1987, Korpimäki & Norrdahl Citation1991, Arroyo & Bretagnolle Citation1999, Poulin et al. Citation2001). Most assessments of home-range size (and breeding density) are made on visual observations of owls, because they do not habitually call to define or defend breeding territories. However, activity by Short-eared Owls during daylight can be quite limited (Swengel & Swengel Citation2009, Calladine et al. Citation2010) and, therefore, the extent to which those daytime visual observations are representative should be questioned.
Despite an extensive distribution across the Holarctic, Neotropics and Pacific (del Hoyo et al. Citation1999), the conservation status of the Short-eared Owl gives cause for concern, or is at best uncertain, across much of its range (BirdLife International Citation2004, Burfield Citation2008, Rich et al. Citation2004). Short-eared Owls breeding in Britain generally migrate shorter distances than those from continental and northern Europe (Calladine et al. Citation2012). Accordingly, areas such as Britain arguably offer more tractable opportunities to contribute towards Short-eared Owl conservation. Most British breeding Short-eared Owls are associated with moorland, here defined as semi-natural vegetation (dominated by graminoid and/or ericaceous vegetation) that generally occurs on acidic and peaty soils. Such plagioclimax habitat typically occurs in upland and exposed areas between the upper limits of enclosed farmland and, where the altitudinal range permits, the alpine zone. Among factors that continue to affect moorland bird communities are changes in regimes of grazing, burning and drainage, and developments such as afforestation and wind turbine construction (Stroud et al. Citation1987, Petty & Avery Citation1990, Fuller & Gough Citation1999, Pearce-Higgins et al. Citation2012).
Knowledge of the ranging behaviour and habitat-use of Short-eared Owls through the breeding season and how they may differ between day and night will aid the interpretation of survey and monitoring data. This can then inform conservation management to maintain or enhance populations, including reduction of impacts from developments. Here we present the findings of a radiotracking study that assessed the ranging behaviour and habitat-use of four Short-eared Owls during the breeding season in Scotland and how these varied through the season and between day and night. We compare the radiotelemetry data with daytime visual observations to assess the representativeness of the latter and also consider habitat-use in relation to seasonal vegetation growth and potential changes in grazing regimes.
METHODS
Radiotelemetry
Radio-transmitters (5 g with twin antennae, glue-mounted via a gauze base onto trimmed back feathers) were attached to four Short-eared Owls (caught in mist nets) on moorland in Perthshire, Scotland (study area centred on 56°30ʹ N, 4°50ʹ W) in May and June 2011. Two breeding males were tagged in early May, the first within a week of its first egg hatching (followed for 54 days) and the second about 12 days before its first egg hatched (followed for 48 days). One breeding female was tagged in mid-June when its young were 3–11 days old (followed for 37 days) and one fledged juvenile (probably fledged for about 2 weeks when caught) was also tagged in mid-June (followed for 35 days).
The movements of the radiotagged Short-eared Owls were followed using hand-held receivers and three-element Yagi antennae. Field protocols aimed to assess ranging behaviour and habitat-use and how they varied through the breeding season and also between day and night. Surveillance of the radiotagged birds was undertaken entirely by volunteers (as was all fieldwork) with limited time and resources and as the principal aims included comparison of diurnal and nocturnal range sizes and habitat-use, priority within diurnal monitoring was given to early mornings and evenings when activity was expected to be greatest (Calladine et al. Citation2010). During daylight, most locations of owls determined from radio signals were confirmed by visual observation, facilitated by the generally open terrain of the study area. Based on this experience, we have a high level of confidence in the accuracy of radio locations determined at night, when visual confirmation was not possible.
For all birds, nearly all hunting was undertaken away from the nest-sites. When birds were close to the nest-site, they were either brooding young (in the case of the female) or otherwise resting on the ground nearby. Therefore, in analyses of habitat-use we have excluded radio locations within 200 m of the nests and, in addition, we consider only locations that are separated by at least 30 minutes, to minimize risks of using auto correlated data; birds were always actively flying or, if settled, remained so for less than 30 minutes when away from the vicinity of nests. For the assessment of overall home-range size, we have included all locations, including those within 200 m of the nest.
For each radio location, the following descriptive variables were determined:
| 1. | Habitat gradient position. Within our study areas, altitude can be considered a readily measurable proxy for both vegetation type and structure with the heather Calluna vulgaris component of moorland vegetation increasing with altitude. Areas most frequently occupied by breeding Short-eared owls in Britain are within mosaics of rough grassland with heather moors (including marginal hill ground and ‘white moor’) but generally avoiding areas with high levels of grazing and the more extensive areas dominated by heather (McGarry Citation1998, Raw Citation2000, Stott Citation2002, Craib Citation2011). Typically, such conditions occur at the interface between ‘white moor’ and more heather-dominated areas on higher ground. The altitude of such favoured areas varies with geology, aspect and local land-use. Given the undulating terrain of our study area and the differing altitudes between the home-ranges that were under surveillance (nests were at 290, 350, 400 and 530 m asl; Figs & ), we have used altitude (the central altitude of 50-m divisions (above mean sea level) determined from contours on published Ordnance Survey maps), standardized between different home-ranges by where they were relative to the altitude of their nest-site, as a proxy to position on that habitat gradient. Note that there were not the resources available for a realistic survey of vegetation types at an appropriate resolution given the heterogeneous mosaics of ericaceous and graminoid patches within each of the bird's home-ranges. | ||||
| 2. | Daylight. Classed as either ‘light’ or ‘dark’. Although assessed on each day of observation, ‘dark’ generally started about 45 minutes after sunset and ended 45 minutes before sunrise; thus twilight was included within the ‘light’ period. | ||||
| 3. | Habitat. For purposes reported here, this was defined as one of two simplified classes: (1) Scrub – sparse young broad-leaved shrubs, either natural or planted, at the time of the study such areas being mostly open, dominated by graminoids and being the only ungrazed areas; and (2) Other – the mosaic of graminoid and ericaceous dwarf shrub-dominated areas grazed by sheep. | ||||
| 4. | Seasonal period. Classed as either ‘early’ or ‘late’. ‘Early’ refers to the period when tagged owls were under surveillance up to and including 15 June (roughly the mid-point) and ‘late’ to the period after that. A principal difference between the early and late periods would have been in the development of ground vegetation resulting from annual summer growth, it generally being taller (especially the graminoids) during the late period. | ||||
Figure 1. The kernels of activity by radiotagged male Short-eared Owls in Scotland during daylight and at night in the 2011 breeding season.
Darker shading shows the areas of core activity of most frequent use (30% kernel density), intermediate shading (60% kernel activity) and lighter shading (90% kernel activity) show the active area of use; the dotted contours show 300 m asl, the dashed contours show 400 m asl and the solid lines 500 m asl; the locations of nest-sites are indicated by star symbols.
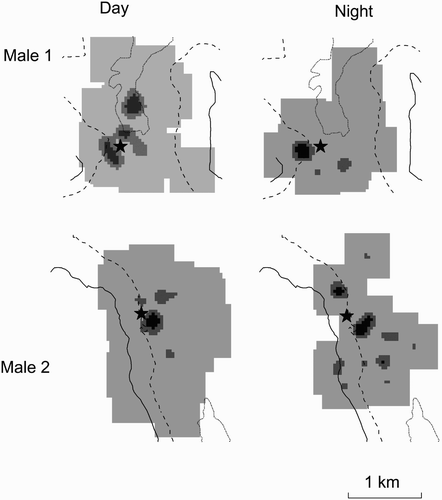
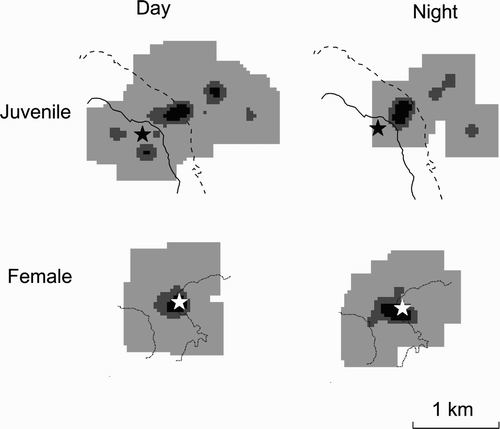
Figure 2. The kernels of activity by radiotagged female (top) and juvenile (bottom) Short-eared Owls in Scotland during daylight and at night in the 2011 breeding season.
Darker shading shows the core areas of most frequent use (30% kernel density), intermediate shading (60% kernel activity) and lighter shading (90% kernel activity) show the active area of use; the dotted contours show 300 m asl, the dashed contours show 400 m asl and the solid lines show 500 m asl; the locations of nest-sites are indicated by star symbols.
Analyses involved
Diurnal and nocturnal activity. Differences in the proportion of time spent hunting were assessed for each bird using contingency tables of recorded behaviour (the frequency of 30-minute samples when hunting against the number when not) during diurnal and nocturnal periods. Likelihood-ratio chi-squared tests (G-tests) were used to assess the statistical significance of any differences for each bird, then using Fisher's method to combine those probabilities. Note that hunting activity at night was assumed when the radio signal showed that the tagged bird was moving.
Ranging extent . Ranging areas used by the individual owls, for both daylight classes and the combined total, were estimated using mean convex polygons (MCPs) drawn around plotted locations of radiotagged owls. These were compared (using t-tests) against comparable measures of home-range size derived from visual observation in other years and in other study areas (see later). For illustrative purposes, the distribution of hunting fixes were derived from fixed kernel analysis performed in ArcGIS with geospatial modelling tools (Beyer Citation2004) using a cell size of 50 m. Maps with kernel density contours of 30% (representing core areas of most frequent use), 60% and 90% (active areas of use) were produced ( & 2).
Hunting position within a habitat gradient . Generalized linear mixed models (glmms) were used to assess the influences on the habitat gradient position (nest altitude minus radio location altitude to standardize for variations in the altitude of suitable habitat, the dependent variable) of: (1) daylight (n = 2 classes); (2) seasonal period (n = 2 classes); and (3) their interaction term (the fixed independent variables). Bird (n = 4) was included in the model as a random repeated variable and the models used a normal distribution and identity link function.
Use of ungrazed areas . Within the study area habitat classes were confounded with altitude (e.g. graminoid-dominated and scrub areas tended to be at lower elevations than areas with a greater component of ericaceous dwarf shrubs). Habitat classes were, therefore, excluded from the aforementioned glmm. Instead, a compositional analysis (Aebischer et al. Citation1993) was used to assess the proportionate use of ungrazed ground relative to its availability within the home-ranges of the tagged owls. Ungrazed areas, although characterized by the presence of sparse scrub (planted and naturally regenerating Betula, Salix and Crataegus species) were, at the time of the study, dominated by graminoids, the scrub cover being patchy and comprising less than 10% of the ungrazed areas. Assessment of habitat-use was restricted to the ungrazed (sparse scrub) areas, not only because of the restrictions associated with the small sample size of radiotagged birds but also because it represented an extreme of the gradient of grazing pressure (total exclusion) within our study area. It was also readily delineated as opposed to the mosaic of graminoid- and ericaceous-dominated patches that dominated the area. The difference between the log-transformed ratios (proportion of scrub/proportion of other habitats) between used habitats (those of independent radio locations when not resting) and available habitats (within the home-range MCP) were calculated for each bird during periods of darkness and daylight and also during the early and late parts of the season. For diurnal or seasonal categories where ungrazed areas (scrub) were not used, the proportion was replaced with a very low value (0.00001) to permit calculations. Paired t-tests were used to assess whether the log-ratio differences differed significantly between (1) the diurnal and nocturnal periods; and (2) the early and late seasonal periods.
Data from all four radiotagged birds were combined for the aforementioned analyses. Although the sample was heterogeneous (two males, a female and a fledged but independent juvenile), combining age and sex classes gave greater power to identify foraging preferences along the moorland habitat gradient, and how that can vary during the breeding season.
Visual observations
Systematic visual observations of breeding Short-eared Owls were made in 2006 (eight territories) and 2007 (ten territories) from ten vantage points within three study areas including where birds were radiotagged in 2011 and two other areas, one centred about 100 km to the southeast and the other about 120 km to the south-southwest (for details of protocols see Calladine et al. [2010]). Observed flight lines were plotted onto large-scale detailed topographic maps. Home-range sizes were estimated from MCPs encompassing the observed flight lines. A t-test was used to compare the home-range size estimates derived from the two methods (radiotracking in 2011 and visual observations in 2006–07).
For each visual flight observation, the following descriptive variables were determined:
| 1. | Habitat gradient position. The central altitude of 50-m divisions (above mean sea level) determined from contours on published Ordnance Survey maps in which the centroid of the plotted flight line occurred. As for the radiotracking data, altitudes were standardized between different territories relative to the altitude of their nest-site so that standardized altitude provided a proxy for the habitat gradient owing to altitude. | ||||
| 2. | Seasonal period. Two classes referring to date within the season, ‘early’ referring to up to and including the 15 June and ‘late’ referring to after that date (as for the radiotracking data). | ||||
A glmm was used to assess the influence on standardized hunting altitude (nest altitude minus central observation altitude, the dependent variable) of seasonal period (n = 2 classes) (the fixed independent variable). Individual home-range (n = 18) nested within study area (n = 3) was included in the model as a random repeated variable and the models used a normal distribution and identity link function.
RESULTS
Diurnal and nocturnal activity
Radiotagged Short-eared Owls were active throughout periods of both daylight and darkness with no detectable difference in the levels of activity within the periods that were sampled (Fisher's combined probability χ2 8 = 5.08, P = 0.80; ). The areas of active use (as indicated by the 60% and 90% kernel densities of radio locations when hunting) remained similar between day and night. However, there were some differences in the core areas of activity as indicated by the 30% kernel densities of radio locations when hunting ( & 2).
Table 1. The proportion of time in which radiotagged Short-eared Owls were recorded actively hunting through the 2011 breeding season during periods of darkness and of daylight.
Home-range sizes
Home-range sizes of four individual radiotagged breeding Short-eared Owls, estimated from the MCPs encompassing their recorded locations ranged from 69 to 311 ha (mean = 201 ± 53 se), with the recorded ranging area (). Apparently smaller nocturnal ranges for two of the tagged individuals (a male and the fledged juvenile) compared with their diurnal home-ranges could potentially be an artifact of their being derived from a lesser number of radio locations (). The home-range size of Short-eared Owls in the breeding season estimated from the MCPs encompassing visual observations of 18 territories in 2006–07 ranged from 35 to 554 ha (mean = 190 ± 34 se; ), much the same as that determined from radiotelemetry (t 20 = 0.15, P = 0.89).
Table 2. The estimated home-range sizes (with diurnal variations) of (a) four radiotagged Short-eared Owls in the 2011 breeding season 2011; and (b) 18 breeding territories (A–R) assessed by visual observation in 2006 and 2007 on moorland in Scotland.
Hunting position within the habitat gradient
The radiotagged Short-eared Owls tended to hunt over the grassier swards found at lower altitudes during daylight than in the dark. During daylight, the mean standardized altitude was 28 m below that of the nest (95% CI –39 to –19 m), in the dark the mean standardized altitude was 1 m above that of the nest-site (95% CI –19 to +21 m relative to the nest), where the sward included a greater ericaceous component, a statistically significant difference (F 1,3 = 17.1, P = 0.03). There was also a trend for the tagged owls to hunt over lower grassier areas in the early part of the breeding season (mean standardized altitude = –13 m, 95% CI –13 to +7 m) than during the later part of the breeding season (mean = –5 m, 95% CI –25 to +41 m), though that trend was arguably marginally non-significant (F 1,2 = 8.7, P = 0.09; note that only three birds were sampled over both seasonal periods).
The trend for a seasonal difference in hunting preference is supported by the 2006–07 visual observations. The mean standardized altitude at which Short-eared Owls were observed hunting before 15 June was –13 m (95% CI –44 to –2 m) and after that date was +28 m (95% CI +15 to +41 m), that difference being statistically significant (F 1,7 = 55.5, P < 0.01) and suggesting an increased preference for areas with a greater ericaceous component later in the season.
Use of ungrazed areas
The ungrazed areas (i.e. those with sparse scrub, either naturally regenerating or planted) comprised 5% of the combined home-ranges of the four radiotagged owls (range 3–6%). There was a marginally non-significant tendency (t 3 = 3.04, P = 0.056) for the four tagged owls to use the ungrazed areas with scrub less frequently at night, relative to their availability, compared with their use of those areas during daylight; the mean difference between the log-ratio differences was –10.1 (95% CI –20.6 to –10.5).
Although there was a suggestion for the ungrazed areas to be more frequently used relative to their availability in the early part of the season compared to their use of those areas in the late season (mean difference in the log-ratio differences = 6.94, 95% CI –14.1 to +28.0), the difference was not statistically significant (t 2 = 1.42, P = 0.29).
DISCUSSION
Despite differences of both times of day and of season in the favoured areas (altitude) where the owls hunted, the areas of active use remained broadly similar, though there were some differences in the areas of core activity between day and night. Therefore, we have no evidence to suggest (from our limited sample size of radiotagged birds) that home-range sizes of actively breeding Short-eared Owls, and, therefore, population densities derived from visual observations during daylight hours, should not be representative. However, reliance on daylight observations alone will not necessarily provide representative information on habitat-use and, therefore, on risks associated with habitat changes (e.g. forestry and grazing changes) and other developments (e.g. wind farm construction). For example, potential collision risks associated with proposed wind turbines or other obstacles (Drewitt & Langston Citation2008) based on diurnal observations may not show true nocturnal risks. Similarly, the relative importance or reliance on a certain part of, or habitat type within, the home-range based on diurnal observations alone may not accurately represent its overall importance.
Nocturnal activity rates closely matched those recorded during the peak diurnal activity periods. The diurnal activity of radiotagged Short-eared Owls in the present study (actively foraging from 52 to 75% of the time in 2011) was generally greater than that recorded by visual observations in 2006–07, when activity was recorded for less than 10% of the time through the season (Calladine et al. Citation2010). Diurnal monitoring of the radiotagged owls was deliberately concentrated during periods when activity rates were expected to be high. Even so, levels of activity tended to be greater in 2011 than in 2006–07 when activity was recorded for 10–40% of the time during peak periods. In 2011, voles Microtis agrestis were particularly abundant within the study area and the breeding success of the monitored Short-eared Owls was generally higher than in 2006–07 (pers. obs.) and so activity rates are likely to have been greater than in earlier years; hunting activity by a predator such as Short-eared Owl will be determined by demand (for itself, dependent mate and young) as well as by the availability (and activity) of prey (Reynolds & Gorman Citation1999). Despite these differences in activity rates, home-range sizes did not differ greatly between years within the present studies (or between methods of their assessment) and were within the ranges reported elsewhere in Europe (40–900 ha [Goddard Citation1935, Lockie Citation1955, Village Citation1987, Shaw Citation1995, Roberts & Bowman Citation1986, Korpimäki & Norrdahl 1991]). Within our restricted sample size of tagged birds, the home-range sizes of the tagged males were greater than that of the female, and that of the tagged juvenile was intermediate. Given the small sample sizes of individual birds for which we have data, we cannot make any assumptions on the generality of those differences.
Short-eared Owls tended to hunt over higher ground that supports a greater ericaceous component within the vegetation at night and also later in the breeding season, the mean altitudinal difference being about 30 m and 40 m respectively, although the seasonal difference was not significantly different for the radiotagged birds. The hunting activity of Short-eared Owls can match the activity patterns of prey, the owls being most active when and where the prey is also active (Mikkola & Sulkava Citation1969, Reynolds & Gorman Citation1999) and both prey availability and foraging preference could be associated with vegetation structure. The annual growth of vegetation in the lower and grassier areas produced a dense sward in the later part of the season, which, although still accommodating voles, could conceivably have made them more difficult for owls to catch. Clearly, vegetation structure would not differ between day and night. However, vole behaviour can differ according to predation risk from diurnal predators (Eccard et al. Citation2008). It is possible that vole activity in the shorter swards at more exposed higher altitudes was greatest at night and, although their overall risk to predation (from all potential predators) may have been less than during the day, they may have provided the more efficient foraging opportunities for owls at night.
As favoured foraging areas within the habitat gradients found on moorland and its fringes varied both diurnally and seasonally, management that delivers a diversity of sward structures within the hunting range of breeding Short-eared Owls could prove useful for their conservation. Such a diversity of swards could be achieved through differential grazing regimes, altitude variations (and therefore exposure) within a hunting range, or through a combination of both. Given past and potential future changes in grazing management in the British uplands (Fuller & Gough Citation1999, Thomson et al. Citation2011), an understanding of how Short-eared Owls use different sward structures and how that varies through the season would inform conservation management for the species. For example, an extensive ungrazed sward may be a productive hunting area early in the breeding season but its value to Short-eared Owls may be reduced later when demands could also be high for chick provisioning. We speculate that a diversity of sward structures within the spatial scale used by Short-eared Owls (in the order of 200 ha) could prove optimal to support them through a breeding season.
ACKNOWLEDGEMENTS
Radiotracking fieldwork was facilitated by grants from the Tay Ringing Group, the Scottish Ornithologists' Club, Mike and Katrina Funsten and the British Trust for Ornithology. Funding from Scottish Natural Heritage and the J & JR Wilson Trust permitted the analyses and reporting. We are grateful to Colin Shand and Mike Martin (of the Tay Ringing Group) for help with fieldwork and to the gamekeepers Stan Riches, Colin McGregor, Roddie McIntosh and Davie Campbell for their cooperation without which the study would not have been possible. Two anonymous referees and the editor greatly improved the manuscript.
REFERENCES
- Aebischer , N. J. , Robertson , P. A. and Kenward , R. E. 1993 . Compositional analysis of habitat use from animal radio-tracking data . Ecology , 74 : 1313 – 1325 . (doi:10.2307/1940062)
- Arroyo , B. E. and Bretagnolle , V. 1999 . Breeding biology of the Short-eared Owl (Asio flammeus) in agricultural habitats of southwestern France . J Raptor Res. , 33 : 287 – 294 .
- Beyer , H. L. 2004 . Geospatial Modelling Environment Version 0.7.1.0 Beta. Available at: www.spatialecology.com/gme
- BirdLife International . 2004 . Birds in Europe: Population Estimates, Trends and Conservation Status , Cambridge , , UK : BirdLife International .
- Burfield , I. J. 2008 . The conservation status and trends of raptors and owls in Europe . Ambio , 37 : 401 – 407 . (doi:10.1579/0044-7447(2008)37[401:TCSATO]2.0.CO;2)
- Calladine , J. , Garner , G. , Wernham , C. and Buxton , N. 2010 . Variation in the diurnal activity of breeding Short-eared Owls Asio flammeus: implications for their survey and monitoring . Bird Study , 57 : 89 – 99 . (doi:10.1080/00063650903437503)
- Calladine , J. , du Feu , C. and du Feu , R. 2012 . Changing migration patterns of the Short-eared Owl Asio flammeus in Europe: an analysis of ringing recoveries . J. Ornithol. , 153 : 691 – 698 . (doi:10.1007/s10336-011-0786-y)
- Craib , J. 2011 . “ Short-eared Owl ” . In The Breeding Birds of North-East Scotland , Edited by: Francis , I and Cook , M. 258 – 259 . Aberdeen : Scottish Ornithologists' Club .
- del Hoyo , J. , Elliot , A. and Sargatal , J. 1999 . Handbook of Birds of the World , Vol. 5 , Barcelona : Lynx Edicions .
- Drewitt , A. L. and Langston , R. H.W. 2008 . Collision effects of wind-power generators and other obstacles on birds . Ann. NY Acad. Sci. , 1134 : 233 – 266 . (doi:10.1196/annals.1439.015)
- Eccard , J. A. , Pusenius , J. , Sundell , J. , Halle , S. and Yionen , H. 2008 . Foraging patterns of voles at heterogeneous avian and uniform mustelid predation risk . Oecologia , 157 : 725 – 734 . (doi:10.1007/s00442-008-1100-4)
- Fuller , R. J. and Gough , S. J. 1999 . Changes in sheep numbers: implications for bird populations . Biol. Conserv. , 91 : 73 – 89 . (doi:10.1016/S0006-3207(99)00039-7)
- Goddard , T. R. 1935 . A census of short-eared owls (Asio f. flammeus) at Newcastleton, Roxburghshire, 1934 . J. Anim. Ecol. , 4 : 113 – 118 . (doi:10.2307/1218)
- Korpimäki , E. 1994 . Rapid or delayed tracking of multi-annual vole cycles by avian predators? . J. Anim. Ecol. , 63 : 619 – 628 . (doi:10.2307/5228)
- Korpimäki , E. and Norrdahl , K. 1991 . Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities . Ecology , 72 : 814 – 826 . (doi:10.2307/1940584)
- Lockie , J. D. 1955 . The breeding habits and food of Short-eared Owls after a vole plague . Bird Study , 2 : 53 – 69 . (doi:10.1080/00063655509475812)
- McGarry , O. C. 1998 . “ Short-eared Owl ” . In Atlas of Breeding Birds of South-east Scotland , Edited by: Murray , R. , Holling , M. , Dott , H. and Vandome , P. 180 – 181 . Edinburgh : Scottish Ornithologists' Club .
- Mikkola , H. 1983 . Owls of Europe , Calton , , UK : T&AD Poyser .
- Mikkola , H. and Sulkava , S. 1969 . On occurrence and feeding habits of Short-eared Owl in Finland 1964–68 . Ornis Fenn. , 46 : 188 – 93 .
- Newton , I. 2006 . Advances in the study of irruptive migration . Ardea , 94 : 433 – 460 .
- Pearce-Higgins , J. W. , Leigh , S , Douse , A. and Langston , R. H. 2012 . Greater impacts of wind farms on bird populations during construction than subsequent operation: results of a multi-site and multi-species analysis . J. Appl. Ecol. , 49 : 386 – 394 . (doi:10.1111/j.1365-2664.2012.02110.x)
- Petty , S. J. and Avery , M. I. 1990 . Forest Bird Communities: A Review of the Ecology and Management of Forest Bird Communities in Relation to Sylvicultural Practices in the British Uplands , Edinburgh : Forestry Commission .
- Poulin , R. G. , Wellicome , T. I. and Todd , L. D. 2001 . Synchronous and delayed numerical responses of a predatory bird community to a vole outbreak on the Canadian prairies . Journal of Raptor Research , 35 : 288 – 295 .
- Raw , D. 2000 . “ Short-eared Owl ” . In A Summer Atlas of the Breeding Birds of County Durham , Edited by: Westerberg , S. and Bowey , K. 96 – 97 . Durham , , UK : Durham Bird Club .
- Reynolds , P. and Gorman , M. L. 1999 . The timing of hunting in short-eared owls (Asio flammeus) in relation to the activity patterns of Orkney voles (Microtus arvalis orcadensis) . J. Zool., Lond. , 247 : 371 – 379 . (doi:10.1111/j.1469-7998.1999.tb01000.x)
- Roberts , J. L. and Bowman , N. 1986 . Diet and ecology of Short-eared Owls Asio flammeus breeding on heather moor . Bird Study , 33 : 12 – 17 . (doi:10.1080/00063658609476885)
- Rich , T. D. , Beardmore , C. J. , Berlanga , H. , Blancher , P. J. , Bradstreet , M. S.W. , Butcher , G. S. , Demarest , D. W. , Dunn , E. H. , Hunter , W. C. , Inigo-Elias , E. E. , Kennedy , J. A. , Martel , A. M. , Pujabi , A. O. , Rosenberg , K. V. , Rustay , C. M. , Wendt , J. S. and Will , T. C. 2004 . Partners in Flight North American Landbird Conservation Plan , Ithaca , NY : Cornell Laboratory of Ornithology .
- Shaw , G. 1995 . Habitat selection by Short-eared Owl Asio flammeus in young coniferous forests . Bird Study , 42 : 158 – 164 . (doi:10.1080/00063659509477161)
- Stott , M. 2002 . The Breeding Birds of Cumbria , Edited by: Stott , M. , Callion , J. , Kinley , I. , Raven , C. and Roberts , J. 202 – 203 . UK : Wood Mitchell, Stoke-on-Trent .
- Stroud , D. A. , Reed , T. M. , Pienkowski , M. W. and Lindsay , R. A. 1987 . Birds Bogs and forestry: The Peatlands of Caithness and Sutherland , Peterborough , , UK : Nature Conservancy Council .
- Swengel , S. R. and Swengel , A. B. 2009 . Variation in the detection of Short-eared and Snowy Owls at Buena vista Grassland 1997–2008 . Passeng. Pigeon , 71 : 377 – 400 .
- Thomson , S. , Holland , J. , Waterhouse , T. and Morgan-Davis , C. 2011 . Response from the Hills: Business as Usual or a Turning Point? An Update of ‘Retreat from the Hills’ , Edinburgh : SAC .
- Village , A. 1987 . Numbers, territory-size and turnover of Short-eared Owls Asio flammeus in relation to vole abundance . Ornis Scand. , 18 : 198 – 204 . (doi:10.2307/3676767)