Abstract
Capsule Across the European wintering range Bar-tailed Godwits Limosa lapponica lapponica selected polychaete worms and especially Ragworms Hediste diversicolor, with differences between areas due to variations in prey availability.
Aims To determine the diet of Bar-tailed Godwits across their wintering range in Europe by the analysis of droppings, collected at five important wintering sites.
Methods Diet was estimated by the identification of undigested prey remains in droppings. We provide the rationale for quantifying the contributions of jawed and non-jawed polychaetes.
Results We identified 18 different prey species in the diet of wintering Bar-tailed Godwits. The Ragworm was the most common prey item and the only one actively selected. Ragworms, on average, contributed 79% to the diet in terms of biomass, followed by King Ragworm Alitta virens (with 17% biomass) and Lugworms Arenicola marina (with 2%). Polychaetes such as Alitta succinea and Scoloplos armiger were also regularly found in the diet. Bivalves, snails and crustaceans contributed less than 1% to the diet.
Conclusion This study highlights and confirms the importance of polychaete worms in the diet of European-wintering Bar-tailed Godwits.
Classical foraging theory predicts that animals select their food rationally, i.e. in such ways that maximum fitness gains are achieved (Stephens & Krebs Citation1986). Such ‘optimal’ foraging decisions vary with ecological context, and their rationale underlies relationships between population level processes and changes in food quality and abundance (e.g. Goss-Custard Citation1977, van Gils et al. Citation2006, Piersma Citation2012). However, any understanding of the relevant food-predator relationships starts off with solid descriptions of diet (e.g. Dekinga & Piersma Citation1993, Moreira Citation1994a, Quaintenne et al. Citation2010). Diets can be reconstructed in direct and indirect ways. Direct methods are: (1) examining the digestive tracts of the birds, (2) taking regurgitation samples, or (3) the lavage method (Verkuil Citation1996). All these methods have limitations, as the birds have to be caught and sometimes euthanized (e.g. Barrett et al. Citation2007). Direct visual observations of foraging birds often yield large amounts of unidentified prey (e.g. Scheiffarth Citation2001), so the alternative is to study the diet based on indirect methods, such as pellet- and dropping analysis (e.g. Alerstam et al. Citation1992, Sanchez et al. Citation2005). Hard parts from prey, such as jaws and chaetae of worms, or hinges of bivalves are indigestible and often remain in birds droppings, which can be used to reconstruct the diet. The advantages of these methods are that they are non-invasive and simple to perform (e.g. Alerstam et al. Citation1992, Dekinga & Piersma Citation1993).
Here we provide a study of the diet of a shorebird species that forages on prey that are difficult to assess: small fragile polychaete worms and similar invertebrates. Shorebirds are gregarious and occur in vast and open landscapes outside the breeding season and have been the focus of a large body of feeding ecological work (e.g. Zwarts & Wanink Citation1991, van de Kam et al. Citation2004, Piersma & van Gils Citation2011, Piersma Citation2012). Although the molluscivore shorebirds in the Wadden Sea have recently shown steady declines, the wintering population of Bar-tailed Godwits Limosa lapponica lapponica has seen an increase (Ens et al. Citation2009). During the non-breeding season in the German Wadden Sea the diet (i.e. prey items) consisted of 99% polychaetes (Scheiffarth Citation2001), whereas in Spain the diet (i.e. prey items) consisted of 83% polychaetes (Perez-Hurtado et al. Citation1997). Extending these studies, we here examine the diet of the lapponica subspecies throughout most of its coastal wintering range in northwest Europe (Scott & Scheiffarth Citation2009). Our diet assessments were based on the analysis of droppings collected on intertidal foraging areas.
METHODS
Study sites
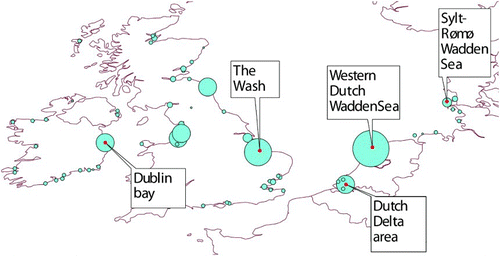
Droppings were collected in intertidal foraging sites at five important wintering areas during the non-breeding season 2010-2011 (). In order to minimize seasonal and year-to-year variations in prey abundance, availability and quality (Zwarts & Wanink Citation1993), as well as in the preferences of birds, because of changes in state (e.g. Piersma Citation2012), we carried out all fieldwork in the briefest possible time period within a single winter season. The German Wadden Sea island of Sylt (55°01'N, 8°26'E) was visited mid October 2010, the Dutch Delta area (51°40'N, 04°07'E) late October 2010, the Wash in the UK (52°56'N, 00°19'E) early November 2010, and Dublin Bay in Ireland (53°19'N, 06°11'W) was visited late November 2010. Finally the Dutch Wadden Sea island of Griend (53°14'N, 05°15'E) was visited early February 2011.
Figure 1. Location of the study sites, with the mean January numbers of Bar-tailed Godwits (1995 – 2005) counted at high tide roosts, based on the Wetlands International midwinter count database. The size of the shaded circles reflects the mean winter abundance of Bar-tailed Godwits: the Western Dutch Wadden Sea had the highest average number with almost 40 000 individuals, followed by the Wash with an average of 14 000 individuals.
Benthic sampling
At locations where we had been observing foraging flocks of Bar-tailed Godwits for over 30 min, 10 random benthic samples (1 / 56 m−2) were taken to a depth of 25–30 cm and sieved through a 1 mm mesh. As the duration of food processing would be ca. 25 min (Scheiffarth Citation2001, pers. obs.), waiting at least 30 min before assigning a spot as the one to sample for benthos, ensured that we collected droppings and food abundance data based on ingestions on that location. All potential prey items were counted per species and stored in 4% formaldehyde saline solution for later analyses in the laboratory, where size classes (lengths) were measured to the nearest mm. To determine the AFDM (g) and shell mass of prey, the fleshy parts were removed from the shell and both shell and flesh were dried to constant mass in a ventilated oven at 55–60°C. Dry mass of both shell and flesh were determined. The dried flesh of all species was incinerated at 560°C for 5 h, after which the remaining ash-mass was subtracted from the dry mass to determine the AFDM.
Dropping analyses
Fifty individual droppings were collected at each site (except in the Sylt-Rømø Wadden Sea area where we collected 40 droppings). At each site we observed the birds at the high tide roost and followed them with the retreating tide. This ensured that we collected fresh droppings and only from specific foraging areas where we observed the birds.
Collectively, droppings were stored frozen at -18oC. Before the analysis, the samples were thawed for at least 60 min and cleaned by using an ultrasonic cleaner (Branson 5510) and consequently sieved over an 80-µm mesh sieve. The samples were initially sorted by using a 40x magnification stereo microscope (Olympus SZ51). All unique parts of prey remains that were visible in the droppings (i.e. hinges and jaws) were taken out and identified to species level whenever possible (). Following this, a 10% sub-sample was taken and re-sorted for polychaete chaetae only. All chaetae were identified whenever possible and counted. Identification referred to the NIOZ reference collections and literature (e.g. Hartmann-Schröder Citation1971).
Table 1. Remains of different prey items found in the droppings used for identification and how to calculate the number of individual prey from these remains.
Estimating the number of prey items
Bar-tailed Godwits were never seen regurgitating pellets, nor were pellets found at feeding or roosting sites, and we therefore considered the prey remains in the droppings to provide a complete and unbiased picture of the diet (see Dekinga & Piersma Citation1993). The occurrence of the different prey items could be calculated from the number of hinges, claw, body whorl, jaws, chaetae, and paleae (). The number of bivalves was calculated as the number of hinges divided by two. Worms of the Nereididae family have paired jaws (Bakken et al. Citation2009), but instead of calculating the number of individuals by dividing the number of jaws by two, we estimated the number of Nereididae by matching left and right jaws, based on the number and position of the teeth on each jaw, considering the maximum size difference of the 5% between the right and left jaws of the Ragworm Hediste diversicolor (Lourenço Citation2007) and the variation in the teeth of its jaw (Hefferan Citation1900). The number of Bloodworms Glycera alba per sample was calculated by dividing the number of jaws by four, since G. alba has four jaws (Hartmann-Schröder Citation1971). The number of individuals of different Nereididae species (Common Clam Worm Alitta succinea, King Ragworm Alitta virens, Clam Worm Eunereis longissima and H. diversicolor) could also be calculated from number of chaetae found in the droppings. The mean number of chaetae per setiger of H. diversicolor is 28.2 (Moreira Citation1995) and the mean setiger per individual is 105 (Chambers & Garwood Citation1992), resulting in a mean number of 2961 chaetae per individual. No references for the number of chaetae of A. succinea, A. virens and E. longissima was found, therefore it was assumed they would have similar numbers of chaetae per setiger as H. diversicolor since they belong to the same subfamily. A. succinea has 160 segments and A. virens 200 segments (Hartmann-Schröder Citation1971) resulting in 4512 and 5640 chaetae per species respectively. The number of occurrence per prey was based on the maximum number of predicted individuals, based on either jaws or chaetae.
For the non-jawed polychaetes, the occurrence of prey in the droppings could only be calculated from number of chaetae, which were identified and counted for 7 polychaeta species. Phyllodoce mucosa has a mean number of 76.5 setiger and each setiger bears six pairs of chaetae (Tzetlin Citation1998), resulting in 918 chaetae per individual. For the Lugworm Arenicola marina and Sand Mason Worm Lanice conchilega no literature values were found. As these two species may contribute a large proportion of the diet (Scheiffarth Citation2001), 25 adult A. marina and 25 adult of Bar-tailed Godwits L. conchilega were collected in the Dutch Wadden Sea and dissected. All chaetae were counted and averaged per individual. This resulted in 1138 and 802 chaetae per species respectively. The equation provided by Scheiffarth (Citation2001) was used to calculate the number of chaetae of Scoloplos armiger from its thoracic hooks, resulting in 1698 chaetae per individual. No information was available concerning the number of paleae of the Ross' Worm Sabellaria spinulosa and therefore, we assumed the number of paleae to follow a similar species, the Honeycomb Worm Sabellaria alveolata with 50 inner paleae, 24 middle paleae, and 28 outer paleae (Ebling Citation1945), consequently summed as 102 paleae per individual.
The remains of three other prey were found in the droppings (unidentified crabs Carcinus spp, the Blue Mussel Mytilus edulis, and Sea Urchin Echinocardium cordatum), but were so rare that we could conveniently exclude them from further analysis. In fact, the spines of E. cordatum may have been collected inadvertently when droppings were collected (Ruiters Citation1992).
Estimating prey sizes
Prey sizes can be estimated from indigestible parts if these correlate with the size of the individual prey eaten (e.g. Zwarts & Esselink Citation1989). Shell length of bivalves was calculated from hinge (and hinge + top) height (Dekinga & Piersma Citation1993). Jaw length was measured in two ways: (1) from the tip of the proximal tooth to the distal end of the jaw, and (2) from the jaw base to the distal end of the jaw. Method 1 was only used (R2 = 0.31), when method 2 (R2 = 0.45; ) could not be used, due to a broken base or tip. When jaws were paired, the mean of the jaw length was used and when no jaws were present, all chaetae in the droppings were counted and from a subsample the length was measured from the tip to the base of the chaetae. The benthic samples collected per area were used as references, with additional references that were collected in the Dutch Wadden Sea. All chaetae and jaws were measured to the nearest 0.1mm, under an inverted microscope (Zeiss Axiovert 200), equipped with digital camera and imaging processing software.
Table 2. Relationships between measurable parts in droppings and prey size.
Prey selection and prey-size selection
Prey selection was determined by means of the Jacobs (s)electivity index (Jacobs Citation1974):where r is the fraction of a prey item in the diet and p is the fraction of a prey item in the habitat. The index J ranges from +1 (complete preference) to –1 (complete avoidance), and the value of 0 indicates that the particular habitat's component was used in proportion to its availability in the study area.
From dietary items to biomass composition
To estimate the energy content of the different prey, the biomass was calculated by using the regression equations (). Whenever no measurements could be taken of an indigestible part of the prey species, the mean AFDM per species was taken from the NIOZ gridded sampling effort (Synoptic Intertidal Benthic Survey, SIBES), which encompasses the entire intertidal Dutch Wadden Sea, consisting out of more than 4500 benthos samples (Compton et al. Citation2013). The AFDM equations and mean AFDM used in calculating biomass per prey species are listed in .
Table 3. Overview of equations and mean AFDM (g) used in calculating prey biomass.
The total biomass of prey consumed by Bar-tailed Godwits per area was calculated as the sum of biomass per species occurring in the droppings. The proportions of prey biomass per area were calculated to determine the importance of prey in diet of Bar-tailed Godwits.
RESULTS
In total we identified 18 different prey species in the diet of wintering Bar-tailed Godwits, at the five study sites (). Hediste diversicolor was the most common prey in the diet at four wintering sites: the Dutch Wadden Sea area, Sylt-Rømø Wadden Sea area, the Wash, and the Dutch delta area, where it represented 80, 41, 91 and 74% of the total number of prey, respectively. In Dublin Bay, H. diversicolor was the second-most common prey, with bloodworms Glycera alba (38%) being the most frequent prey found. Even though they were present in smaller proportions, other polychaetes such as Alitta succinea, Arenicola marina, and Scoloplos armiger were also regularly found in the droppings.
Table 4. Diet composition of Bar-tailed Godwits in the 5 wintering areas, based on frequency of occurrence.
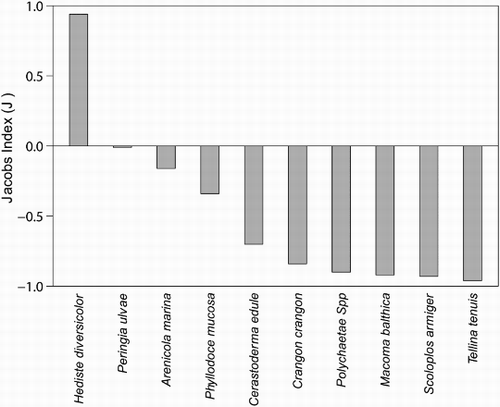
Compared with the relative abundance, Hediste diversicolor was the only prey actively selected by Bar-tailed Godwits (). The composition of the diet indicates a negative selection for other polychaetes and bivalves such as the Thin Tellin Angulus tenuis and the Baltic Tellin Macoma balthica (). However, when categorizing the diet into four groups (i.e. bivalves, crustaceans, snails and polychaetes), the Jacobs' selectivity index indicates a positive selection for polychaetes. Small snails were positively selected in the Dutch Delta area and in the Sylt-Rømø Wadden Sea area.
Figure 2. Bar-tailed Godwits prey preferences determined with Jacobs' index (J) calculated from five wintering sites at different prey densities. When the index is positive, the prey species is preferred.
H. diversicolor was not only the most preferred prey item, but it was also the most important prey item in terms of biomass (AFDM content; i.e. 79% ). Next were A. virens and A. marina, with 17 and 2% of the biomass respectively, as the most important species, while the other prey combined only contributed 2% of energy intake. Although other prey items were taken regularly, 99.6% of the diet's biomass consisted out of polychaetes and remained the most preferred and important prey. In Dublin bay, a relative high proportion of biomass consisted out of bivalves (i.e. the Thin Tellin), although they were not preferred ().
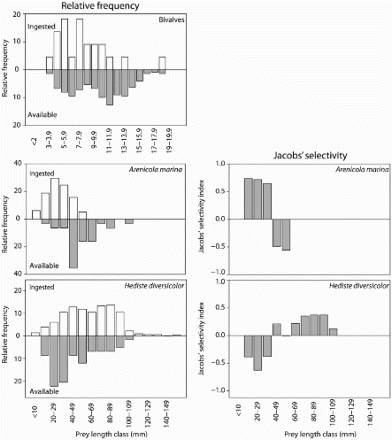
We aggregated the size distribution of all bivalve species of all areas, both in the droppings and in the sediment, due to low sample size of bivalve prey occurring in the droppings (n = 19). The available size distribution for bivalves varied between 3 to 19 mm of shell length (n = 222), however, only the smaller bivalves were consumed (mean size ± se 8.2 ± 0.8 mm; range 4–11 mm; ). Based on Jacobs' selectivity, we compared the size of the Lugworm and Ragworm between the sediment and their diet. Bar-tailed Godwits showed positive selection of Ragworm with length between 40 to 110 mm, while they positively selected Lugworms shorter than 40 mm. The mean lengths of the Ragworms and Lugworms eaten by Bar-tailed Godwits were 62.5 ± 1.1 mm and 29.4 ± 0.7 mm, respectively ().
Figure 3. The relative size distributions of three bivalve species (Macoma balthica, Cerastoderma edule and Angulus tenuis), the polychaete Arenicola marina and Hediste diversicolor ingested by Bar-tailed Godwits at the five study areas (upward histograms), in comparison with the size distributions of those prey species present on the feeding sites (downward histograms) are shown in the left panels. The right panels represent the prey preferences of A. marina and H. diversicolor, as determined with Jacobs' index (J) based on the five wintering sites. When the index is positive, the prey species is preferred.
DISCUSSION
With at least 18 prey species contributing to the diet of Bar-tailed Godwits across the European wintering range, the diet showed high diversity compared with the recorded diets of molluscivores such as Red Knots (e.g. Dekinga & Piersma Citation1993, Moreira Citation1994b, Piersma et al. Citation1994) and European Oystercatchers (e.g., Goss-Custard et al. Citation1977a, Durell et al. Citation1993). Confirming previous studies (e.g. Goss-Custard et al. Citation1977b, Perez-Hurtado et al. Citation1997, Scheiffarth Citation2001), Bar-tailed Godwits selected polychaetes. Not surprisingly, the diet varied between wintering sites. In Dublin Bay for example, the bivalve Abra tenuis occurred frequently in their diet, which would be explained by these bivalves being the most abundant benthic species in the core samples. The preference for snails in two areas (i.e. the Dutch Delta area and the Sylt-Rømø Wadden Sea area), is likely caused by benthic sampling design. Mudsnails Peringia ulvae have a very patchy occurrence (e.g. Bocher et al. Citation2007), and thus easily missed in 10 benthic cores. As they only contributed a small fraction of the total consumed biomass, this issue seems a minor one.
There was a positive selection for the smaller Arenicola marina (< 4 cm) which is perhaps surprising. This might be due to their burying depth. Benthic prey are buried deeper in winter than in summer, as is also the case for A. marina (Zwarts & Wanink Citation1993), and is also related to the length of the individual. A. marina exceeding 4 cm in length, are buried 17 cm and deeper (Zwarts & Wanink Citation1993), mostly out of the bill length of (female) Bar-tailed Godwits (i.e. mean bill length females 9.9 cm; Prater et al. Citation1977), whereas the smaller individuals are closer to the surface. Additionally, Bar-tailed Godwits rely on cast formation by Lugworms to detect them, and at ambient temperatures below 3oC, casts are not produced (Smith Citation1975). Therefore, the low ambient temperatures may explain a shift to H. diversicolor.
The preference for the larger H. diversicolor can be expected for birds wintering in temperate climates because these polychaetes, even in winter, never really bury much beyond the length of the bill (Esselink & Zwarts Citation1989). The largest, and most profitable (Zwarts & Esselink Citation1989) individual Ragworms (13 cm), have a maximum burying depth of approximately 16 cm in winter, which will put them out of reach even for females. That H. diversicolor is such an important prey species throughout their wintering range concurs with their widespread distribution, e.g. occurring in 30% of all sampled locations (n > 4257) in the entire Dutch Wadden Sea (Compton et al. Citation2013). Furthermore, of all polychaetes, Ragworms contributed the third highest biomass contribution across the Wadden Sea in three years (Compton et al. Citation2013).
This study brings together information about the Bar-tailed Godwit's diet in different wintering areas across Europe. The scale of the comparison, at least with respect to shorebird studies (but see Quaintenne et al. Citation2010, Alves et al. Citation2013), is quite novel. Although some variation in diet composition was found, it is apparent that across the wintering range Bar-tailed Godwits rely on polychaete worms.
ACKNOWLEDGEMENTS
This study depended on colleagues throughout Europe providing us with valuable information on where to find the birds in various places. We thank Karsten Laursen for information about the situation in Denmark, and Gregor Scheiffarth, Gundolf Reichert and Klaus Günther for Germany. Lesley Lewis, the late Oscar Merne and Stewart Holohan helped us in Ireland, whereas Jennifer Gill and Simon Delany helped us to make the most of the UK tour. Rob Dekker and Mardik Leopold are acknowledged for their help in identifying species. Bernard Spaans, Job ten Horn and Maarten Brugge assisted with the benthic sampling. We thank Piet van den Hout and Allert Bijleveld for their help in identifying and measuring bivalve hinges and Tanya Compton for helping accessing the SIBES data. We thank Simon Delany from Wetlands International for providing midwinter high tide count data. We also thank Anneke Bol, Pieternella Luttikhuizen and Judith van Bleijswijk for trying to make the sexing of droppings work. Finally, we thank Will Cresswell and two anonymous reviewers for their comments on earlier versions of this paper. This study was supported by operational grants from NIOZ to TP.
REFERENCES
- Alerstam, T., Gudmundsson, G.A. & Johannesson, K. 1992. Resources for long distance migration - Intertidal exploitation of Littorina and Mytilus by Knots Calidris canutus in Iceland. Oikos, 65: 179–189. doi: 10.2307/3545008
- Alves, J.A., Gunnarsson, T.G., Potts, P.M., Sutherland, W.J. & Gill, J.A. 2013. Sex-biases in distribution and resource use at different spatial scales in a migratory shorebird. Ecol. Evol., 3: 1079–1090. doi: 10.1002/ece3.503
- Bakken, T., Glasby, C.J. & Wilson, R.S. 2009. A review of paragnath morphology in Nereididae (Polychaeta). Zoosymposia, 2: 305–316.
- Barrett, R.T., Camphuysen, K., Anker-Nilssen, T., Chardine, J.W., Furness, R.W., Garthe, S., Huppop, O., Leopold, M.F., Montevecchi, W.A. & Veit, R.R. 2007. Diet studies of seabirds: a review and recommendations. ICES J. Mar. Sci., 64: 1675–1691. doi: 10.1093/icesjms/fsm152
- Bocher, P., Piersma, T., Dekinga, A., Kraan, C., Yates, M.G., Guyot, T., Folmer, E.O. & Radenac, G. 2007. Site- and species-specific distribution patterns of molluscs at five intertidal soft-sediment areas in Northwest Europe during a single winter. Mar. Biol., 151: 577–594. doi: 10.1007/s00227-006-0500-4
- Chambers, S. & Garwood, P. 1992. Polychaetes from Scottish waters, a guide to identification: 3. Family Nereidae. NMSE - Publishing Ltd., Edinburgh.
- Compton, T.J., Holthuijsen, S., Koolhaas, A., Dekinga, A., ten Horn, J., Smith, J., Galama, Y., Brugge, M., van der Wal, D., van der Meer, J., van der Veer, H.W. & Piersma, T. 2013. Distinctly variable mudscapes: Distribution gradients of intertidal macrofauna across the Dutch Wadden Sea. J. Sea Res., 83: 103–116. doi: 10.1016/j.seares.2013.02.002
- Dekinga, A. & Piersma, T. 1993. Reconstructing diet composition on the basis of faeces in a mollusc-eating wader, the Knot Calidris canutus. Bird Study, 40: 144–156. doi: 10.1080/00063659309477140
- Durell, S.E. A. Le V. Dit, Goss-Custard, J.D. & Caldow, R.W.G. 1993. Sex-related differences in diet and feeding method in the Oystercatcher Haematopus ostralegus. J. Anim. Ecol., 62: 205–215. doi: 10.2307/5495
- Ebling, F.J. 1945. Formation and nature of the opercular chaetae of Sabellaria alveolata. Q. J. Microsc. Sci., 2: 153–176.
- Ens, B.J., van Winden, E.A.J., van Turnhout, C.A.M., van Roomen, M.W.J., Smit, C.J. & Jansen, J.M. 2009. Changes in the abundance of intertidal birds in the Dutch Wadden Sea in 1990–2008: differences between East and West [In Dutch with English summary]. Limosa, 82: 100–112.
- Esselink, P. & Zwarts, L. 1989. Seasonal trend in burrow depth and tidal variation in feeding activity of Nereis diversicolor. Mar. Ecol.-Prog. Ser., 56: 243–254. doi: 10.3354/meps056243
- Goss-Custard, J.D. 1977. Ecology of wash III. Density-related behavior and possible effects of a loss of feeding grounds on wading birds (Charadrii). J. Appl. Ecol., 14: 721–739. doi: 10.2307/2402805
- Goss-Custard, J.D., Jones, R.E. & Newbery, P.E. 1977a. The ecology of the Wash. I. Distribution and diet of wading birds (Charadrii). J. Appl. Ecol., 14: 681–700. doi: 10.2307/2402803
- Goss-Custard, J.D., Jenyon, R.A., Jones, R.E., Newbery, P.E. & Williams, R. Le B. 1977b. The ecology of the Wash. II. Seasonal variation in the feeding conditions of wading birds (Charadrii). J. Appl. Ecol., 14: 701–719. doi: 10.2307/2402804
- Hartmann-Schröder, G. 1971. Annelida, Borstenwürmer, Polychaeta. Gustav Fischer Verlag, Jena.
- Hefferan, M. 1900. Variation in the teeth of Nereis. Biological Bulletin, 2: 129–143. doi: 10.2307/1535673
- Jacobs, J. 1974. Quantitative measurement of food selection - A modification of forage ratio and Ivlev's electivity index. Oecologia, 14: 413–417. doi: 10.1007/BF00384581
- Lourenço, P.M. 2007. Analysing faecal samples of Ragworm predators: not just a matter of counting mandibles. Ardea, 95: 151–155. doi: 10.5253/078.095.0117
- Moreira, F. 1994a. Diet, prey-size selection and intake rates of Black-tailed Godwits Limosa limosa feeding on mudflats. Ibis, 136: 349–355. doi: 10.1111/j.1474-919X.1994.tb01106.x
- Moreira, F. 1994b. Diet and feeding rates of Knots Calidris canutus in the Tagus estuary (Portugal). Ardea, 82: 133–135.
- Moreira, F. 1995. The winter feeding ecology of Avocets Recurvirostra avosetta on emerged intertidal areas 2. Diet and feeding mechanisms. Ibis, 137: 99–108. doi: 10.1111/j.1474-919X.1995.tb03225.x
- Perez-Hurtado, A., Goss-Custard, J.D. & Garcia, F. 1997. The diet of wintering waders in Cadiz Bay, southwest Spain. Bird Study, 44: 45–52. doi: 10.1080/00063659709461037
- Piersma, T. 2012. What is habitat quality? Dissecting a research portfolio on shorebirds. In Fuller, R.J. (ed.) Birds and habitat: relationships in changing landscapes series: ecological reviews: 383–407. Cambridge University Press, Cambridge.
- Piersma, T. & van Gils, J.A. 2011. The flexible phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford University Press, Oxford.
- Piersma, T., Verkuil, Y. & Tulp, I. 1994. Resources for long-distance migration of Knots Calidris canutus islandica and C. c. canutus: How broad is the temporal exploitation window of benthic prey in the western and eastern Wadden Sea? Oikos, 71: 393–407. doi: 10.2307/3545827
- Prater, A.J., Marchant, J.H. & Vuorinen, J. 1977. Guide to the identification and ageing of holarctic waders. BTO, Thetford.
- Quaintenne, G., van Gils, J.A., Bocher, P., Dekinga, A. & Piersma, T. 2010. Diet selection in a molluscivore shorebird across Western Europe: does it show short- or long-term intake rate-maximization? J. Anim. Ecol., 79: 53–62. doi: 10.1111/j.1365-2656.2009.01608.x
- Ruiters, P.S. 1992. Relaties tussen verspreiding en dieetkeuzes van steltlopers en het voorkomen van macrozoöbenthos in de Westerschelde, Report 1992–04. NIOO-CEMO, Yerseke.
- Sanchez, M.I., Green, A.J. & Castellanos, E.M. 2005. Seasonal variation in the diet of Redshank Tringa totanus in the Odiel Marshes, southwest Spain: a comparison of faecal and pellet analysis. Bird Study, 52: 210–216. doi: 10.1080/00063650509461393
- Scheiffarth, G. 2001. The diet of Bar-tailed Godwits Limosa lapponica in the Wadden Sea: Combining visual observations and faeces analyses. Ardea, 89: 481–494.
- Scott, D. & Scheiffarth, G. 2009. Bar-tailed Godwit. In Delany, S., Scott, D., Dodman, T. & Stroud, D. (eds.), An atlas of wader populations in Africa and Western Eurasia: 291–297. Wetlands International, Wageningen.
- Smith, P.C. 1975. A study of the winter feeding ecology and behaviour of the Bar-tailed Godwit (Limosa lapponica). PhD thesis, University of Durham, UK.
- Stephens, D.W. & Krebs, J.R. 1986. Foraging theory. Princeton University Press, Princeton, New Jersey.
- Tzetlin, A.B. 1998. Giant pelagic larvae of Phyllodocidae (Polychaeta, Annelida). J. Morphol., 238: 93–107. doi: 10.1002/(SICI)1097-4687(199810)238:1<93::AID-JMOR8>3.0.CO;2-O
- van de Kam, J., Ens, B., Piersma, T. & Zwarts, L. 2004. Shorebirds: An illustrated behavioural ecology. KNNV Publishers, Utrecht.
- van Gils, J.A., Piersma, T., Dekinga, A., Spaans, B. & Kraan, C. 2006. Shellfish dredging pushes a flexible avian top predator out of a marine protected area. PLoS Biol., 4: 2399–2404. doi: 10.1371/journal.pbio.0040376
- Verkuil, Y. 1996. Stomach-pumping of waders does not necessarily provide more information on diet than faecal analysis. Wader Study Group Bull., 79: 60–63.
- Zwarts, L. & Esselink, P. 1989. Versatility of male Curlews Numenius arquata preying upon Nereis diversicolor: deploying contrasting capture modes dependent on prey availability. Mar. Ecol.-Prog. Ser., 56: 255–269. doi: 10.3354/meps056255
- Zwarts, L. & Wanink, J.H. 1991. The macrobenthos fraction accessible to waders may represent marginal prey. Oecologia, 87: 581–587. doi: 10.1007/BF00320424
- Zwarts, L. & Wanink, J.H. 1993. How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body weight, biomass, burying depth and behavior of tidal-flat invertebrates. Neth. J. Sea Res., 31: 441–476. doi: 10.1016/0077-7579(93)90059-2