Abstract
Capsule The Mute Swan, a large generalist herbivore, showed patterns of habitat use influenced by social grouping and season. Territorial swans showed strong preferences for river and lake habitat in all seasons, while the non-territorial birds known to cause grazing conflicts preferred river in summer–autumn and pasture in winter–spring.
Aims To quantify the habitat preferences across different seasons of two types of Mute Swan social group, territorial and non-territorial, and assess the implications for the grazing conflict between swans and valuable plant communities.
Methods Repeated surveys of the River Frome catchment, Dorset, UK, over a two-year period allowed us to record the numbers of swans in each habitat type. An electivity index was used to calculate habitat preference scores for territorial and non-territorial swans across different seasons.
Results We found strong seasonal switches in habitat use for both territorial and non-territorial swans, but preferences for some habitats differed between these social groupings. In particular, non-territorial swans preferred pasture in winter and spring, and river in summer and autumn, while territorial swans preferred river and lake habitats throughout the year.
Conclusion The Mute Swan, a large generalist herbivore that can cause grazing damage to river plant communities, showed patterns of habitat use influenced by social grouping and season. These seasonal patterns of habitat use suggest that the grazing conflict with the river plant community caused by flocks of non-territorial swans is currently limited to summer and autumn.
Herbivorous birds can affect the abundance and composition of plant communities in both aquatic and terrestrial habitats through foraging, trampling and faecal deposition (Marklund et al. Citation2002, Wood et al. Citation2012a). In particular, waterfowl can cause substantial reductions in plant abundance and thereby affect ecosystem structure, functioning and service provision (Klaassen & Nolet Citation2007, Gayet et al. Citation2011a, Citation2012, Wood et al. Citation2012a). Recent research has shown that swans (Order: Anseriformes) can have a greater per-capita impact on plant abundance, compared with smaller-bodied waterfowl, due to their larger size and highly herbivorous diet (Wood et al. Citation2012a). Such grazing impacts may require management where they lead to ecological or socioeconomic damage (Watola et al. Citation2003, Ellis & Elphick Citation2007, Wood et al. Citation2013). Such management can be expensive and ethically controversial, thus to avoid wasted effort and opposition any management decisions must be underpinned by robust data on swan use of the grazed habitats (Spray et al. Citation2002). A prerequisite of successful management is a comprehensive understanding of the timing, social groupings and numbers, of swans using the grazed habitats.
Mute Swans (Cygnus olor Gmelin, 1789) are large (∼ 10 kg) herbivorous birds capable of consuming up to 4 kg of fresh vegetation daily (Mathiasson Citation1973). In Britain, the Mute Swan population has undergone a substantial increase in population size, rising from around 17 600 individuals in 1978 to 31 700 in 2002, and may have increased further since then (Kirby et al. Citation1994, Ward et al. Citation2007). Mute Swans are among the most abundant water birds within the lowland river catchments of Britain (Mason & Macdonald Citation2000, Mason et al. Citation2006). Such rivers, typically rich in vegetation, are highly productive ecosystems (Berrie Citation1992). In particular, the lowland rivers dominated by cretaceous chalk geology (hereafter ‘chalk rivers’), support diverse and productive wildlife communities as well as socioeconomically valuable agriculture and game fishing (Environment Agency Citation2004). Chalk rivers are numerous in southern and eastern Britain but are uncommon internationally, and so their aquatic plant communities are protected under international legislation for their conservation value (e.g. EU Habitats Directive 92/43/EEC). However, grazing conflicts with this protected plant community have been reported for non-territorial flocks of swans in summer, including reductions in abundance and flowering (O'Hare et al. Citation2007, Wood et al. Citation2012b). The exploitation of river habitat at other times of year, and by territorial swans, has not yet been addressed. The absence of complaints for grazing damage outside of the summer period may indicate that swan use of river habitat is lower in other seasons.
The type of social group involved in any grazing conflict has implications for management. Non-territorial swans are highly mobile and are observed to move between feeding sites frequently, whereas territorial groups typically exhibit a high fidelity to a particular area (Birkhead & Perrins Citation1986, Holm Citation2002, Gayet et al. Citation2011b). Management which manipulates the relative quality of the grazed and adjacent habitats may be effective in shifting non-territorial groups away from the grazed area at any time (Vickery et al. Citation1994, Spray et al. Citation2002), but is only likely to be effective for territorial groups if carried out before nest-site selection, because after this time movement will be highly restricted due to the low dispersal ability of the young (Birkhead & Perrins Citation1986). An understanding of swan habitat use across different seasons, and by different swan social groupings, is required to advise when and where management could be targeted. However, we currently lack such data for Mute Swans in chalk river catchments.
In this study we used data from repeated surveys over two years to quantify the numbers of territorial and non-territorial swans and their habitat preferences in a chalk river catchment which has had recent reports of grazing damage to aquatic plants (O'Hare et al. Citation2007, Wood et al. Citation2012b). We tested three hypotheses regarding Mute Swan habitat use within our study system. Our first hypothesis (1) was that we would observe different swan habitat use in different seasons (spring, summer, autumn and winter). Our second hypothesis (2) was that habitat use would differ between two social groupings of swan (territorial and non-territorial). Our third hypothesis (3) was that observed differences in habitat use between social groupings would vary between seasons, thus habitat use would be influenced by interactions between season and social grouping.
METHODS
Study area
This study was undertaken in the catchment of the River Frome and its main tributary the River Piddle (Dorset, UK), mesotrophic chalk rivers that flows through a landscape used primarily for mixed pastoral and arable agriculture (Wood et al. Citation2012b). The main river channel, and associated side streams, are dominated by the aquatic plant Stream Water Crowfoot Ranunculus penicillatus ssp. pseudofluitans Webster (Wood et al. Citation2012b, Citation2012c). The river is typically bordered by pasture fields dominated by Perennial Ryegrass (Lolium perenne L.), Creeping Bentgrass (Agrostis stolonifera L.), and Yorkshire Fog (Holcus lanatus L.), and small patches of damp woodland of Black Alder (Alnus glutinosa L.) and Willow (Salix spp.). Arable fields predominantly contain Wheat (Triticum spp.), Barley (Hordeum vulgare L.) and Maize (Zea mays L.). Drainage ditches, often connected to the main river channel, frequently separate pasture fields. Numerous shallow lakes are also found within the catchment. Due to the mild climate and influx of groundwater water temperatures never decline below 5°C and so ice formation does not occur (Berrie Citation1992, Wood et al. Citation2012b). Complaints of grazing damage to aquatic plants by swans started around 1996 (Ilsington Angling Club and Moreton Estate, pers. comms).
Catchment surveys
Surveys of the catchment were carried out in January, March, May, July, September and December 2009, and monthly between February and November 2010. Thus in each year we surveyed during all of the major events that swans undergo in a typical year such as nesting, moulting, fledging of young and overwintering (Birkhead & Perrins Citation1986). On each occasion the river and the adjacent land approximately 500 m either side, including any other waterbodies, were surveyed from the Wareham Channel estuary (50°43ʹN, 02°02ʹW) 56.5 km upstream to Maiden Newton (50°46ʹN, 02°34ʹW) on the River Frome, and 12.0km to Warren Heath (50°43ʹN, 02°12ʹW) on the River Piddle. Swans were identified using a Swarovski STS 80HD (20 × 60) tripod-mounted telescope (Swarovski AG, Austria); for each individual the social grouping (territorial or non-territorial), age category (adult, juvenile, or cygnet) and habitat (pasture, river, lake, ditch, estuary, arable, woodland or urban, representing all of the available habitat types) were recorded. Swans were considered to be territorial if they were observed to exhibit territorial behaviours, as described in Birkhead & Perrins Citation(1986), such as aggressive exclusion of other swans, nesting behaviour or the presence of cygnets. Such territorial behaviours are observed in all seasons in shallow river catchments due to the intense competition for territories (Scott Citation1984). Cygnets (≤ 6 months old) have greyish-brown plumage; juveniles (7–18 months old) possess pinkish-grey bill colouration and some greyish-brown feathers; adults (> 18 months old) possess all-white plumage and orange bill colouration (Birkhead & Perrins Citation1986). To reduce subjectivity all surveys were undertaken by a single observer (KW). Such repeated monthly site visits are a well-established method of quantifying the use of a site by Mute Swans (Scott & Birkhead Citation1983, Scott Citation1984, Gayet et al. Citation2011c, Wood et al. Citation2012b). Swans have a very high detection probability (e.g. 0.94; Gayet et al. Citation2011c) due to their large size, conspicuous plumage and tolerance of humans. Each catchment survey took 4 days, and was only conducted during daylight hours. We cannot exclude the possibility that swan movements during a survey may have resulted in individuals being either undetected or double-counted. However, we argue that this was unlikely as approximately one-third of the population were fitted with a coloured leg ring, allowing individual identification as part of a long-term monitoring project in southern England (Watola et al. Citation2003), and no colour-ringed individual was ever observed twice during the same survey. We observed a mean (± se) of 28 ± 5 ringed individuals per month.
Habitat use and preferences
To quantify habitat availability, the spatial extent of each habitat category type within the study area was estimated visually during a survey of the catchment. We observed eight habitat types: Arable, Ditch, Estuary, Lake, Pasture, River, Urban and Woodland. The spatial extent of each habitat was recorded onto Explorer Maps 117 and OL15 (Ordinance Survey, UK) from which the total area of each habitat was measured (± 0.001 km2). Swan habitat preferences were examined by electivity analysis. For each month for each habitat category, Ivlev's electivity index (s) was calculated as:
Statistical analyses
We used a general linear model to test the effects of season, swan social group (territorial or non-territorial) and the interaction between season and social group, on electivity for (i) pasture, (ii) river, (iii) lake, (iv) ditch, (v) estuarine, and (vi) arable habitat; each habitat type was tested in a separate model. In our model we included season, social grouping and year as fixed effects, and the interaction between season and social grouping. To test between-season effects, we sorted electivity values for a given habitat type for each month into groups on the basis of season; spring (March, April), summer (May, June, July, August), autumn (September, October), or winter (November, December, January, February). These seasons reflected the annual changes in meteorological conditions within our study area and also influence swan phenology as changes in meteorological conditions such as light, temperature and precipitation, drive vegetation dynamics and hydrological conditions (Birkhead & Perrins Citation1986). Electivity values for each month were rescaled between 0 and 1, and arcsine square root-transformed to meet the assumptions of the model. Analyses were carried out using SPSS version 19 (IBM, US), with a statistically significant result attributed where P < 0.05. Normality of the residuals was confirmed with the Kolmogorov–Smirnov test.
RESULTS
Swan population structure
We observed a mean (± 95% CI) population of 298.2 ± 18.9 swans over our entire study period, with the total population size typically highest in winter and lowest in spring and autumn (). Both the territorial and non-territorial subpopulations were primarily composed of adult birds, with smaller numbers of juveniles and cygnets (). As expected, the territorial subpopulation was greatest in autumn due to the birth of cygnets over the preceding summer and return from the summer moulting grounds of failed breeders to their territories. In contrast, the non-territorial subpopulation was lowest during autumn and greatest during winter. A greater mean (± 95% CI) number of discrete non-territorial groups was observed during winter (18.8 ± 4.4) relative to spring (11.7 ± 5.3), summer (8.2 ± 2.0) or autumn (10.7 ± 2.4), but mean group size was relatively stable at 17.7 ± 4.5 individuals. Swans in territorial groups were most numerous in summer and autumn, but never decreased below 70 individuals outside of this period.
Table 1. Between season changes in the mean (± 95% CI) numbers of individuals of each age class which comprised the total population, territorial subpopulation and non-territorial subpopulation.
Swan habitat use
Comparisons of habitat availability and use by swans suggested that habitats were not used in proportion to their availability; strong preferences and avoidances of particular habitats was evident (). Urban and woodland habitat constituted 15.1% and 7.7% of the available habitat, respectively, but no swans were ever observed in either habitat and so we excluded these from further analyses. We found some evidence of between-season differences in swan habitat use. In particular, the river was preferred in summer and autumn, while pasture was preferred in winter and spring (). Consequently, habitat electivity values differed significantly between seasons for both pasture and river habitat; electivity for river habitat was greater in summer and autumn relative to winter and spring, while the reverse pattern was observed for pasture (, ). Arable habitat was little used despite being widely available within the catchment; a small number of birds were observed in a single barley field in January and March 2009 but at no other time. Electivity for arable habitat did not differ between seasons or social group.
Table 2. A comparison of the availability and mean (± 95% CI) percentage of the swan population using each of six habitats in the River Frome catchment.
Table 3. The influence of year, season, swan social group and the swan*social group interaction on the electivity values for six habitat categories, as illustrated by a general linear model. Biological significance is shown in .
Figure 1. Seasonal differences in mean (± se) habitat electivity (s) of the non-territorial (dark bars) and territorial (light bars) subpopulations, indicating habitat preference (positive values) or avoidance (negative values).
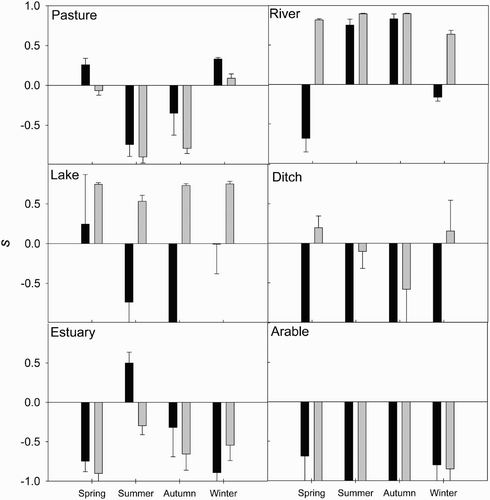
We detected some differences in habitat use between territorial and non-territorial swans. Habitat electivity values differed significantly between the two social groups for pasture, river, lake and ditch habitats (). Territorial birds had a stronger preference for river, ditch and lake habitats, while non-territorial swans had the stronger preference for pasture habitat (). Additionally, we also found some evidence of interactions between season and social grouping on swan habitat use. We detected a significant interaction between season and social grouping such that preference for river habitat was greatest for non-territorial birds in summer and autumn (, ). Additionally, non-territorial birds in spring showed significantly lower electivity values for river. Furthermore, we detected a significant interaction between season and social grouping for estuary habitat, such that non-territorial individuals in summer and to a lesser extent autumn showed a greater preference for the estuary (, ). We detected no effect of year on electivity for five out of six habitats; the exception was arable habitat, which showed a less negative electivity value for 2009 compared with 2010 (0.16 ± 0.07 se parameter estimate for 2009; ).
DISCUSSION
We demonstrated the influence of season and swan social grouping on Mute Swan habitat use, through catchment-scale field data collected over two years. By quantifying the use of river habitat, our study identifies the temporal extent of the grazing conflict between Mute Swans and the river plant community; management efforts can be focussed within this period. Further studies which address the spatial distribution and local movements of such flocks of non-territorial individuals are required to predict how grazing pressure at any given site may respond to changes in the number of swans present within the catchment.
In terms of numbers observed, river was the single most important habitat type for territorial birds throughout the year, and the most important for non-territorial swans in summer and autumn (May–September). Estuarine habitat was important in the summer as the principal moulting site in the catchment; previous research has also documented the use of large, open sites such as estuaries and lagoons as moulting sites (Holm Citation2002). We found that the swan population was predominantly comprised of non-territorial individuals living in flocks, with smaller proportions of territorial birds, in common with previous studies of swan populations (Minton Citation1971, Coleman et al. Citation2001). The mean size of non-territorial flocks remained relatively constant across seasons; the winter influx of swans to flocks resulted in the establishment of additional flocks rather than increasing the size of existing flocks.
We detected differences in Mute Swan habitat preferences between different seasons, in accordance with our first hypothesis. A clear seasonal switch in habitat use was observed, with pasture preferred in spring and winter and river preferred in summer and autumn. Foraging decisions were likely the key determinant of habitat selection in our Mute Swan population, rather than other factors such as disturbance or predation. For example, Tuite et al. Citation(1984) reported no effects of human disturbance on Mute Swan abundances on inland waters. After the cygnet stage predation risk for swans is very low (< 3% of all mortality; Brown et al. Citation1992), thus outside of the early breeding season we expect foraging decisions to determine habitat selection. For non-territorial swans we expect foraging decisions to determine habitat selection during all seasons (Birkhead & Perrins Citation1986, Holm Citation2002). Habitat availability was constant between seasons, but the relative quality and thus profitability of these habitats probably varied with season. In particular, the preference for river habitat in summer and autumn coincides with the periods of lowest river flow and turbidity and highest aquatic plant biomass (O'Hare et al. Citation2007, Wood et al. Citation2012b), which are all likely to increase the feeding profitability of the river habitat, relative to other habitats. Further research could explore the mechanisms which underpin seasonal changes in the feeding profitability of different habitats and their role in driving habitat shifts. In particular, plant abundance, plant nutritional quality, and the metabolic costs associated with foraging, are all known to influence the profitability of plants to herbivores (Vickery et al. Citation1995, Nolet et al. Citation2002). The absence of swans in wooded habitat was consistent with the results of studies from other regions both within and outside the native range of the Mute Swan (Fuller et al. Citation2005, Weaver et al. Citation2012). We found a stronger, albeit highly seasonal, preference for pasture habitat than reported for other regions (Fuller et al. Citation2005). The improved pasture fields in our study were adjacent to the river and underwent partial flooding each winter (Environment Agency Citation2004), offering large areas of shallow water habitat with higher food abundance compared to the shallow lake habitat typically used as overwintering habitat by swans in many other regions (Rees et al. Citation1997, Holm Citation2002, Carss et al. Citation2012). Pasture fields surrounding dairy farms seemed particularly attractive to non-territorial swans, presumably because of the higher nutritional quality of these fertilizer-enriched fields; the preference of foraging waterfowl for fertilizer-enriched fields is well-documented (Vickery & Gill Citation1999). In contrast to some other swan populations (Chisholm & Spray Citation2002), arable fields were typically avoided in our study area. This difference may be explained by the different patterns of land use in the different study areas; in particular, the River Frome catchment lacked fields of Oilseed Rape (Brassica napus L.) which were a preferred habitat in the River Tweed catchment, Scotland (Chisholm & Spray Citation2002, Spray et al. Citation2002). Patterns of habitat use may thus depend, at least in part, on the relative availability, quality and distribution of preferred food plants within the landscape.
We found some differences in habitat preferences for non-territorial and territorial individuals, in accordance with our second hypothesis. Territorial individuals consistently preferred freshwater habitats, particularly river and lake habitat with ditches typically only preferred in winter. Freshwater habitats offer both abundant plant food and security for cygnets from terrestrial predators during the breeding period, making them attractive breeding habitats and therefore territories (Birkhead & Perrins Citation1986). Lakes in particular are known as a key breeding habitat for swans and so the preference we observed of territorial birds for lake habitat was expected (Birkhead & Perrins Citation1986, Tuite et al. Citation1984, Gayet et al. Citation2011b). Territorial individuals continued to exhibit a preference for river habitat even during periods of high flow in winter, presumably because of the need to defend it from other pairs due to the strong competition for territories among swans (Scott Citation1984). In contrast, non-territorial birds switched from river to pasture habitat during winter and spring, probably due to the change in relative habitat profitability. Inferior profitability of lake habitat, perhaps due to lower plant biomass and nutritional quality, or the dominance of unpalatable species, may have been responsible for the avoidance of lakes by non-territorial birds; further research into relative habitat profitability is required to help explain our observed patterns fully. We also found some support for our third hypothesis that habitat use across seasons would differ according to social grouping. Preference for river habitat in spring was lower for non-territorial relative to territorial birds. Intense competition for territories probably forces swans which aim to breed to remain within their river territories despite adverse hydrological conditions (i.e. high water velocities) and low food availability (Scott Citation1984). Under the more benevolent conditions of summer and autumn the preference of non-territorial birds for the river was greater than that of territorial swans, because territorial birds also used lake and ditch habitat as breeding areas. During summer months large numbers of non-territorial swans moved to the estuary to moult, as observed in other study areas (Gillham Citation1956), whereas territorial birds with young remained within their breeding territories to moult.
In this study we found strong influences of both season and swan social grouping on swan habitat use. Interactions between season and social group were also found. These results have implications for potential management of the swan grazing conflict in river catchments. Pasture field use was limited to winter and spring, but high usage during these periods could cause depletion of grasses used to feed livestock. The use of deterrent tape could be used during this period to alleviate such a grazing conflict (McKay & Parrott Citation2002). However, the avoidance of arable habitat suggests that no protection will be required for crop fields during any period. Aquatic plant communities are particularly vulnerable to grazing damage during periods of low abundance (Marklund et al. Citation2002), which for chalk rivers is the winter period (Wood et al. Citation2012b). However, swan use of river habitat in winter was very low, indicating low grazing pressure and thus no need for management during this period. Territorial swans also used river habitat in smaller numbers than those for which grazing conflicts have been reported (O'Hare et al. Citation2007, Wood et al. Citation2012b).
Non-territorial swans, which have previously been linked with the grazing conflict (Trump et al. Citation1994, Wood et al. Citation2012b, Citation2013) showed the strongest preference for river habitat, and used river habitat in the largest numbers, in summer and autumn. This period coincides with plant reproduction, which can be negatively affected by swan grazing (Wood et al. Citation2012b). The period of peak river use also coincides with the game fishing season (April–September), which likely exacerbates the conflict between swan grazing and angling (Trump et al. Citation1994, Parrott & McKay Citation2001). Such high grazing pressure indicates that management may be required principally during the summer to autumn period. River reaches could be protected from grazing by flocks of non-territorial swans by encouraging a pair to nest and breed, because territorial swans aggressively exclude other individuals (Birkhead & Perrins Citation1986). However, previous attempts to manipulate swan territory formation and nesting have been unsuccessful, and so a better understanding of swan breeding habitat requirements is needed (Parrott & McKay Citation2001).
ACKNOWLEDGEMENTS
We thank Lucy Mulholland for field assistance and the riparian landowners for access. Thanks to David Stone and members of the Radipole Ringing Group for information on ringed birds. Andy Green, James Bullock, Will Cresswell and two anonymous reviewers provided useful comments on an earlier draft of the manuscript. KW was supported by a Centre for Ecology & Hydrology Algorithm (Natural Environment Research Council) studentship.
REFERENCES
- Berrie , A. D. 1992 . The chalk-stream environment . Hydrobiologia , 248 : 3 – 9 . (doi:10.1007/BF00008881)
- Birkhead , M. and Perrins , C. M. 1986 . The Mute Swan , London : Croom Helm .
- Brown , M. J. , Linton , E. and Rees , E. C. 1992 . Causes of mortality among wild swans in Britain . Wildfowl , 43 : 70 – 79 .
- Carss , D. , Spears , B. M. , Quinn , L. and Cooper , R. 2012 . Long-term variations in waterfowl populations in Loch Leven: identifying discontinuities between local and national trends . Hydrobiologia , 681 : 85 – 104 . (doi:10.1007/s10750-011-0927-6)
- Chisholm , H. and Spray , C. J. 2002 . Habitat useage and field choice by Mute and Whooper Swans in the Tweed Valley, Scotland . Waterbirds , 25 ( Special Publication 1 ) : 177 – 182 .
- Coleman , A. E. , Coleman , J. T. , Coleman , P. A. and Minton , C. D.T. 2001 . A 39 year study of a Mute Swan Cygnus olor population in the English midlands . Ardea , 89 : 123 – 133 .
- Ellis , M. M. and Elphick , C. S. 2007 . Using a stochastic model to examine the ecological, economic and ethical consequences of population control in a charismatic invasive species: Mute Swans in North America . J. Appl. Ecol. , 44 : 312 – 322 . (doi:10.1111/j.1365-2664.2006.01265.x)
- Environment Agency . 2004 . The State of England's Chalk Rivers A report by the UK biodiversity action plant steering group for chalk rivers. Environment Agency, Bristol
- Fuller , R. M. , Devereux , B. J. , Gillings , S. , Amable , G. S. and Hill , R. A. 2005 . Indices of bird-habitat preference from field surveys of birds and remote sensing of land cover: a study of south-eastern England with wider implications for conservation and biodiversity assessment . Global Ecol. Biogeogr. , 14 : 223 – 239 . (doi:10.1111/j.1466-822X.2005.00145.x)
- Gayet , G. , Guillemain , M. , Fritz , H. , Mesleard , F. , Begnis , C. , Costiou , A. , Body , G. , Curtet , L. and Broyer , J. 2011a . Do Mute Swan (Cygnus olor) grazing, swan residence and fishpond nutrient availability interactively control macrophyte communities? . Aquat. Bot. , 95 : 110 – 116 . (doi:10.1016/j.aquabot.2011.04.003)
- Gayet , G. , Guillemain , M. , Benmergui , M. , Mesleard , F. , Boulinier , T. , Bienvenu , J. P. , Fritz , H. and Broyer , J. 2011b . Effects of seasonality, isolation and patch quality for habitat selection processes by Mute Swans Cygnus olor in a fishpond landscape . Oikos , 120 : 801 – 812 . (doi:10.1111/j.1600-0706.2010.18750.x)
- Gayet , G. , Eraud , C. , Benmergui , M. , Broyer , J. , Mesleard , F. , Fritz , H. and Guillemain , M. 2011c . Breeding Mute Swan habitat selection when accounting for detectability: a plastic behaviour consistent with rapidly expanding populations . Eur. J. Wildl. Res. , 57 : 1051 – 1056 . (doi:10.1007/s10344-011-0518-x)
- Gayet , G. , Croce , N. , Grillas , P. , Nourry , C. , Deschamps , C. and du Rau , P. D. 2012 . Expected and unexpected effects of waterbirds on Mediterranean aquatic plants . Aquat. Bot. , 103 : 98 – 105 . (doi:10.1016/j.aquabot.2012.07.002)
- Gillham , M. E. 1956 . Feeding habits and seasonal movements of Mute Swans on two south Devon estuaries . Bird Study , 3 : 205 – 212 . (doi:10.1080/00063655609475849)
- Holm , T. E. 2002 . Habitat use and activity patterns of Mute Swans at a molting and a wintering site in Denmark . Waterbirds , 25 ( Special Publication 1 ) : 183 – 191 .
- Ivlev , V. S. 1961 . Experimental Ecology of the Feeding of Fishes , New Haven , CT : Yale University Press .
- Jacobs , J. 1974 . Quantitative measurements of food selection. A modification of the forage ratio and Ivlev's electivity index . Oecologia , 14 : 413 – 417 . (doi:10.1007/BF00384581)
- Kirby , J. , Delany , S. and Quinn , J. 1994 . Mute Swans in Great Britain: a review, current status and long-term trends . Hydrobiologia , 279/280 : 467 – 482 . (doi:10.1007/BF00027878)
- Klaassen , M. and Nolet , B. A. 2007 . The role of herbivorous water birds in aquatic systems through interactions with aquatic macrophytes, with special reference to the Bewick's Swan – Fennel Pondweed system . Hydrobiologia , 584 : 205 – 213 . (doi:10.1007/s10750-007-0598-5)
- Marklund , O. , Sandsten , H. , Hansson , L. A. and Blindow , I. 2002 . Effects of waterfowl and fish on submerged vegetation and macroinvertebrates . Freshwater Biol. , 47 : 2049 – 2059 . (doi:10.1046/j.1365-2427.2002.00949.x)
- Mason , C. F. and Macdonald , S. M. 2000 . Numbers of wintering waterbirds on rivers in eastern England . Wildfowl , 51 : 215 – 219 .
- Mason , C. F. , Hofmann , T. A. and Macdonald , S. M. 2006 . The winter bird community of river corridors in eastern England in relation to habitat variables . Ornis Fennica , 83 : 73 – 85 .
- Mathiasson , S. 1973 . A moulting population of non-breeding Mute Swans with special reference to flight feather moult, feeding ecology and habitat selection . Wildfowl , 24 : 43 – 63 .
- McKay , H. V. and Parrott , D. 2002 . Mute Swan grazing on winter crops: evaluation of three grazing deterrents on oilseed rape . Int. J. Pest Manage. , 48 : 189 – 194 . (doi:10.1080/09670870110102585)
- Minton , C. D.T. 1971 . Mute Swan flocks . Wildfowl , 22 : 71 – 88 .
- Nolet , B. A. , Bevan , R. M. , Klaassen , M. , Langevoord , O. and van der Heijden , Y. G.J.T. 2002 . Habitat switching by Bewick's Swans: maximization of average long-term energy gain? . J. Anim. Ecol. , 71 : 979 – 993 . (doi:10.1046/j.1365-2656.2002.00662.x)
- O'Hare , M. T. , Stillman , R. A. , McDonnell , J. and Wood , L. R. 2007 . Effects of Mute Swan grazing on a keystone macrophyte . Freshwater Biol. , 52 : 2463 – 2475 . (doi:10.1111/j.1365-2427.2007.01841.x)
- Parrott , D. and McKay , H. 2001 . “ Habitat preferences and nest site selection by Mute Swans: an investigation into the potential for managing swan distribution in leisure fisheries ” . In Advances in Vertebrate Pest Management – Volume II , Edited by: Pelz , H. J. , Cowan , D. P. and Feare , C. J. 263 – 282 . Furth : Filander Verlag .
- Rees , E. C. , Kirby , J. S. and Gilburn , A. 1997 . Site selection by swans wintering in Britain and Ireland; the importance of habitat and geographic location . Ibis , 139 : 337 – 352 . (doi:10.1111/j.1474-919X.1997.tb04633.x)
- Scott , D. K. 1984 . Winter territoriality of Mute Swans Cygnus olor . Ibis , 126 : 168 – 176 . (doi:10.1111/j.1474-919X.1984.tb07996.x)
- Scott , D. K. and Birkhead , M. E. 1983 . Resources and reproductive performance in Mute Swans Cygnus olor . J. Zool. , 200 : 539 – 547 . (doi:10.1111/j.1469-7998.1983.tb02814.x)
- Spray , C. J. , Morrison , N. and Chisholm , H. 2002 . Utilisation of Oilseed Rape fields by Mute Swans Cygnus olor in Scotland and implications for management . Aspect. Appl. Biol. , 67 : 67 – 74 .
- Trump , D. P.C. , Stone , D. A. , Coombs , C. F.B. and Feare , C. J. 1994 . Mute Swans in the Wylye Valley: population dynamics and habitat use . Int. J. Pest Manage. , 40 : 88 – 93 . (doi:10.1080/09670879409371860)
- Tuite , C. H. , Hanson , P. R. and Owen , M. 1984 . Some ecological factors affecting winter wildfowl distributions on inland waters in England and Wales, and the influence of water-based recreation . J. Appl. Ecol. , 21 : 41 – 62 . (doi:10.2307/2403036)
- Vickery , J. A. and Gill , J. A. 1999 . Managing grassland for wild geese in Britain: a review . Biol. Conserv. , 89 : 93 – 106 . (doi:10.1016/S0006-3207(98)00134-7)
- Vickery , J. A. , Watkinson , A. R. and Sutherland , W. J. 1994 . The solutions to the Brent goose problem: an economic analysis . J. Appl. Ecol. , 31 : 371 – 382 . (doi:10.2307/2404551)
- Vickery , J. A. , Sutherland , W. J. , Watkinson , A. R. , Lane , S. J. and Rowcliffe , J. M. 1995 . Habitat switching by Dark-bellied Brent Geese Branta b. bernicla (L.) in relation to food depletion . Oecologia , 103 : 499 – 508 . (doi:10.1007/BF00328689)
- Ward , R. M. , Cranswick , P. A. , Kershaw , M. , Austin , G. E. , Brown , A. W. , Brown , L. M. , Coleman , J. C. , Chisholm , H. K. and Spray , C. J. 2007 . Numbers of Mute Swans Cygnus olor in Great Britain: results of the national census in 2002 . Wildfowl , 57 : 3 – 20 .
- Watola , G. V. , Stone , D. A. , Smith , G. C. , Forrester , G. J. , Coleman , A. E. , Coleman , J. T. , Goulding , M. J. , Robinson , K. A. and Milsom , T. P. 2003 . Analyses of two Mute Swan populations and the effects of clutch reduction: implications for population management . J. Appl. Ecol. , 40 : 565 – 579 . (doi:10.1046/j.1365-2664.2003.00811.x)
- Weaver , J. E. , Conway , T. M. and Fortin , M. J. 2012 . An invasive species' relationship with environmental variables changes across multiple spatial scales . Landscape Ecol. , 27 : 1351 – 1362 . (doi:10.1007/s10980-012-9786-4)
- Wood , K. A. , Stillman , R. A. , Clarke , R. T. , Daunt , F. and O'Hare , M. T. 2012a . The impact of waterfowl herbivory on plant standing crop: a meta-analysis . Hydrobiologia , 686 : 157 – 167 . (doi:10.1007/s10750-012-1007-2)
- Wood , K. A. , Stillman , R. A. , Clarke , R. T. , Daunt , F. and O'Hare , M. T. 2012b . Understanding plant community responses to combinations of biotic and abiotic factors in different phases of the plant growth cycle . PLoS ONE , 7 : e49824 (doi:10.1371/journal.pone.0049824)
- Wood , K. A. , Stillman , R. A. , Clarke , R. T. , Daunt , F. and O'Hare , M. T. 2012c . Measuring submerged macrophyte standing crop in shallow rivers: a test of methodology . Aquat. Bot. , 102 : 28 – 33 . (doi:10.1016/j.aquabot.2012.04.006)
- Wood , K. A. , Stillman , R. A. , Daunt , F. and O'Hare , M. T. 2013 . Evaluating the effects of population management on a herbivore grazing conflict . PLoS ONE , 8 : e56287 (doi:10.1371/journal.pone.0056287)