Abstract
Capsule Recent annual productivity of Arctic Terns Sterna paradisaea in Iceland has been persistently low, primarily because of very low rates of chick fledging.
Aims To quantify current levels of productivity in Arctic Terns in Iceland, and the extent to which this varies among 10 colonies across the Snaefellsnes peninsula, West Iceland.
Methods Between 2008 and 2011, up to 10 Arctic Tern colonies were monitored annually and a total of 635 nests were monitored from laying to fledging or failure.
Results Arctic Tern productivity in colonies across the peninsula varied little between years; the clutch size of 635 nests did not vary significantly between colonies or years, and hatching success was consistently high (80–100%). However, fledging success was low in all years, although with some variation between colonies.
Conclusion Population declines are evident among Arctic Terns and many other seabird populations around the North Atlantic, but little is known about the status of the huge populations breeding in Iceland. The low productivity across the colonies and years suggests that environmental changes are also impacting Arctic Tern populations in Iceland. We discuss the potential causes of these demographic patterns and the implications for the conservation of this species in Iceland.
During the past decade, breeding densities of several seabird species have decreased around the North Atlantic (Frederiksen Citation2010, ICES Citation2012b). Although population growth rates in long-lived species, such as seabirds, are most sensitive to changes in adult survival (Ezard et al. Citation2006, Stahl & Oli Citation2006), repeated low breeding success in some species has been sufficient to result in population declines (Jenouvrier et al. Citation2005a, Citation2005b, Citation2009, Rolland et al. Citation2009). Reports of repeated annual breeding failures from most major North Atlantic seabird areas (Frederiksen Citation2010) coincide with the measures of population declines for numerous species across the North Atlantic (Mavor et al. Citation2008, Barrett et al. Citation2012). Some studies have shown that environmental conditions were poor enough to negatively affect adult survival during a period of population decline (Sandvik et al. Citation2005, Frederiksen et al. Citation2008), and declines in productivity have been widely linked to declines in food availability (Frederiksen et al. Citation2006, Heath et al. Citation2009), in particular during chick-rearing (Anker-Nilssen et al. Citation1997, Davis et al. Citation2005, Barrett Citation2007). High rates of nest and chick predation (Becker Citation1995) and extreme weather conditions (Thompson & Furness Citation1991, Aebischer Citation1993, Robinson et al. Citation2002) are also known to affect productivity in colonial seabirds.
To date, the most detailed information on the past and current status of seabird populations in the North Atlantic has come from colonies around the North Sea and along the Norwegian coast (see overview in Frederiksen Citation2010). In this region, both large-scale, long-term population monitoring and detailed within-colony studies have provided evidence that seabird population trends are strongly influenced by variation in prey abundance (Thompson & Ollason Citation2001, Frederiksen et al. Citation2006, ICES Citation2009, Jennings et al. Citation2012), which can in turn be influenced by fishing pressure and environmental conditions such as changes in sea temperature (Dulvy et al. Citation2008). Ongoing depletion of fishing stocks and observed and future predicted shifts in fish distributions in response to rising sea temperatures (Perry et al. Citation2005) are, therefore, of growing concern for the status of seabird populations. At high latitudes, human impacts on marine ecosystems such as fishing (Halpern et al. Citation2008) may be generally lower, but climate-driven environmental changes are predicted to be particularly severe (IPCC Citation2007). Very large seabird populations are found in the sub-Arctic and Arctic areas (Mitchell et al. Citation2004, Frederiksen Citation2010) but, with the exception of Norwegian coastal populations (Barrett et al. Citation2012), very few high latitude seabird populations have been studied in detail, and information on these populations is rarely available (Frederiksen Citation2010). The implications of current and future environmental changes for the demography of seabirds breeding at higher latitudes are, therefore, largely unknown.
A large proportion of many of the largest seabird populations in the North Atlantic can be found in Iceland (Gardarsson Citation1995), highlighting the international importance of Icelandic waters for seabirds. Iceland is in the middle of the North Atlantic, where ocean areas of the South and West coast are influenced by warm and saline Atlantic water while areas of the North and East coast are more variable and are influenced by Atlantic, Arctic and even Polar water masses to different degrees. These oceanic conditions support a wide range of sea life, including large commercial fish and huge populations of seabirds (Astthorsson et al. Citation2007). Dietary studies indicate that these seabird populations rely mainly on sandeel (Ammodytes spp.) to the South and the West of Iceland, and capelin (Mallotus villosus) as well as krill (Euphausiacea) in the waters of the North and East (Thompson et al. Citation1999, Lilliendahl Citation2009). In contrast to the North Sea, sandeel populations are not harvested in Icelandic waters, but capelin populations, the largest pelagic fish stock in Icelandic waters (Astthorsson et al. Citation2007), are heavily exploited, with average annual total landings of ∼800 thousand tons since 1978 (ICES Citation2012a).
In 2005–2008, a country-wide population census of cliff-breeding seabird species (Fulmars Fulmarus glacialis, Kittiwakes Rissa tridactyla, Razorbills Alca torda, Common Uria aalge and Brünnich's Guillemots Uria lomvia) in Iceland showed that these populations had declined by 16–43% since 1983–86 (Gardarsson et al. Citation2011; A. Gardarsson pers. comm.). Additionally, studies at 5- to 10-year intervals between the mid-1980s and 2005 in colonies in Southwest and Northeast Iceland showed that the numbers of apparently occupied nests (AON) of Fulmars and Brünnich's Guillemots had declined by 2–3% and 7% per annum, respectively, and 9% annual declines in AON were evident for Razorbills and Common Guillemots between 1999 and 2005. Kittiwake populations showed evidence of regional declines, with AON in the largest colonies in the northeast declining by 75% (Gardarsson Citation2006a). All these populations have been shown to use the same food resources; the sandeel (Ammodytes marinus) in the South and West of Iceland, while on the North coast the capelin was the most important food item for five of the six species (Lilliendahl & Solmundsson Citation1998; Thompson et al. Citation1999). Despite the indication from these studies that Icelandic seabird populations may be in decline, there have been no studies explicitly aimed at identifying the environmental and demographic drivers of these changes.
Arctic Terns Sterna paradisaea provide an opportunity to explore the problems these seabird populations are encountering. They are ground-nesters that often breed in dense colonies, allowing easy access to both eggs and chicks for productivity measures. Iceland supports numerous Arctic Tern colonies that are widely distributed and are exposed to a broad range of local environmental conditions, including weather, access to feeding grounds and oceanic conditions. In addition, their pole-to-pole migration (Egevang et al. Citation2010) and late arrival on the Icelandic breeding grounds (Gunnarsson & Tómasson Citation2011) means that individuals are on a tight schedule for breeding, which may make them particularly sensitive to environmental changes. Their body condition could reflect environmental conditions experienced during migration but this is also likely to be influenced by local conditions in Iceland, because nesting typically occurs 3–4 weeks after arrival. Furness & Tasker (Citation2000) identified breeding success of terns as being particularly sensitive to changes in abundance of small pelagic fish stocks, and tern breeding success can also be strongly affected by the impacts of predators and weather conditions (Mavor et al. Citation2008).
Around 20–30% of the world's Arctic Terns breed in Iceland (Asbirk et al. Citation1997), in colonies ranging from tens to tens of thousands of pairs, and their breeding status in Iceland is, therefore, a key issue for the global conservation of the species. Arctic Tern colonies around the coast of the Snaefellsnes peninsula in West Iceland support tens of thousands of breeding pairs, and the area provides a wide range of oceanic, estuarine and freshwater foraging opportunities. In this study, we quantify the current levels of productivity in Arctic Tern colonies throughout the Snaefellsnes peninsula and assess the extent of between-year and between-colony variations in different components of productivity, in order to identify the current status of breeding conditions for Arctic Terns in Iceland, and the scale of spatial and temporal variation in breeding success.
METHODS
Study area and colonies
The study was conducted on Snaefellsnes peninsula from 2008 to 2011. Snaefellsnes is situated in West Iceland and has mountainous terrain surrounded by coastal lowlands. The peninsula stretches 100 km into the Atlantic Ocean, between the bays of Faxafloi and Breidafjordur (). The peninsula supports numerous seabird colonies, of both cliff-breeders and ground-nesters. Arctic Tern colonies are found along the whole coastline of the peninsula ().
Figure 1. Location and approximate size (number of pairs) of Arctic Tern study colonies on Snaefellsnes peninsula and the location of the peninsula in western Iceland (inset). Sty, Stykkisholmur; Ber, Berserkseyri; Ska, Skallabudir; Tho, Thordisarstadir; Gru, Grundafjordur; Lar, Larvadall; Rif, Rif; Arn, Arnarstapi; Hra, Hraunsmuli; Lan, Langaholt; Sta, Stakkhamarsnes.
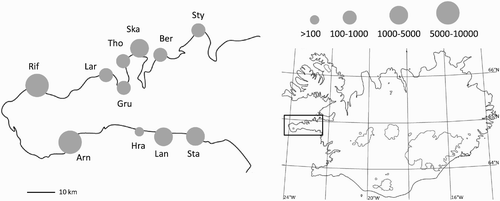
During the study period, a total of between 66 and 272 Arctic Tern nests located in 4–10 colonies were monitored through to fledging or failure each year (). Study nests were located close to the middle of colonies to avoid sampling of nests at colony edges, which may disproportionately comprise inexperienced breeders. The distances between the colonies ranged from 10 to 90 km. Arctic Tern foraging ranges are typically <10 km from the colony (Rock et al. Citation2007, Thaxter et al. Citation2012), and so the foraging ranges of individuals from these colonies are not likely to overlap much. Colonies also varied in size, ranging from 50 to 10 000 pairs. Colony size was estimated in orders of magnitude as the approximate maximum number of birds in the air when the birds were flushed from the ground while researchers were in the colony (). The nesting habitat within the colonies typically comprised dry sand and gravel, sometimes with thick grass up to 10–30 cm height.
Table 1. Numbers of Arctic Tern nests monitored in each colony in each year of the study.
Measuring Arctic Tern productivity
Arctic Tern clutch size, hatching success and productivity were measured in study nests in each colony and in each year (). To aid nest and chick relocation, these nests were enclosed within low (30 cm high) chicken-mesh wire fences. In 2008 and 2009, between two and four large (10–20 m2) fences were erected around groups of 5–12 nests in each colony, and in 2010 and 2011 most nests were fenced individually using fences of ca. 1–2 m2. To minimize disturbance and the risks of nest desertion, enclosures were erected after the terns had completed, on average, more than half of the incubation in each colony. Fences were designed to be as unobtrusive as possible, to ensure that predators were not attracted to them or hindered by them, and no predators or predation events were recorded within the fences.
In 2008, clutch size, hatching success and the number of fledglings per pair were measured in four colonies, and for between 11 and 25 nests per colony (). Clutch size was measured for each nest but colony visits in this year were too infrequent to allow hatching and fledging success of individual nests to be measured; thus, only the overall colony averages could be estimated for these metrics in this year.
In 2009–11, 5–10 colonies were studied and an average of 25 nests. sd: 11.03, range: 9–51) were monitored within each colony. Individual fencing of nests and more regular visits allowed the calculation of the following metrics: clutch size (eggs per nest), hatching success (% hatched eggs per nest), number of fledged chicks per egg laid, number of fledged chicks per egg hatched and number of fledged chicks per nest.
Colonies were first visited after the first few days of egg-laying, and egg floatation (Rahn & Paganelli Citation1989) was used to estimate egg incubation stages and predicted hatching dates. Clutch size was determined after the mid-point of the incubation period (ca. 10 days post-egg laying), and nests were revisited at least once before hatching so that any additions to clutches could be detected. Nests were visited every 1–3 days around the predicted hatching date to measure the hatching success of each nest. Arctic Terns are semi-precocial and chicks typically remain in the nest for the first 3 days after hatching. Eggs that were not hatched and were cold by at least 10 days after the predicted hatching date were considered to be unsuccessful. Chicks were ringed and measured just after hatching and the colonies were revisited every 3–5 days in order to relocate chicks. At each recapture, measurements of mass (measured to the nearest 0.1 g with an electronic scale), wing length (measured to the nearest 1 mm with a wing ruler), and the combined head plus bill length (or skull) (measured to the nearest 0.1 mm with digital callipers) were recorded for each chick. Chicks were considered to have fledged successfully if they reached at least 80 g in mass and/or 100 mm wing length and were not relocated on subsequent visits.
Data analysis
Annual and between-colony variation in clutch size, hatching success, number of fledglings per egg laid, fledglings per chicks hatched and fledglings per nest were explored with generalized linear models. Year and colony were defined as fixed factors. The dependent variable was either the number of eggs (clutch size), the number of eggs hatched (hatching success) or the number of chicks fledged (fledging success) per nest and was modelled with a Poisson distribution and a log-link function. For hatching success, the number of eggs in each clutch was included as an offset, and fledging success was modelled with offsets of (a) number of eggs (chicks fledged/egg), (b) number of hatched eggs (chicks fledged/egg hatched) or (c) no offset (chicks fledged per pair). Statistical analyses were conducted in spss 16.0 and minimum models are presented.
RESULTS
General variation in productivity
Between 2008 and 2011, Arctic Tern productivity in colonies across the Snaefellsnes peninsula varied little between years (). Overall, the modal clutch size was 2 and ranged from 1 to 3, with 15% of nests having one egg, 83% having two and 2% having three. Hatching success (chicks hatched per egg laid) was consistently high, with around 80–100% of all eggs hatching in all years (), and 96.6% of two- and three-egg clutches hatched all eggs. However, fledging success was low in all years, ranging from 0.05 to 0.5 chicks per pair, and was lowest in 2008 and highest in 2010 ().
Table 2. Annual variation in components of productivity of Arctic Tern nests on colonies across the Snaefellsnes peninsula, West Iceland.
Between colony-variation in productivity
Arctic Tern clutch sizes did not vary significantly between colonies () and were typically around 1.5–2 eggs per nest on average (). However, there was weak significant annual variation, with clutch sizes being slightly smaller in 2011 than in previous years. There was no significant interaction between colony and year, indicating that clutch sizes in 2011 were generally lower in all colonies.
Table 3. Results of generalized linear models of annual and between-colony variation in five components of productivity of Arctic Terns on Snaefellsnes peninsula, West Iceland, between 2009 and 2011.
Figure 2. Annual and between-colony variation in mean (±se) Arctic Tern (a) clutch size, (b) hatching success, (c) fledged chicks per eggs laid, (d) fledged chicks per chick hatched and (e) fledged chicks per nest. Colony order is presented according to colony size (from smallest (left) to largest (right)). Black dashed lines indicate zero values. See for colony locations and details.
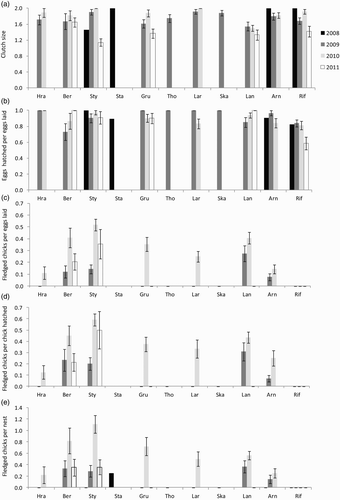
Hatching success was consistently high, with at least 70% of eggs hatching in all colonies and years except for Rif in 2011 (), and there was no significant variation among years or colonies ().
The number of fledglings per egg laid, per hatchling and per nest all varied significantly both between colonies and between years, and the annual variation did not differ significantly between colonies (). Most colonies produced less than half a chick per egg laid () and per egg hatched (), and only one colony in one year reached an average of one fledgling per nest (). Fledging success in most colonies was higher in 2010 than in other years; in 2009, none of the measured hatchlings fledged in 6 out of 10 colonies and in 2011 only 2 out of 5 colonies fledged any of the measured hatchlings ().
Overall, two colonies, Berserkseyri (Ber) and Stykkisholmur (Sty), had higher fledging success across all years (c–e). Both of these colonies are located on the eastern part of the north coast of the peninsula (). Rif, which is located at the western end of the peninsula and is the largest colony, had the poorest success overall, with no monitored nests producing fledglings in any of the 4 years (c–e).
DISCUSSION
The Snaefellsnes area in West Iceland supports tens of thousands of breeding Arctic Terns, in colonies spread all along the coastal fringe of the peninsula. Between 2008 and 2011, the productivity of colonies all around the peninsula was consistently low, with several colonies fledging no chicks from the nests that were monitored, and no colonies exceeding 0.5 chicks/egg laid. Fledging success was slightly higher in 2010 than in the other years but, even in that year, only one colony (Stykkisholmur) had an average productivity exceeding one fledgling per nest. Thus, despite some between-year variation, productivity in all colonies and years was generally lower than the ∼0.7–1.5 chicks per pair that has been considered elsewhere as moderate or good breeding success (Monaghan et al. Citation1989, Suddaby & Ratcliffe Citation1997, Robinson et al. Citation2001). The lowest fledging success was recorded on Rif, in the far west of the peninsula, where no fledgling success was measured in any of the 4 years. In absolute terms, the higher fledging success at smaller colonies is likely to have resulted in approximately half the number chicks fledging (∼0.5 chicks per pair in colonies of ∼500 pairs) than in the larger colonies with lower per capita fledging success (∼0.1 chicks per pair in colonies of ∼5000 pairs, & 2e). Anecdotal information from local landowners suggested that poor productivity in these Arctic Tern colonies had been evident since 2005, and similar low levels of productivity have been reported for Lesser Black-backed Gulls Larus fuscus in Southwest Iceland since 2005 (Hallgrimsson Citation2011). Historically, Rif has been a large colony supporting thousands of pairs, suggesting that conditions at this location must have been sufficiently favourable in the past to attract large numbers of breeding birds and/or to facilitate high levels of productivity and natal recruitment.
Across all colonies in all years, clutch sizes were close to the modal clutch of two and hatching success was usually around 90%. This suggests that conditions for adults were sufficiently good for egg production and incubation to hatch the eggs. The low productivity is primarily a consequence of the post-hatching phase of the breeding season, when conditions for chick rearing appear to be constraining fledging success.
Possible reason for why so few hatchlings fledged could include mortality of chicks caused by predation, unfavourable weather condition or starvation. A wide range of predators occurs on the Snaefellsnes peninsula, including mammalian (Arctic Fox Vulpes lagopus and American Mink Neovison vison) and avian (Herring Gulls Larus argentatus, Lesser Black-backed Gulls and Black-headed Gulls Chroicocephalus ridibundus, Arctic Skua Stercorarius parasiticus, Short-eared Owl Asio flammeus, Gyr Falcon Falco rusticolus, White-tailed Eagle Haliaeetus albicilla, Raven Corvus corax) predators. However, there is no evidence to suggest any significant recent increases in predator numbers or activity and the high hatching success (and the fact that very few unhatched eggs went missing) on all colonies would suggest a limited impact of predator activity at least at the egg stage. Predation of chicks could be implicated in the low productivity, but only one event of predation of a chick (by a black-headed gull) was observed during the four seasons of fieldwork in all colonies. Large numbers of chick carcasses were also retrieved from within these colonies (Vigfusdottir Citation2012), again suggesting a very limited influence of predation on chicks.
Extreme and prolonged unfavourable weather conditions are known to impact ground-nesting, colonial birds with semi-precocial chicks (Robinson et al. Citation2002) and the Snaefellsnes peninsula can experience periods of low temperatures and high winds. Given the consistency of the low productivity on colonies right across the peninsula, weather-induced mortality would likely have to involve large-scale weather events, rather than local variations in temperature and rainfall. Additionally, no extreme weather events or anomalies were apparent during these years, e.g. minimum temperatures recorded at the five weather stations around the Snaefellsnes peninsula (www.vedur.is) were never lower than 2.6–5.9°C during these four breeding seasons.
Starvation is also a known cause of mortality and driver of seabird productivity. Starvation can result from low prey abundance, insufficient prey size and/or low nutritious value of the available resources (Wanless et al. Citation2005). Changes in the spatial or temporal distributions of prey can also influence their availability, resulting in insufficient food during chick rearing (Burthe et al. Citation2012). Over the last decade, population declines and low productivity have been reported for many seabird populations (Gardarsson Citation2006a, Citation2006b, Gardarsson et al. Citation2011), including cliff breeding auks, fulmars and kittiwakes as well as ground-nesting gulls and burrowing puffins. What all of these species have in common is summer diet (Lilliendahl & Solmundsson Citation1998, Thompson et al. Citation1999). On the South and West coasts of Iceland, these seabirds depend primarily on sandeels during the period of chick growth. Sandeel abundance has been monitored in selected locations in South and West Iceland annually since 2006 by the Icelandic Marine Research Institute. These surveys show that sandeel densities were much lower than elsewhere in the North Atlantic (Greenstreet et al. Citation2006) and that annual density estimates declined during the survey period (Bogason & Lilliendahl Citation2008). Additionally, some year classes were absent from the surveys. Declines in sandeel abundance in response to overfishing have been reported in the North Sea, together with implications for the species preying on sandeel (Frederiksen et al. Citation2004), but sandeels are not fished in Icelandic waters. Thus, although there are insufficient data to quantify long-term trends in sandeel abundance in Icelandic waters, there would appear to be a strong possibility that constraints on the availability of sandeels during the breeding season may be implicated in recent, low productivity of seabirds.
During the last 15 years, the waters in the South and West of Iceland have warmed by 1–2°C and many southern commercially fished species have extended their ranges further north, and northern species' ranges have retreated further north (Valdimarsson et al. Citation2012). Range shifts of fish species have already lead to changes such as marked increases in numbers of mackerel in the South and West of Iceland (Astthorsson et al. Citation2012), a species which is known to predate sandeel in the North Sea (Furness Citation2002). Warming sea temperatures in the North Sea have been linked to declines in sandeel recruitment (Arnott & Ruxton Citation2002) and long-term changes in sandeel sizes (Frederiksen et al. Citation2011). Further hydrographic changes with warming sea temperatures have been predicted in the future at high latitudes, including Icelandic waters (IPCC Citation2007). The implications of such events in Iceland would be of international importance for seabirds as Iceland supports such large population sizes of many species. To evaluate the possible impacts of the predicted environmental changes on seabird populations, further research is needed to identify the drivers of demographic changes in seabirds and to quantify the spatial and temporal patterns and magnitude of changes in breeding success.
ACKNOWLEDGEMENTS
We thank the numerous field workers who helped out in the field, in particular: Birgitta Stefansdottir, Cat Morrison, David Showler, Ellen Magnusdottir, Hen Bellman, Harry Charnock, Karen Jordan, Karl Phillips, Livia Benson, Jon Einar Jonsson, Jonas P. Jonasson, Rebecca Laidlaw, Richard Hillard, Rosa Sigurjonsdottir and Steinunn Gudmundsdottir. Local farmers and landowners get special thanks for local information and logistic support. We thank Snaefellsnes Research Centre and Icelandic Institute of Natural History for logistic support. For financial support, we thank the University of East Anglia, British Chevening Scholarship, Palmi Jonsson's Fund for Nature Conservation, Rannis Icelandic Research Fund of Graduate Students, Icelandic Innovation Fund and British Ornithologists' Union.
References
- Aebischer , N. J. 1993 . Immediate and delayed-effects of a gale in late spring on the breeding of the Shag Phalacrocorax aristotelis . Ibis , 135 : 225 – 232 . (doi:10.1111/j.1474-919X.1993.tb02838.x)
- Anker-Nilssen , T. , Barrett , R. T. and Krasnov , J. V. 1997 . Long- and short-term responses of seabirds in the Norwegian and Barents Seas to changes in stocks of prey fish Proceedings Forage Fishes in Marine Ecosystems . 97 : 683 – 698 .
- Arnott , S. A. and Ruxton , G. D. 2002 . Sandeel recruitment in the North Sea: demographic, climatic and trophic effects . Mar. Ecol. Prog. Ser. , 238 : 199 – 210 . (doi:10.3354/meps238199)
- Asbirk , S. , Berg , L. , Hardeng , G. , Koskimies , P. and Petersen , A. 1997 . Population sizes and trends of birds in the Nordic countries: 1978–1994 . TemaNord , 614 : 1 – 88 .
- Astthorsson , O. S. , Gislason , A. and Jonsson , S. 2007 . Climate variability and the Icelandic marine ecosystem . Deep-Sea Res. Pt. II , 54 : 2456 – 2477 . (doi:10.1016/j.dsr2.2007.07.030)
- Astthorsson , O. S. , Valdimarsson , H. , Gudmundsdottir , A. and Oskarsson , G. J. 2012 . Climate-related variations in the occurrence and distribution of mackerel (Scomber scombrus) in Icelandic waters . ICES J. Mar. Sci. , 69 : 1289 – 1297 . (doi:10.1093/icesjms/fss084)
- Barrett , R. T. 2007 . Food web interactions in the southwestern Barents Sea: black-legged kittiwakes Rissa tridactyla respond negatively to an increase in herring Clupea harengus . Mar. Ecol. Prog. Ser. , 349 : 269 – 276 . (doi:10.3354/meps07116)
- Barrett, R., Nilssen, T.A., Bustnes, J.O., Dalsgaard, S.C., Descamps, S., Erikstad, K.E., Lorentsen, S.H., Strøm, H. & Systad, G.H. 2012. Key site monitoring in Norway 2011. SEAPOP Short Report 1–2012. NINA, NP & TMU, Trondheim, Norway.
- Becker , P. H. 1995 . Effects of coloniality on gull predation on Common Tern (Sterna hirundo) chicks . Colon Waterbirds , 18 : 11 – 22 . (doi:10.2307/1521394)
- Bogason , V. and Lilliendahl , K. 2008 . An initiation of sandeel monitoring in Iceland . Hafrannsoknir , 145 : 36 – 41 . (in Icelandic, English summary)
- Burthe , S. , Daunt , F. , Butler , A. , Elston , D. , Frederiksen , M. , Johns , D. , Newell , M. , Thackeray , S. and Wanless , S. 2012 . Phenological trends and trophic mismatch across multiple levels of a North Sea pelagic food web . Mar. Ecol. Prog. Ser. , 454 : 119 – 133 . (doi:10.3354/meps09520)
- Davis , S. E. , Nager , R. G. and Furness , R. W. 2005 . Food availability affects adult survival as well as breeding success of Parasitic Jaegers . Ecology , 86 : 1047 – 1056 . (doi:10.1890/04-0989)
- Dulvy , N. K. , Rogers , S. I. , Jennings , S. , Stelzenmller , V. , Dye , S. R. and Skjoldal , H. R. 2008 . Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas . J. Appl. Ecol. , 45 : 1029 – 1039 . (doi:10.1111/j.1365-2664.2008.01488.x)
- Egevang , C. , Stenhouse , I. J. , Phillips , R. A. , Petersen , A. , Fox , J. W. and Silk , J. R.D. 2010 . Tracking of Arctic Terns Sterna paradisaea reveals longest animal migration . Proc. Natl. Acad. Sci. USA , 107 : 2078 – 2081 . (doi:10.1073/pnas.0909493107)
- Ezard , T. H.G. , Becker , P. H. and Coulson , T. 2006 . The contributions of age and sex to variation in common tern population growth rate . J. Anim. Ecol. , 75 : 1379 – 1386 . (doi:10.1111/j.1365-2656.2006.01162.x)
- Frederiksen , M. 2010 . Appendix 1: seabirds in the North East Atlantic. A review of status, trends and anthropogenic impact . TemaNord , 587 : 47 – 122 .
- Frederiksen , M. , Wanless , S. , Harris , M. P. , Rothery , P. and Wilson , L. J. 2004 . The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes . J. Appl. Ecol. , 41 : 1129 – 1139 . (doi:10.1111/j.0021-8901.2004.00966.x)
- Frederiksen , M. , Edwards , M. , Richardson , A. J. , Halliday , N. C. and Wanless , S. 2006 . From plankton to top predators: bottom-up control of a marine food web across four trophic levels . J. Anim. Ecol. , 75 : 1259 – 1268 . (doi:10.1111/j.1365-2656.2006.01148.x)
- Frederiksen , M. , Daunt , F. , Harris , M. P. and Wanless , S. 2008 . The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird . J. Anim. Ecol. , 77 : 1020 – 1029 . (doi:10.1111/j.1365-2656.2008.01422.x)
- Frederiksen , M. , Elston , D. , Edwards , M. , Mann , A. and Wanless , S. 2011 . Mechanisms of long-term decline in size of lesser sandeels in the North Sea explored using a growth and phenology model . Mar. Ecol. Prog. Ser. , 432 : 137 – 147 . (doi:10.3354/meps09177)
- Furness , R. W. 2002 . Management implications of interactions between fisheries and sandeel-dependent seabirds and seals in the North Sea . ICES J. Mar. Sci. , 59 : 261 – 269 . (doi:10.1006/jmsc.2001.1155)
- Furness , R. W. and Tasker , M. L. 2000 . Seabird-fishery interactions: quantifying the sensitivity of seabirds to reductions in sandeel abundance, and identification of key areas for sensitive seabirds in the North Sea . Mar. Ecol. Prog. Ser. , 202 : 253 – 264 . (doi:10.3354/meps202253)
- Gardarsson , A. 1995 . Numbers and distribution of common Murre Uria aalge, Thick-billed Murre U. lomvia and Razorbill Alca torda in Iceland . Bliki , 16 : 47 – 65 . (in Icelandic, English summary)
- Gardarsson , A. 2006a . Breeding success of Kittiwake in Iceland 2005 . Bliki , 27 : 23 – 26 . (in Icelandic, English summary)
- Gardarsson , A. 2006b . Recent changes in numbers of cliff-breeding seabirds in Iceland . Bliki , 27 : 13 – 22 . (in Icelandic, English summary)
- Gardarsson , A. , Gudmundsson , G. A. and Lilliendahl , K. 2011 . Numbers of Northern Fulmars Fulmarus glacialis in Iceland: notes on early records, and changes between 1983–86 and 2005–09 . Bliki , 31 : 1 – 10 . (in Icelandic, English summary)
- Greenstreet , S. , Armstrong , E. , Mosegaard , H. , Jensen , H. , Gibb , I. , Fraser , H. , Scott , B. , Holland , G. and Sharples , J. 2006 . Variation in the abundance of sandeels Ammodytes marinus off southeast Scotland: an evaluation of area-closure fisheries management and stock abundance assessment methods . ICES J. Mar. Sci. , 63 : 1530 – 1550 . (doi:10.1016/j.icesjms.2006.05.009)
- Gunnarsson , T. G. and Tómasson , G. 2011 . Flexibility in spring arrival of migratory birds at northern latitudes under rapid temperature changes . Bird Study , 58 : 1 – 12 . (doi:10.1080/00063657.2010.526999)
- Hallgrimsson, G.T. 2011. Ecological constraints on two species of large gulls. Unpublished PhD Thesis, University of Iceland, Reykjavik.
- Halpern , B. S. , Walbridge , S. , Selkoe , K. A. , Kappel , C. V. , Micheli , F. , D'Agrosa , C. , Bruno , J. F. , Casey , K. S. , Ebert , C. , Fox , H. E. , Fujita , R. , Heinemann , D. , Lenihan , H. S. , Madin , E. M.P. , Perry , M. T. , Selig , E. R. , Spalding , M. , Steneck , R. and Watson , R. 2008 . A global map of human impact on marine ecosystems . Science , 319 : 948 – 952 . (doi:10.1126/science.1149345)
- Heath, M., Edwards, M., Furness, R., Pinnegar, J. & Wanless, S. 2009. A view from above: changing seas, seabirds and food sources. In Baxter, J.M., Buckley, P.J. & Frost, M.T. (eds) Marine Climate Change Ecosystems Linkages Report Card 2009. Online science reviews.
- ICES. 2009. Report of the Working Group on Seabird Ecology (WGSE), 23–27 March 2009, Bruges, Belgium. ICES CM 2009/LRC:05.
- ICES. 2012a. Report of the North-Western Working Group (NWWG), 26 April–3 May 2012, ICES Headquarters, Copenhagen. ICES CM 2012/ACOM:07.
- ICES. 2012b. Report of the Working Group on Seabird Ecology (WGSE), 1–4 November 2011, Madeira, Portugal. ICES CM 2011/SSGEF:07.
- IPCC . 2007 . “ Climate change 2007: synthesis report ” . In Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Pachauri , R. K. and Reisinger , A. 1 – 104 . Geneva , , Switzerland : IPCC .
- Jennings , G. , McGlashan , D. J. and Furness , R. W. 2012 . Responses to changes in sprat abundance of common tern breeding numbers at 12 colonies in the Firth of Forth, east Scotland . ICES J. Mar. Sci. , 69 : 572 – 577 . (doi:10.1093/icesjms/fss022)
- Jenouvrier , S. , Barbraud , C. , Cazelles , B. and Weimerskirch , H. 2005a . Modelling population dynamics of seabirds: importance of the effects of climate fluctuations on breeding proportions . Oikos , 108 : 511 – 522 . (doi:10.1111/j.0030-1299.2005.13351.x)
- Jenouvrier , S. , Barbraud , C. and Weimerskirch , H. 2005b . Long-term contrasted responses to climate of two Antarctic seabird species . Ecology , 86 : 2889 – 2903 . (doi:10.1890/05-0514)
- Jenouvrier , S. , Barbraud , C. , Weimerskirch , H. and Caswell , H. 2009 . Limitation of population recovery: a stochastic approach to the case of the emperor penguin . Oikos , 118 : 1292 – 1298 . (doi:10.1111/j.1600-0706.2009.17498.x)
- Lilliendahl , K. 2009 . Winter diets of auks in Icelandic coastal waters . Mar. Biol. Res. , 5 : 143 – 154 . (doi:10.1080/17451000802279636)
- Lilliendahl , K. and Solmundsson , J. 1998 . The summer diets of six Icelandic seabird species . Bliki , 19 : 1 – 12 . (in Icelandic, English summary)
- Mavor , R. A. , Heubeck , M. , Schmitt , S. and Parsons , M. 2008 . Seabird Numbers and Breeding Success in Britain and Ireland, 2006 , Peterborough : JNCC .
- Mitchell , P. I. , Newton , S. F. , Ratcliffe , N. and Dunn , T. E. 2004 . Seabird Populations of Britain and Ireland , London : T and A.D. Poyser .
- Monaghan , P. , Uttley , J. D. , Burns , M. D. , Thaine , C. and Blackwood , J. 1989 . The relationship between food supply, reproductive effort and breeding success in Arctic Terns Sterna paradisaea . J. Anim. Ecol. , 58 : 261 – 274 . (doi:10.2307/4999)
- Perry , A. L. , Low , P. J. , Ellis , J. R. and Reynolds , J. D. 2005 . Climate change and distribution shifts in marine fishes . Science , 308 : 1912 – 1915 . (doi:10.1126/science.1111322)
- Rahn , H. and Paganelli , C. V. 1989 . The initial density of avian eggs derived from the tables of Schönwetter . J. Ornithol. , 130 : 207 – 215 . (doi:10.1007/BF01649755)
- Robinson , J. A. , Hamer , K. C. and Chivers , L. S. 2001 . Contrasting brood sizes in Common and Arctic Terns: The roles of food provisioning rates and parental brooding . Condor , 103 : 108 – 117 . (doi:10.1650/0010-5422(2001)103[0108:CBSICA]2.0.CO;2)
- Robinson , J. A. , Hamer , K. C. and Chivers , L. S. 2002 . Developmental plasticity in Arctic Terns Sterna paradisaea and Common Terns S. hirundo in response to a period of extremely bad weather . Ibis , 144 : 344 – 346 . (doi:10.1046/j.1474-919X.2002.00061.x)
- Rock , J. C. , Leonard , M. L. and Boyne , A. B. 2007 . Do co-nesting Arctic and Common Terns partition foraging habitat and chick diets? . Waterbirds , 30 : 579 – 587 . (doi:10.1675/1524-4695(2007)030[0579:DCAACT]2.0.CO;2)
- Rolland , V. , Barbraud , C. and Weimerskirch , H. 2009 . Assessing the impact of fisheries, climate and disease on the dynamics of the Indian yellow-nosed Albatross . Biol. Conserv. , 142 : 1084 – 1095 . (doi:10.1016/j.biocon.2008.12.030)
- Sandvik , H. , Erikstad , K. E. , Barrett , R. T. and Yoccoz , N. G. 2005 . The effect of climate on adult survival in five species of North Atlantic seabirds . J. Anim. Ecol. , 74 : 817 – 831 . (doi:10.1111/j.1365-2656.2005.00981.x)
- Stahl , J. T. and Oli , M. K. 2006 . Relative importance of avian life-history variables to population growth rate . Ecol. Model. , 198 : 23 – 39 . (doi:10.1016/j.ecolmodel.2006.04.001)
- Suddaby , D. and Ratcliffe , N. 1997 . The effects of fluctuating food availability on breeding Arctic terns (Sterna paradisaea) . Auk , 114 : 524 – 530 . (doi:10.2307/4089260)
- Thaxter , C. B. , Lascelles , B. , Sugar , K. , Cook , A. S.C.P. , Roos , S. , Bolton , M. , Langston , R. H.W. and Burton , N. H.K. 2012 . Seabird foraging ranges as a preliminary tool for identifying candidate Marine protected areas . Biol Conserv. , 156 : 53 – 61 . (doi:10.1016/j.biocon.2011.12.009)
- Thompson , K. R. and Furness , R. W. 1991 . The influence of rainfall and nest-site quality on the population-dynamics of the Manx Shearwater Puffinus puffinus on Rhum . J. Zool. , 225 : 427 – 437 . (doi:10.1111/j.1469-7998.1991.tb03826.x)
- Thompson , P. M. and Ollason , J. C. 2001 . Lagged effects of ocean climate change on fulmar population dynamics . Nature , 413 : 417 – 420 . (doi:10.1038/35096558)
- Thompson , D. R. , Lilliendahl , K. , Solmundsson , J. , Furness , R. W. , Waldron , S. and Phillips , R. A. 1999 . Trophic relationships among six species of Icelandic seabirds as determined through stable isotope analysis . Condor , 101 : 898 – 903 . (doi:10.2307/1370085)
- Valdimarsson , H. , Astthorsson , O. S. and Palsson , J. 2012 . Hydrographic variability in Icelandic waters during recent decades and related changes in distribution of some fish species . ICES J. Mar. Sci. , 69 : 816 – 825 . (doi:10.1093/icesjms/fss027)
- Vigfusdottir, F. 2012. Drivers of productivity in a subarctic seabird: Arctic Terns in Iceland. Unpublished PhD Thesis, University of East Anglia, Norwich, UK.
- Wanless , S. , Harris , M. P. , Redman , P. and Speakman , J. R. 2005 . Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea . Mar. Ecol. Prog. Ser. , 294 : 1 – 8 . (doi:10.3354/meps294001)