Abstract
Capsule We found significant differences in the composition and diversity of diet among House Martins, Barn Swallows and Swifts breeding in the same village in Poland.
Aims To evaluate the character and extent of diversity, specialization and overlap of diet between trophically similar nestlings of three species of aerial feeding birds breeding at the same location and differing considerably in foraging height.
Methods Diet was determined based on faecal analysis. Differences in composition and diversity of diet and food niche overlap were assessed through multivariate analysis of variance (manova), Shannon Diversity Index and the Pianka index. Diet specialization was measured by application of the Berger–Parker index of dominance.
Results manova indicated significant differences in diet composition among all three species. House Martins showed the most diverse diet, Swallows were intermediate and Swifts least diverse. Average body mass of all prey found in the diet of Swifts was nearly three times smaller than in Swallows and two times smaller than in House Martins.
Conclusion Our findings show that these three species consume the same types of insect prey, but they take different proportions, and hence biomass, of the major prey groups. House Martins had the widest niche and greatest overlap.
Studies on diet and niche overlap have critical importance for understanding the co-occurrence of species with similar morphological and ecological features (Gonzalez-Solis et al. Citation1997, Garcia & Arroyo Citation2005, Vieira & Port Citation2007). A high degree of overlap does not necessarily mean that interspecific competition is taking place (Jones & Barmuta Citation1998, Whitfield et al. Citation2013), especially if resources are abundant. However, if resources are limited, overlap between co-existing species is expected to increase (Gonzalez-Solis et al. Citation1997). Moreover, Ricklefs (Citation1979) asserts that increased ecological overlap allows more species to occupy an area, which increases species diversity.
The ecological and ornithological literature is replete with analyses of dietary breadth and overlap between species pairs or triads (Azevedo et al. Citation2006), explaining dietary differences in the context of alternative feeding strategies (i.e. generalist vs. specialist) or intrinsic niche differentiation (i.e. disparity in habitat requirements or body size; Waugh Citation1978, Turner Citation1982a,Citationb, Yalden Citation1985, Dyrcz & Flinks Citation2003, Garcia & Arroyo Citation2005). Several such studies have been conducted on swifts and hirundines. The food resources of these aerial feeding birds are variable in distribution and abundance and are dependent mainly on weather conditions, phenology or habitat diversity (Dąbrowska-Prot & Karg Citation1976, Ryszkowski & Karg Citation1977, Karg Citation1989, Grüebler et al. Citation2008). In general, these aerial insectivores are recognized as opportunistic feeders with a broad trophic niche based on various prey types that are only temporarily available (Bryant Citation1973, Citation1975, Waugh Citation1978, Turner Citation1982a,Citationb, Cucco et al. Citation1993).
Three species of aerial feeding birds occur broadly across the northern part of the western Palearctic almost exclusively within human settlements and urbanized areas, namely Common Swift Apus apus (henceforth Swift), Barn Swallow Hirundo rustica (henceforth Swallow) and House Martin Delichon urbicum (Møller Citation1983, Cramp Citation1998, Turner Citation2006). Results of dietary studies have shown that these and other aerial foraging insectivores partition food partly as a result of different foraging heights and prey size (Bryant Citation1973, Waugh Citation1978, Bryant & Turner Citation1982). According to Bryant and Turner (Citation1982), for example, Swallows, House Martins and Sand Martins Riparia riparia use different air layers (foraging at different heights). However, to our knowledge, there has never been a comparative, simultaneous investigation of food composition and/or dietary overlap for Swifts, Swallows and House Martins breeding at the same location. Earlier comparative studies on the similarity of prey composition have been conducted between Swallows and House Martins (Kožená Citation1983) and all three species, but the analytical material of these studies was collected at breeding sites separated by a few to several kilometres or had been compiled from other studies (Waugh Citation1978, Turner Citation1980, Citation1982a,b, Bryant & Turner Citation1982).
The primary purpose of the present study was to compare and characterize the diet fed to trophically similar nestlings of three species of sympatric, aerial feeding birds: Swift, Swallow and House Martin. We present for the first time new dietary data over the entire breeding period of three species breeding simultaneously at the same location, complementing earlier dietary studies (Waugh Citation1978, Bryant & Turner Citation1982). In particular, our investigation of diet, besides two related species of hirundines, Swallows and House Martins, includes Swifts – a species with similar foraging modes from another taxonomic group – most probably foraging in different air layers and covering considerably larger distances to their foraging grounds (Waugh Citation1978, Bryant & Turner Citation1982). We hypothesized that diet among Swifts, Swallows and House Martins might vary in four ways: (1) occurrence of major food types, i.e. most numerous taxa of prey species, (2) prey diversity and food niche overlap between bird species, (3) body mass of prey and (4) composition of prey expressed as their ecological and economic roles. To test these hypotheses, we analysed the potential dietary overlap/segregation among these species in terms of the taxonomy, biomass and trophic specialization (= type of food consumed) of the prey species. Unlike some previous studies, we identified prey to the lowest taxonomic level possible to provide a more complete picture of the dietary overlap of these species of aerial insectivores.
METHODS
The diets of nestling Swifts, Swallows and House Martins were investigated by identifying prey items from faecal sacs collected under active nests of these species. All three species breed in Stary Gołe˛bin, a village in southwestern Poland (51° 10′ 57″N, 16° 57′ 18″E) located in an agricultural region of western Wielkopolska. Primary crops cultivated within 600 m of the village included alfalfa (37.5%), winter cereals (wheat and triticale; 37.5%) and winter oil-seed rape (25%). A large farm with cattle (1370 head), pigs (25 head) and c. 50 poultry is present in the village. Nests of the three study species were distributed in various sites within a 3 ha area within the village. The maximum distance between nests was about 110 m. No detailed observations on feeding sites of the study birds were conducted, however, on calm days, these species were often seen flying at various heights near the breeding sites. Faecal sacs were collected from 2 large breeding colonies of House Martins with 60 and 35 nests located on two 4-storey house blocks, 3 nests of Swifts located in ventilation holes of a low building and 4 nests of Swallows located in 4 small farmstead buildings, in 2 of which were kept one or two pigs. We collected 98 faecal sacs from Swifts (some directly from ventilation holes after the departure of nestlings), 138 from Swallows and 238 from House Martins. Faecal sacs were collected between the beginning of July and 5 September 2011 in 3–7-day intervals under occupied nests of these species. In practice, we collected all faecal sacs of Swift and Swallow found, and only a small fraction of faecal sacs available in the House Martin colonies. For Swifts, we collected 20 faecal sacs in July, 21 in August and 57 in September; for Swallows numbers per month were 23, 72 and 43, respectively; and for House Martins, 37, 141 and 60, respectively.
Prior to analysis, droppings were manually crushed and separated on Petri dishes and examined with 40× binocular microscopes (Orłowski & Karg Citation2011). In most cases, food components from individual faecal sacs were identified, however, because some faecal sacs broke up or became stuck to others, some initial identifications involved more than one faecal sac. The number of prey items representing particular insect species and spiders was established on the basis of the quantity of fragments of chitin parts, chiefly the elytra (for different families and genera of Coleoptera, Homoptera or Heteroptera), wings (in the case of Diptera, Hymenoptera, Odonata), mouthparts (most orders) and other preserved organs (e.g. limbs, petiolus, clypeus, mandible). To determine the number of prey belonging to particular species, we applied the rule of summation of different chitin parts to the level of one individual. For example, two or more different fragments of chitin parts (e.g. head, mandibles, six legs and other parts in the case of ants) from one dropping would be treated as belonging to the same individual of a given species (Orłowski & Karg Citation2011). Special attention was paid to the presence of small prey items, mainly Diptera and Hymenoptera. In most analyses, diet composition for each bird species was assessed as the number, proportions and mass of 14 main food types representing the most numerous family or order of insect prey. Prey was identified to family for the orders Coleoptera (Curculionidae, Chrysomelidae, Staphylinidae, Coccinellidae, Carabidae and other Coleoptera), Hymenoptera (Ichneumonidae, Formicidae [winged ants], Chalcidoidea and other Hymenoptera) and Heteroptera (Pentatomidae and other Heteroptera). The group ‘other insects’ included Homoptera rarely occurring among our material and other insects as well as spiders; see list of taxa in Electronic Appendix 1. Prey mass was expressed as a value calculated for dry mass (i.e. mg d.w.); these values were obtained from detailed measurements of insect mass based on analysis of 479 087 individuals of different taxa of insects (Karg Citation1989) and which had been used in dietary studies of the Swallow (Orłowski & Karg Citation2011, Citation2013). Dominant food types were defined as those taxa exceeding 5% of total abundance and biomass in the samples for each species. Analysis of faeces is likely to yield a reliable picture of the diet of aerial insectivores, since earlier findings of the experimental feeding of a nestling Swallow conducted by Waugh (Citation1978) showed that the proportions of different prey types ingested (including some soft-bodied prey types such as small Diptera) and the proportions recovered in the faeces are in very close agreement. This essentially means that no significant differential digestion exists between types with soft bodies and flexible wings and heavily chitinized prey (Waugh Citation1978).
As aerial foragers are potentially important in the biological control of insect pests (Lack & Owen Citation1955), we also characterized insect taxa ecologically (i.e. herbivorous; saprophagous, including coprophilous and coprophagous species; predators and parasites) and economically (i.e. neutral, agricultural pests and beneficial) (division of taxa after Da˛browska-Prot & Karg 1976, Ryszkowski & Karg Citation1977, Karg Citation1989, Orłowski & Karg Citation2011).
DATA ANALYSES
Our primary comparisons of the diversity and specialization of diet and weight of prey are based on the composition of diet determined by identification of prey items from single, whole faecal sacs (Swifts, n = 98; Swallows, n = 89 and House Martins, n = 171). These data were used to assess differences in diet composition using multivariate analysis of variance (manova). Two main indices of diet diversity – Shannon Diversity Index and Berger–Parker index of dominance – and body weight of prey were compared between the three study species using GLMs.
We also pooled the data from all the faecal samples of each species to obtain the percentage composition of major food types (defined by insect order) and the body weight and numbers of prey of different ecological and economic types (, Electronic Appendix 1). Because of the large number of nests in the House Martin colonies, we were not able to ascribe faecal sacs to particular nests and did not incorporate nest as a potential covariate in our analyses.
Table 1. General percentage composition of major food types/order of insects (number and biomass) in the diets of nestlings of three species of aerial feeding birds breeding at the same location in southwestern Poland.
We used manova to assess differences in diet among species as expressed as the percentage of the 14 major food types. In this analysis, all data were square root-arcsine transformed.
To determine the diet diversity and the level of diet specialization of each species, we determined values of the Shannon Diversity Index (using ecosim 7.72 software; Gotelli & Entsminger Citation2009) and the index of dominance of Berger–Parker (d). The index of dominance is a simple measure of the numerical importance of the most abundant species. It was calculated as d = Nmax/N, where Nmax is the number of individuals of the most abundant species and N is the total number of individuals in the sample (Berger & Parker Citation1970). The Berger–Parker index (d) varies between 1/N and 1; values closer to 1 indicate higher specialization in the choice of food, while values closer to 0 are typical for polyphagous species; this surprisingly simple index has been considered to be one of the best to assess diet specialization (May Citation1975).
The food niche overlap index was calculated for all dietary data (from analysis of all faecal samples, Electronic Appendix 1) using the Pianka index using ecosim 7.72 software (Gotelli & Entsminger Citation2009). This index is symmetrical and it assumes values between 0 and 1, where 0 indicates a resource used by a single species and indicates complete diet overlap. Values >0.60 indicate overlap between species (Gotelli & Entsminger Citation2009).
Chi-square tests were used to compare the distribution of body mass of prey and composition of prey in terms of their ecological and economic roles.
Apart from the above-mentioned statistical programs, statistical analyses were conducted using statistica 7.0 (StatSoft Citation2006) and excel software.
RESULTS
Overall, we identified 15 274 prey items in the faecal sacs that we examined (). Among Swift, Swallow and House Martin faecal sacs, we found 78, 76 and 113 taxa of prey, respectively (Electronic Appendix 1). A general description of the diets is presented in .
Diet composition, expressed as proportions of 14 taxonomic groups derived from identifications from individual whole faecal sacs (), clearly shows statistical differences among the three study species (manova: Wilks's Lambda, λ = 0.144; df = 28, 658; P < 0.0001). Similarly, we found significant differences in pair-wise comparison in the diet composition between Swifts and House Martins (Wilks's Lambda, λ = 0.293, df = 14, 253; P < 0.0001), Swifts and Swallows (Wilks's Lambda, λ = 0.265; df = 14, 159; P < 0.0001) and House Martins and Swallows (Wilks's Lambda, λ = 0.328; df = 14, 233; P < .0001) ().
Table 2. Comparison of proportions (%) of 14 main food types (in terms of their number) per individual faecal sac (number of faecal sacs given in brackets) from nestlings of three species of aerial feeding birds breeding at the same location in southwestern Poland.
Our analyses of diversity of diet using the Shannon Diversity Index, Berger–Parker index of dominance and body weight of prey show statistically significant differences among the study species (Fig. 1). Furthermore, post hoc analysis using all these three measures showed significant differences in all possible pair-wise comparisons. In general, House Martins had the most diverse diet (as measured by the Shannon Diversity Index) and Swifts the least diverse (anova GLM, F2,348 = 39.7, P < 0.0001). A reversed ranking was obtained using the Berger–Parker index, which showed that Swifts were the most specialized (feeding mainly on one or a few types of insects, see below), and Swallows and House Martins fed on the intermediate and greatest variety of prey, respectively (anova GLM, F2,348 = 34.9, P < 0.0001). With regard to prey weight, Swallows took the heaviest, House Martins intermediate and Swifts the lightest insects; prey taken by Swifts was approximately 2.2 times and 1.9 times lighter than in House Martins and Swallows, respectively (anova GLM, F2,348 = 41.5, P < 0.0001; ).
Figure 1. Comparison of the main characteristics (indices) of the diet (mean ± se) calculated per individual faecal sac of nestlings of Swifts (n = 98), Swallows (n = 89) and House Martins (n = 171) breeding at the same location in southwestern Poland.
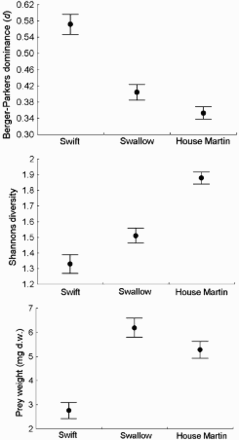
The average weight of prey calculated from individual faecal samples () was higher than the mean calculated from all collected material analysed together (cf. ), including faecal samples of Swifts which were all individually analysed.
Table 3. Comparison of body weight of prey (mg d.w.) identified in all analysed faecal sacs of nestlings of three species of aerial feeding birds breeding at the same location in southwestern Poland calculated for the complete list and number of insect prey found, see and Electronic Appendix 1 for numerical details.
GENERAL DIETARY OVERLAP AND PREY CHARACTERISTICS
We found much overlap in diet of the three bird species, which was the greatest in the case of House Martins. In pair-wise comparisons, Pianka index values were similar and exceeded 0.6 which is an indication of similarity: 0.781 for Swifts vs. 0.743 for Swallows; 0.807 for Swifts vs. 0.786 for House Martins and 0.762 for Swallows vs. 0.884 for House Martins.
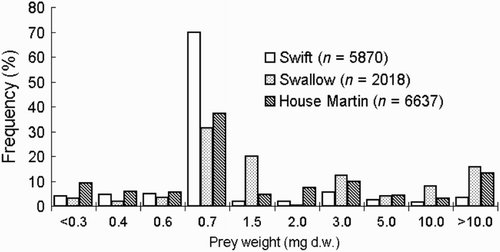
Body mass of prey taken by Swifts ranged from 0.09 to 37.27 mg d.w., corresponding values for Swallows ranged from 1.18 to 84.75 mg d.w. and for House Martins from 0.09 to 102.0 mg d.w. with significantly different distribution patterns among three study species (; chi-square test, χ2 = 71.5, df = 18, P < 0.0001). Average body mass of Swift prey was nearly three times smaller than that of Swallows and two times smaller than that of House Martins (). For Swifts and House Martins, prey weighing less than 0.7 mg represented 83% and 58%, respectively, of all prey (), which was a consequence of the predominance of the cabbage seed weevil Ceutorhynchus assimilis (family: Curculionidae) a phytophagous pest of oil-seed rape (Electronic Appendix 1). For Swallows, only 39% of its prey weighed below 0.7 mg (). C. assimilis was the most numerous prey in the diet of all three species, constituting 63.3%, 23.2% and 20.4% of all identified prey in Swifts, Swallows and House Martins, respectively (Electronic Appendix 1): these weevils comprised a proportionately greater part of the diet of Swifts than in House Martins and Swallows (χ2 = 1052.7, df = 1, P < 0.0001).
Figure 2. Comparison of the frequency distribution of insect prey weight taken by three species of aerial feeding birds breeding at the same location in southwestern Poland based on known weight of all identified prey, see Electronic Appendix 1.
For prey items comprising >5% of a species’ diet, only a single species – C. assimilis – was numerically dominant in Swifts; four taxa (C. assimilis, Formica sp. and unidentified Diptera and Ichneumonidae) comprised 54.0% of all prey items in Swallows; and five taxa (C. assimilis, Lasius sp., Coccinella septempunctata, Psylliodes sp. and Ichneumonidae) comprised 48.2% of all prey in House Martins (Electronic Appendix 1).
In spite of its high numerical frequency, total biomass of C. assimilis constituted only 21.1%, 3.2% and 3.5% of the diet in Swifts, Swallows and House Martins, respectively. In Swifts and House Martins, another species of large phytophagous curculionid, Otiorhynchus sp., had the greatest biomass, constituting 41.6% and 26.8%, respectively; in Swallows, the prey with greatest biomass was a large bug, Eurygaster sp. (19.8%) (Electronic Appendix 1).
The diet of House Martins contained a large contribution of aposematic ladybirds (nine taxa of Coccinellidae), a much higher frequency than was found in the Swifts' diet (χ2 = 568.0, df = 1, P < 0.0001) and Swallows' diet (χ2 = 221.6, df = 1, P < 0.0001) ().
The diet of all three species comprised mostly herbivorous insects: 77.5% (Swifts), 45.2% (Swallows) and 40.0% (House Martins) by total number in diets (a). Ecological groupings of prey varied significantly among all three species (χ2 = 38.2, df = 6, P < 0.001), as well as pair-wise comparison: Swifts vs. Swallows and Swifts vs. House Martins (χ2 ≥ 25.8, df = 3, P < 0.001) but not between Swallows and House Martins (χ2 = 1.2, df = 3, P > 0.50) (a).
Figure 3. Comparison of proportions of various groups of insects in terms of their ecological (a) and economic (b) roles in the diet of three species of aerial feeding birds based on the complete list of insect prey found, see Electronic Appendix 1; total number of prey assigned to at least one role is indicated.
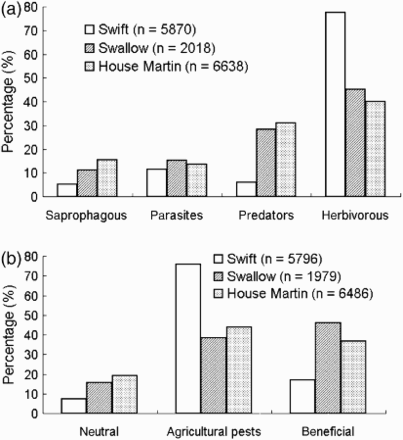
Partitioning prey with regard to their economic importance showed that agricultural pests represented 75.9% (Swifts), 43.9% (House Martins) and 38.2% (Swallows) of prey and the general distribution of prey according to economic importance showed statistically significant differences (χ2 = 34.5, df = 4, P < 0.001; b). Pair-wise comparisons showed significant differences between Swifts vs. Swallows (χ2 = 29.1, df = 2, P < 0.001) and Swifts vs. House Martins (χ2 = 21.5, df = 2, P < 0.001) but not between Swallows vs. House Martins (χ2 = 1.7, df = 2, P > 0.30) (b).
DISCUSSION
Our findings showed that the diet of these three aerial insectivores clearly differs in the four measures that we examined: (1) proportions of major food types, (2) diversity of diet, (3) specialization of diet and (4) weight of prey. However, a general assessment of dietary overlap based on the Pianka index shows a high degree of diet similarity among Swifts, House Martins and Swallows. Our findings show that these species consume the same type of insect prey when breeding at the same place, indicating shared exploitation of abundant food resources. However, we clearly identify differences in major food types, resulting in apparent differences in diet diversity and prey mass suggesting a mechanism of food niche differentiation. Moreover, several of our results suggest that there is separation of these species on some ecological dimension (Cucco et al. Citation1993, Dyrcz & Flinks Citation2003, Vieira & Port Citation2007, Whitfield et al. Citation2013). Furthermore, it is worth noting that the three measures we used to compare diets (i.e. assessment of dietary overlap, comparison of proportion of major food types and weight of prey) are equally important and provide insight into different components of diet composition.
The findings from our study could be partly explained by foraging niche differences in the use of different air layers – that is, foraging at different heights (cf. Bryant & Turner Citation1982). This is especially evident with C. assimilis (Curculionidae) a species found periodically swarming at various heights, including higher layers (Ferguson et al. Citation1999), where Swifts could feed. Alternatively, these aggregated and abundant insects may be a prey type which is easy to catch because they form large aerial aggregations (Lack & Owen Citation1955, Cucco et al. Citation1993). Large prey species such as Calliphoridae, Otiorrhynchus or Eurygaster (cf. Electronic Appendix 1) are not expected to fly at great heights; therefore, they would more likely constitute a large part of the diet of Swallows. Waugh (Citation1978) showed that prey mobility is important in influencing the prey type taken by aerial insectivores. We found that Swallows took some large Diptera which were strong fliers (cf. Electronic Appendix 1). House Martins – with the widest niche and the greatest overlap in this study – forage in air layers between the other two species and seem to show also the greatest flexibility in foraging niche and greatest adjustments of foraging heights to environmental conditions (Waugh Citation1978, Bryant & Turner Citation1982). Alternatively, these three species may forage at various locations within a small distance from the nests (≤400–500 m), that in practice could result in different composition of their diets, as has been suggested by other studies analysing prey type at nest sites separated by several kilometres (cf. Introduction section). Swifts in particular can cover large distances to foraging grounds (Lack & Owen Citation1955).
In general, the pattern of prey weight (≈size) that we found among our three study species is consistent with other studies in showing little variation despite different habitats being studied. It seems that the size composition of insects flying in different air layers could be quite constant over different habitats, because the flight activity and height of insects are size dependent. In this aspect, our data correspond to earlier findings on prey weight for these three species of aerial insectivores (Waugh Citation1978, Bryant & Turner Citation1982, Kožená Citation1983, Cramp Citation1998, Glutz von Blotzheim Citation2001). Waugh (Citation1978) identified two reasons to explain the pattern of prey size among aerial feeding birds (besides habitat effects, including the partitioning of the air space, which are probably also very variable and could overlap in some conditions or circumstances). First, the morphology of the feeding apparatus (i.e. the size and shape of the bill) can affect the size of prey selected and, secondly, flight manoeuverability (as defined by body morphology and wing and tail shape) will also influence successful prey capture. Considering these factors, Swallows are adapted to take the largest prey types followed by House Martins and Swifts (Waugh Citation1978). In this sense, morphological differences among these species are most likely to be the main explanation defining the optimal weight of prey (Waugh Citation1978). This is the case when resources are abundant and potential diet overlap is limited; conversely, if resources are limited, diet overlap between co-existing species will increase (Gonzalez-Solis et al. Citation1997). Diet overlap could be especially clear during bad weather: Waugh (Citation1978) compared diets of Swallows and House Martins feeding at the same site in bad weather and found greater overlap in prey size because each species took a narrower range of insect sizes at this time. This suggests that during poor weather conditions (with very limited food availability, due to the absence of flying insects), all three species of aerial insectivores could reduce foraging height, exploit the same air layer and so probably increase their trophic overlap during these times.
An important consideration for understanding our results is the location of our study within an area of crop fields. This is especially clear because the superabundant cabbage seed weevil C. assimilis was predominant in the diet of all three species. The high contribution of this herbivorous beetle and other agricultural pests in the diet is undoubtedly the result of our study site being in an area with a high coverage of oil-seed rape cultivation. The high proportion of some species of agricultural pests has been mentioned in earlier studies of Swift diet (Lack & Owen Citation1955), which may indicate that aerial insectivores may play an important role as agents of biological control (Wenny et al. Citation2011).
An additional factor which may favour the co-existence of aerial insectivores is the degree of species-specific elements of the diet, such as the case of aposematic ladybirds being consumed almost solely by House Martins in our study (cf. , Electronic Appendix 1). To our knowledge, such a high proportion of ladybirds in the diet of nestling House Martins is unusual. Other studies have found that ladybirds are avoided by Swifts even if occurring in large numbers. Ladybirds have shown up in food-balls delivered to nestling Swifts at low frequencies (1.4%, Lack & Owen Citation1955, Cramp Citation1998, Glutz von Blotzheim Citation2001) and have been taken by hirundines (up to 18% by number) in their winter quarters in Africa (Kopij Citation2000). Apparent differences in the contribution of ladybirds to the diets of aerial insectivores could indicate that House Martins favour this kind of prey, which is usually avoided by other insectivorous passerines (Dolenská et al. Citation2009 and references therein). Alternatively, differences in feeding sites may determine whether ladybirds are present in the diet: these insects may not occur at high altitudes and in open terrain, where Swifts and Swallows forage, but may occur around trees and other vegetation where House Martins feed.
Finally, the evaluation of diet similarity should consider body mass of prey as a feature of the diet or potential diet overlap (Hespenheide Citation1971, Kožená Citation1980, Cucco et al. Citation1993). We believe that a more complete ‘biological’ picture of prey weight needs to analyse all material (rather than individual faecal sacs, cf. and ), which permits a better measure of diet and better comparisons with other studies (Hespenheide Citation1971, Waugh Citation1978, Turner Citation1980, Citation1982a,b, Bryant & Turner Citation1982, Kožená Citation1983, Cucco et al. Citation1993).
Ultimately, it should be remembered that, for aerial foragers, the natural variation in food abundance is probably more important in determining nestling growth than variation in diet quality (Johnston Citation1993). The exploitation of abundant, swarming insects (such as some agricultural pests) facilitates access to appropriate amounts of food – as is the case with Swifts which generally prefer highly clustered prey (discussed in Cucco et al. Citation1993). We recommend further dietary studies on aerial foraging insectivorous species, especially in areas of sympatry in order to explain how niche overlap varies with environmental conditions.
SUPPLEMENTAL DATA
Electronic for this article can be accessed here [http://dx.doi.org/10.1080/09593330.2013.839622].
Appendix 1
Download MS Word (41.8 KB)ACKNOWLEDGEMENTS
We are very grateful to Will Cresswell and the anonymous reviewers for valuable comments and discussion, which greatly improved the value of the article and Joanna Czarnecka for statistical advice. We appreciate the improvements in English usage made by Peter Lowther through the Association of Field Ornithologists’ program of editorial assistance and by Wenesa Synowiec.
REFERENCES
- Azevedo, F.C.C., Lester, V., Gorsuch, W., Larivie, S., Wirsing, A.J. & Murray, D.L. 2006. Dietary breadth and overlap among five sympatric prairie carnivores. J. Zool. 269: 127–135. doi: 10.1111/j.1469-7998.2006.00075.x
- Berger, W.H. & Parker, F.L. 1970. Diversity of planktonic foraminifera in deep sea sediments. Science 168: 1345–1347. doi: 10.1126/science.168.3937.1345
- Bryant, D.M. 1973. The factors influencing the selection of food by the House Martin (Delichon urbica (L.)). J. Anim. Ecol. 42: 539–564. doi: 10.2307/3123
- Bryant, D.M. 1975. Breeding biology of house martin Delichon urbica in relation to aerial abundance. Ibis 117: 180–216. doi: 10.1111/j.1474-919X.1975.tb04206.x
- Bryant, D.M. & Turner, A.K. 1982. Central place foraging by swallows (Hirundinidae): The question of load size. Anim. Behav. 30: 845–856. doi: 10.1016/S0003-3472(82)80158-9
- Cramp, S. (ed.) 1998. The Complete Birds of the Western Palaearctic on CD-ROM. Oxford University Press, Oxford, UK.
- Cucco, M., Bryant, D.M. & Malacarne, G. 1993. Differences in the diet of the Common (Apus apus) and Pallid (A. pallidus) Swifts. Avocetta 17: 131–138.
- Dąbrowska-Prot, E. & Karg, J. 1976. Occurrence and flight activity of Diptera in an agricultural landscape. Pol. Ecol. Stud. 2: 87–93.
- Dolenská, M., Nedveˇd, O., Veselý, P., Tesarˇová, M. & Fuchs, R. 2009. What constitutes optical warning signals of ladybirds (Coleoptera: Coccinellidae) towards bird predators: Colour, pattern or general look? Biol. J. Linn. Soc. 98: 234–242. doi: 10.1111/j.1095-8312.2009.01277.x
- Dyrcz, A. & Flinks, H. 2003. Nestling food of the congeneric and sympatric Rusty-margined and Social flycatchers. J. Field Ornithol. 74: 157–165.
- Ferguson, A.W., Williams, I.H., Klukowski, Z., Walczak, B. & Perry, J. 1999. Spatial population dynamics of a pest and its parasitoid in an oilseed rape crop. Asp. Appl. Biol. 53: 1–6.
- Garcia, J.T. & Arroyo, B.T. 2005. Food-niche differentiation in sympatric Hen Circus cyaneus and Montagu's Harriers Circus pygargus. Ibis 147: 144–154. doi: 10.1111/j.1474-919x.2004.00377.x
- Glutz von Blotzheim, U.N. (ed) 2001. Handbuch der Vögel Mitteleuropas. Band 10/I. Passeriformes (1. Teil): Alaudidae – Hirundidae. Lerchen und Shwalben. Aula-Verlag, eBook, Wiesbaden.
- Gonzalez-Solis, J., Oro, D., Jover, L., Ruiz, X. & Pedrocchi, V. 1997. Trophic niche width and overlap of two sympatric gulls in the southwestern mediterranean. Oecologia 112: 75–80. doi: 10.1007/s004420050285
- Gotelli, N.J. & Entsminger, G.L. 2009. EcoSim: Null Models Software for Ecology. Version 7. Acquired Intelligence and Kesey-Bear, Jericho, VT. Available from: http://garyentsminger.com/ecosim.htm
- Grüebler, M.U., Morand, M. & Naef-Daenzer, B. 2008. A predictive model of the density of airborne insects in agricultural environments. Agric. Ecosys. Environ. 123: 75–80. doi: 10.1016/j.agee.2007.05.001
- Hespenheide, H.A. 1971. Food preference and the extent of overlap in some insectivorous birds, with special reference to the Tyrannidae. Ibis 113: 59–72. doi: 10.1111/j.1474-919X.1971.tb05123.x
- Johnston, R.D. 1993. Effects of diet quality on the nestling growth of a wild insectivorous passerine, the house martin Delichon urbica. Funct. Ecol. 7: 255–266. doi: 10.2307/2390203
- Jones, M.E. & Barmuta, L.A. 1998. Diet overlap and relative abundance of sympatric dasyurid carnivores: A hypothesis of competition. J. Anim. Ecol. 67: 410–421. doi: 10.1046/j.1365-2656.1998.00203.x
- Karg, J. 1989. Differentiation in the density and biomass of flying insects in the agricultural landscape of Western Poland. Roczniki Akademii Rolniczej w Poznaniu. 188: 1–78. (in Polish).
- Kopij, G. 2000. Diet of swifts (Apodidae) and swallows (Hirundinidae) during the breeding season in South African grassland. Acta Ornithol. 35: 203–206. doi: 10.3161/068.035.0201
- Kožená, I. 1980. Dominance of items and diversity of the diet of young swallows (Hirundo rustica). Fol. Zool. 29: 143–156.
- Kožená, I. 1983. Comparison of the diets of young swallows (Hirundo rustica) and House Martins (Delichon urbica). Fol. Zool. 32: 41–50.
- Lack, D. & Owen, D.F. 1955. The food of the swift. J. Anim. Ecol. 24: 120–136. doi: 10.2307/1882
- May, R.M. 1975. Patterns of species abundance and diversity. In Cody, M.L. & Diamond, J.M. (eds) Ecology and Evolution of Communities: 81–120. Harvard University Press, Cambridge.
- Møller, A.P. 1983. Breeding habitat selection in the Swallow Hirundo rustica. Bird Study 30: 134–142. doi: 10.1080/00063658309476788
- Orłowski, G. & Karg, J. 2011. Diet of nestling Barn Swallows Hirundo rustica in rural areas of Poland. Cent. Eur. J. Biol. 6: 1023–1035. doi: 10.2478/s11535-011-0070-4
- Orłowski, G. & Karg, J. 2013. Partitioning the effects of livestock farming on the diet of an aerial insectivorous passerine, the Barn Swallow Hirundo rustica. Bird Study 60: 111–123. doi: 10.1080/00063657.2012.748717
- PECBMS. 2007. State of Europe's Common Birds, 2007. CSO/RSPB, Prague, Czech Republic, 23.
- Ricklefs, R.E. 1979. Ecology. Chiron Press, New York.
- Ryszkowski, L. & Karg, J. 1977. Variability of biomass of epigeic insects in the agricultural landscape. Ekol. Pol. 25: 501–517.
- StatSoft. 2006. Statistica© (Data Analysis Software System), Version 7.1. Tulsa.
- Turner, A.K. 1980. The use of time and energy by aerial feeding birds. PhD Thesis. Department of Biology, University of Stirling.
- Turner, A.K. 1982a. Optimal foraging by the swallow (Hirundo rustica, L.): Prey size selection. Anim. Behav. 30: 862–872. doi: 10.1016/S0003-3472(82)80160-7
- Turner, A.K. 1982b. Timing of laying by swallows (Hirundo rustica) and sand martins (Riparia riparia). J. Anim. Ecol. 51: 29–46. doi: 10.2307/4308
- Turner, A.K. 2006. The Barn Swallow. Poyser, London.
- Vieira, E.M. & Port, D. 2007. Niche overlap and resource partitioning between two sympatric fox species in southern Brazil. J. Zool. 272: 57–63. doi: 10.1111/j.1469-7998.2006.00237.x
- Waugh, D.R. 1978. Predation strategies in aerial feeding birds. PhD Thesis. Department of Biology, University of Stirling.
- Wenny, D.G., DeVault, T.L., Johnson, M.D., Kelly, D., Sekercioglu, C.H., Tomback, D.F. & Whelan, C.J. 2011. The need to quantify ecosystem services provided by birds. Auk 128: 1–14. doi: 10.1525/auk.2011.10248
- Whitfield, D.P., Marquiss, M., Reid, R., Grant, J., Tingay, R. & Evans, R. 2013. Breeding season diets of sympatric White-tailed Eagles and Golden Eagles in Scotland: No evidence for competitive effects. Bird Study 60: 67–76. doi: 10.1080/00063657.2012.742997
- Yalden, D.W. 1985. Dietary separation of owls in the Peak District. Bird Study 32: 122–131. doi: 10.1080/00063658509476867
- Zar, J.H. 1984. Biostatistical Analysis, 2nd edn. Prentice Hall, London.