Abstract
Capsule Structural heterogeneity was the most important factor influencing the distribution of Barred Warbler Sylvia nisoria, Common Whitethroat S. communis and Lesser Whitethroat S. curruca in linear habitats in farmland of Western Poland.
Aims To investigate the occurrence of three species of Sylvia warblers in relation to the spatial structure of linear habitats in the agricultural landscape of Western Poland where, in contrast to Western Europe, field boundaries are not managed in terms of their size or spatial structure.
Methods In 2008, the distribution of breeding territories of Sylvia warblers in linear habitats was estimated in farmland of Western Poland. Redundancy detrended analysis was used to assess the relationship between bird abundance and seven linear habitat variables in ninety-four 150 m sections.
Results Sylvia warblers differed in habitat requirements, however heterogeneity affected their distribution to the greatest extent. In addition, Barred Warbler preferred high shrub volume and wider sections, whereas Common Whitethroat was attracted by brambles and nettles and Lesser Whitethroat favoured shrubs. All species avoided a high proportion of low vegetation.
Conclusion Structural heterogeneity resulted in highly preferred linear habitats for Sylvia warblers. Thus, maintaining or increasing structural heterogeneity of linear habitats may be a very effective tool for the conservation of farmland bird populations.
Many farming landscapes comprise a network of field boundaries such as hedgerows, roads lined by trees or shrubs, lines of trees along meadow edges, drainage ditches with or without shrubs, and road verges covered by herbaceous vegetation. Most of these linear habitats were created by man as a byproduct of farmland intensification and/or landscape planning, and they appear to increase the landscape complexity of rural areas (Benton et al. Citation2003). Moreover, because of their semi-natural character, they play a key role in maintaining farmland biodiversity (Green et al. Citation1994, Tattersall et al. Citation2002, Croxton et al. Citation2005, Tscharntke et al. Citation2005). Consequently, linear habitats have drawn the attention of conservation biologists, especially those interested in bird conservation (Fuller et al. Citation2004). Studies have shown that the width and height of the hedgerows and the shrub cover are the most important factors, positively affecting the overall richness of bird species (Hinsley & Bellamy Citation2000).
Barred Warbler Sylvia nisoria, Common Whitethroat Sylvia communis, and Lesser Whitethroat Sylvia curruca are among several European species dependent on the structure of the agricultural landscape (Donald et al. Citation2006). In the farmland of Western Poland, in which a continuous habitat is limited, these Sylvia warblers are known as shrub-specific species that most frequently choose field boundaries as their breeding habitat (Tryjanowski et al. Citation2009). Nevertheless, the value of linear habitats to each species is not the same (Wuczyński et al. Citation2011). Habitat selection by Sylvia warblers has been previously investigated in the context of interspecific territoriality (Cody Citation1978, Tsiakiris et al. Citation2009, Polak Citation2012), migration (Turrian & Jenni Citation1989, Vickery et al. Citation1999) and many aspects of breeding biology and ecology (Persson Citation1971, Macdonald Citation1979, Bairlein et al. Citation1980, Bocheński Citation1985, Hedenström & Åkesson Citation1991, Hałupka et al. Citation2002, Brauze Citation2012), but detailed studies carried out in linear habitats are sparse. To the best of our knowledge, only Stoate and Szczur (Citation2001) have focused on the influence of field boundary structure on the breeding territory establishment of Common Whitethroat. More general studies have identified the structure of linear habitats preferred by Common and Lesser Whitethroat (Arnold Citation1983, Green et al. Citation1994, Sparks et al. Citation1996), however Bramble Rubus spp. coverage, which is important to Sylvia warblers (Bocheński Citation1985), was not taken into account. Finally, most studies have been conducted in Western Europe, outside the range of the Barred Warbler (Hinsley & Bellamy Citation2000).
Agricultural landscapes in Western and Central Europe contrast in many aspects and thus, the ecological conditions experienced by bird populations may differ markedly (Tryjanowski et al. Citation2011). For example, in contrast to Western Europe where field boundaries are managed (e.g. hedgerows are coppiced and laid), linear habitats in Western Poland are usually not managed, which results in large variation in their spatial structure. Therefore, we expect that the habitat requirements of species inhabiting field boundaries may differ between Western and Central Europe. We hypothesize that the heterogeneity of unmanaged linear habitats in Poland may be more attractive to Lesser Whitethroat and Common Whitethroat than the width of hedgerows (Green et al. Citation1994, Sparks et al. Citation1996). In this study, we investigate the distribution of three species of Sylvia warblers in relation to the spatial structure of linear habitats in the agricultural landscape of Western Poland and discuss our findings in relation to results obtained particularly from hedgerows in Western Europe.
METHODS
The study was conducted in the agricultural landscape of Western Poland, near Leszno (51°51′N, 16°34′E). The study plot covered 10.0 km2 of farmland dominated by arable fields (55.7%) with Scots Pine Pinus sylvestris forests (27.3%) which were uninhabitable for the studied species. Meadows occupied about 6.0% and rural buildings covered nearly 7.0% of the total area (for details see Szymański Citation2010). Linear habitats were located along edges of meadows, surfaced and unsurfaced roads and drainage ditches, or a mixture of these elements. Roadside ditches, road verges and non-shrub areas were covered by Stinging Nettle Urtica dioica, Bramble Rubus spp. and other herbaceous vegetation. The dominant species of shrubs were Elderberry Sambucus nigra, Hawthorn Crataegus spp., Blackthorn Prunus spinosa and Wild Rose Rosa canina. The dominant species of trees were Black Alder Alnus glutinosa, Poplar Populus spp., Ash Fraxinus spp. and, in some sections, fruit trees: Plum Prunus spp. and Crabapple Malus spp.
We estimated Barred Warbler, Common Whitethroat and Lesser Whitethroat abundance in 2008. Beginning from about the last week in April, six morning (05:00–11:00) visits were made. The number of breeding territories was assessed using the combined version of the mapping method (Tomiałojc´ Citation1980). However, in most cases, direct nest searching was not used due to the high sensitivity of Sylvia warblers to disturbance, especially during nest building and incubation (Cramp Citation1998, P. Szymański, own observations). Therefore, nests locations were identified based on behavioural observations of birds (e.g. nest building, chick feeding, removal of faecal sacs from the nest). The centre of each breeding territory was determined as the nest site or, if the position of the nest was unknown, based on at least three observations of a singing male at a song post. Particular attention was paid to simultaneous records of singing males and their aggressive interactions (Tomiałojc ´ Citation1980).
We designated ninety-four 150 m (total 14.10 km) sections within 16 linear habitats (total 18.0 km). The maximum distance between main linear habitats was 3.8 km and a few of them intersected. In particular, linear habitat sections were contiguous to each other. The average width of the linear sections was 9.91 m (range = 2.0–28.0, sd = 4.31). All breeding territories of studied species were assigned to particular sections based on the location of its centre and only territories located within linear habitats were considered in the statistical analysis. Detailed data on habitat measurements of sections of linear habitats associated with the presence of breeding territories of Barred Warbler, Common Whitethroat and Lesser Whitethroat are given in . The spatial structure of each section was characterized by seven habitat variables:
Number of trees (TRN): the number of trees (>15 cm diameter at breast height) per section,
Brambles and nettles (BRA): the area (m2) covered by brambles and nettles,
Low vegetation (LVE): the area (m2) covered by herbaceous vegetation,
Shrub volume (SHR): total volume (m3) of bushes per section,
Ditch occurrence (DIT): no ditch – 0, a single-line ditch – 1, and a ditch on both sides – 2,
Heterogeneity (HET): the sum of number (n) of separate clumps of brambles and nettles, low vegetation, patches of shrub and single trees or stands of tree found in each section. Clumps of brambles and nettles, low vegetation and patches of shrub were considered separate if the distance between them was more than 2 m; single trees or stands of trees were considered separate if the distance between them was over 15 m. The heterogeneity index can be written by the equation: HET = (TRN)n + (LVE)n + (BRA)n + (SHR)n,
Width (WID): the width of the field boundary measured at ground level (m).
Table 1. Mean values, standard errors and ranges for habitat measurements of sections of linear habitats associated with the presence of a breeding territory of Barred Warbler, Common Whitethroat and Lesser Whitethroat.
We used redundancy detrended analysis (RDA) in combination with an associated Monte Carlo permutation test (1000 permutations) in CANOCO 4.5 for Windows (ter Braak & Šmilauer Citation2002) to assess differences in habitat requirements of Sylvia warblers in linear habitats. This method assumes that species abundance depends linearly on environmental variables. This approach allowed for the identification of environmental gradients along which the studied species were distributed. Moreover, this method detects the possible interdependence of analysed variables. In our data, heterogeneity and shrub volume were significantly correlated (rs = 0.29) and so a variance partitioning procedure was applied according to suggestions in Lepš and Šmilauer (Citation2003). We present both individual and automatic methods of selection of environmental variables to demonstrate the independence of our model results from the order of inclusion of variables. The significance of each variable was tested with a Monte Carlo permutation test. Signed-Rank Wilcoxon tests were used to compare the structure of occupied and unoccupied sections. Sections with a breeding territory of a species were compared to the nearest unoccupied sections with respect to the seven habitat variables.
RESULTS
In 2008, Common Whitethroat was the most numerous species, while Barred Warbler was the least abundant. In the study plot, the majority of breeding territories were located within linear habitats. Detailed data on bird numbers and densities are given in .
Table 2. The number (t) and density of breeding territories of studied Sylvia warblers within the 10.0 km2 study area, and their number, number per km and percentage located in linear habitats within the study area.
The RDA ordination diagram () indicated that the studied Sylvia warblers differed in habitat requirements with their distribution being governed by linear habitat variables associated with the first and second ordination axes (F = 2.9, df = 6, P = 0.003). Barred Warblers preferred wider sections with a heterogeneous structure and high shrub volume. Common Whitethroats were attracted by brambles and nettles as well as by heterogeneity and it was the only species observed in non-shrubby and treeless sections. Lesser Whitethroats showed clear preferences for shrub volume and heterogeneity. All study species avoided sections with a high proportion of low vegetation. Ditch presence and number of trees had a relatively small effect on the distribution of birds. In general, the studied Sylvia warblers were distributed along one major environmental gradient of increasing heterogeneity and shrub volume in one direction and the growth of low vegetation cover in the opposite direction of the first ordination axis (F = 12.3, df = 6, P = 0.008; variance accounted for = 67.7%). An increasing gradient of brambles and nettles was associated with the second ordination axis (3.8% of variance explained). The correlation coefficients of habitat variables and ordination axes are given in . The variance partitioning procedure showed that heterogeneity and shrub volume accounted for 56.7% and 19.1% of variance in species data, respectively, whereas 9.8% was shared by both habitat variables. A forward stepwise variable selection showed that the most important habitat variable which affected the distribution of the studied Sylvia warblers was heterogeneity (). The spatial structure of occupied and unoccupied sections differed significantly in terms of heterogeneity for all studied species ().
Figure 1. The RDA ordination diagram (showing first – horizontal and second – vertical ordination axes) displaying the habitat preferences (black arrows) of Barred Warbler (BW), Common Whitethroat (CW) and Lesser Whitethroat (LW) among the seven linear habitat variables (grey arrows). The angle between axes of the ordination diagram and species and habitat variable arrows show the power of the correlation. Abbreviations of habitat variables are as follows: TRN – the numbers of trees, BRA – brambles and nettles, LVE – herbaceous vegetation, SHR – shrub volume, DIT – a ditch occurrence, HET – heterogeneity and WID – the width of the section.
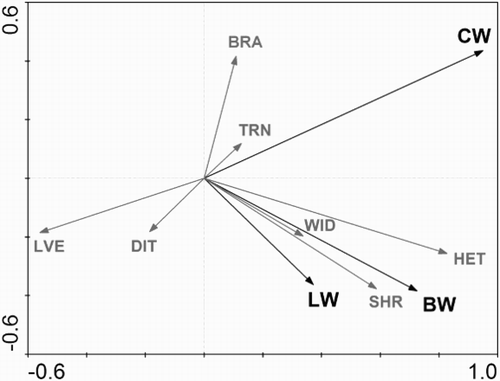
Figure 2. Mean (±se, ±1.96*se) heterogeneity of occupied (grey boxes) and unoccupied (white boxes) sections of linear habitats of Barred Warbler (BW), Common Whitethroat (CW) and Lesser Whitethroat (LW). The heterogeneity of occupied and unoccupied sections was compared using Ranked-Sign Wilcoxon tests (Barred Warbler – T = 2.00, n = 7, P = 0.043; Common Whitethroat – z = 3.45, n = 30, P = 0.00053; Lesser Whitethroat – T = 2.00, n = 6, P = 0.028).
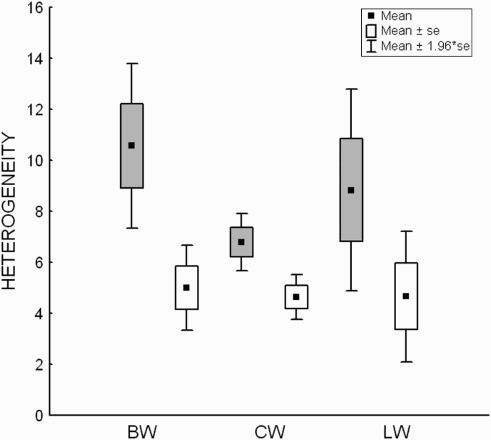
Table 3. Correlation coefficients between habitat variables and the first two axes in the RDA ordination.
Table 4. Results of Monte Carlo permutation testing of the significance of habitat variables affecting the distribution of studied Sylvia warbler species, with use of both individual and automatic selection procedures.
DISCUSSION
We showed that, in the farmland of Western Poland, Barred Warbler, Common Whitethroat and Lesser Whitethroat were most common in heterogeneous linear habitats. All species preferred sections with structural components such as trees, shrubs, brambles and nettles or low vegetation separated into several stands or clumps rather than in a single homogeneous patch. Such a relationship between habitat and birds might be caused by several factors. Firstly, a heterogeneous structure of a breeding territory provides ample resources required during the reproductive season, e.g. a diversity of tree and shrub species supplies a variety of and season-round food availability (Hałupka et al. Citation2002, Waldenström et al. Citation2004). Secondly, structurally diverse linear habitats are more difficult to penetrate by predators, increase nest concealment and consequently decrease predation risk (Newton Citation1998). The positive effect of structural complexity of hedgerows on other species of farmland birds has been shown by several authors, though the spatial heterogeneity of hedgerows had been measured in different ways, including the number of gaps (Lack Citation1992), the number of shrub (Parish et al. Citation1995) and tree species (Osborne Citation1984) or overgrowth with vegetation (O’Connor Citation1987). We suggest that predicting the overall value of linear habitats for birds based on different estimates of heterogeneity of linear habitats might therefore be unreliable. As Hinsley and Bellamy (Citation2000) suggest, no single type of hedgerow is suitable for all bird species. In addition, agricultural landscapes in Western and Central Europe contrast in many aspects of the farming system (Báldi & Batáry Citation2011, Tryjanowski et al. Citation2011). In fact, typical hedgerows do not exist in Polish farmland and most field boundaries are rarely or never managed by farmers, except for total removal of vegetation or controlled burning in spring. Consequently, there is a larger variation in their type, size and structure than in Western Europe. In order that heterogeneity of linear habitats could be estimated in different regions, we used a different approach and built a simple index of structural complexity (heterogeneity) that included a set of environmental variables: vegetation type and the spatial arrangement of habitat components and the discontinuity/patchiness of vegetation cover. Our detailed analysis revealed that this index reliably measures the micro-scale heterogeneity of different types of linear habitats. However, we emphasize the need for further comparative studies of bird communities in linear habitats in agricultural landscapes of Western and Central Europe to provide further evidence for the effects of heterogeneity generally.
The second linear habitat variable that most affected the studied species was shrub volume. Barred Warbler and Lesser Whitethroat, species considered to be hedgerow specialist (Fuller et al. Citation2001), were positively influenced by increasing shrub volume. In contrast, Common Whitethroats were not affected: it is known that Common Whitethroats establish territories in non-shrubby habitats and may even occasionally occupy patches of Oilseed Rape Brassica napus (Persson Citation1971). Several studies have shown that the width of hedgerows is one of the most important variables for the overall richness of bird species (see review Hinsley & Bellamy Citation2000). In our study, all Sylvia warblers were attracted by the width of the linear habitat. This was most important for Barred Warblers and least important for Common Whitethroats. However, our findings suggest that in structurally diverse linear habitats, width is not the most important environmental predictor for the occurrence of the Sylvia warblers studied. We hypothesize that heterogeneity functions in the same manner as width, providing birds with varied niches. Brambles and nettles had the greatest influence on the distribution of Common Whitethroat, which uses these habitats most frequently for nesting (Persson Citation1971, Mason Citation1976, Bocheński Citation1985, Hałupka et al. Citation2002). Our results also support those reported by Stoate and Szczur (Citation2001) who showed that Common Whitethroats in field boundaries use brambles and nettles most frequently as nest sites. Tree number had a relatively small effect on the occurrence of Sylvia warblers within particular sections. Because birds preferred only low tree density we assume, as Polak (Citation2012), that trees may function as song posts or may be used for foraging (Hałupka et al. Citation2002) rather than for nesting (Mason Citation1976, Bocheński Citation1985). All species avoided a high proportion of low vegetation which may be related to reducing foraging (Hałupka et al. Citation2002). Only Common Whitethroats may be attracted by low vegetation at the beginning of the breeding season when the foliage of brambles or shrubs is not well developed (Persson Citation1971). Nevertheless, even a small clump of herbaceous vegetation may be entirely sufficient for Common Whitethroat to build a nest.
In addition, our results showed that, depending on the species, 66.7% to 87.5% of territories in the study plot were located within linear habitats. This means that such landscape elements supported the majority of Barred Warbler, Common Whitethroat and Lesser Whitethroat populations. Although this study concentrated on only three species we have also provided further evidence that provision of non-cropped linear habitats can be highly beneficial to bird populations (Tscharntke et al. Citation2005), and especially for Barred Warbler, a species included in Annex I of the Directive 2009/147/EC, the so-called Birds Directive. Since linear habitats in farmland are easy to create, management practices should focus on increasing the spatial heterogeneity of field boundaries. Under such conditions, this can be a very effective tool of conservation and management of farmland bird populations.
ACKNOWLEDGEMENTS
We thank Paweł Re˛k, Will Cresswell and two anonymous referees for their critical comments on the first draft of this manuscript. We also thank Tim H. Sparks for linguistic corrections and helpful suggestions.
REFERENCES
- Arnold, G.W. 1983. The influence of ditch and hedgerow structure, length of hedgerows, and area of woodland and garden on bird numbers on farmland. J. Appl. Ecol. 20: 731–750. doi: 10.2307/2403123
- Bairlein, F., Berthold, P., Querner, U. & Schlenker, R. 1980. Die Brutbiologie der Grasmücken Sylvia atricapilla, borin, communis und curruca in Mittel – und N-Europa. J. Ornithol. 121: 325–369 (in German). doi: 10.1007/BF01643331
- Báldi, A. & Batáry, P. 2011. Spatial heterogeneity and farmland birds: different perspectives in Western and Eastern Europe. Ibis 153: 875–876. doi: 10.1111/j.1474-919X.2011.01169.x
- Benton, T.G., Vickery, J.A. & Wilson, J.D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18: 182–188. doi: 10.1016/S0169-5347(03)00011-9
- Bocheński, Z. 1985. Nesting of the Sylvia Warblers. Acta Zool. Cracov. 29: 241–328.
- ter Braak, C.J.F. & Šmilauer, P. 2002. CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination (Version 4.5). Microcomputer Power, Ithaca, NY.
- Brauze, T. 2012. Tree-stand age preferences of breeding Lesser Whitethroats Sylvia curruca in a forest in Central Poland. Bird Study 59: 376–379. doi: 10.1080/00063657.2012.665831
- Cody, M.L. 1978. Habitat selection and interspecific territoriality among the Sylviid Warblers of England and Sweden. Ecol. Monogr. 48: 351–396. doi: 10.2307/2937239
- Cramp, S. (ed.). 1998. The complete birds of the Western Palearctic. CD-ROM Version 1.0, Oxford University Press, Oxford.
- Croxton, P.J., Hann, J.P., Greatorex-Davies, J.N. & Sparks, T.H. 2005. Linear hotspots? The floral and butterfly diversity of green lanes. Biol. Conserv. 121: 579–584. doi: 10.1016/j.biocon.2004.06.008
- Donald, P.F., Sanderson, F.J., Burfield, I.J. & van Bommel, F.P.J. 2006. Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric. Ecosyst. Environ. 116: 189–196. doi: 10.1016/j.agee.2006.02.007
- Fuller, R.J., Chamberlain, D.E., Burton, N.H.K. & Gough, S.J. 2001. Distributions of birds in lowland agricultural landscapes of England and Wales: how distinctive are bird communities of hedgerows and woodland? Agric. Ecosyst. Environ. 84: 79–92. doi: 10.1016/S0167-8809(00)00194-8
- Fuller, R.J., Hinsley, S.A. & Swetnam, R.D. 2004. The relevance of non-farmland habitats, uncropped areas and habitat diversity to the conservation of farmland birds. Ibis 146: 22–31. doi: 10.1111/j.1474-919X.2004.00357.x
- Green, R.E., Osborne, P.E. & Sears, E.J. 1994. The distribution of passerine birds in hedgerows during the breeding season in relation to characteristics of the hedgerow and adjacent farmland. J. Appl. Ecol. 31: 677–692. doi: 10.2307/2404158
- Hałupka, K., Borowiec, M., Karczewska, A., Kunka, A. & Pietrowiak, J. 2002. Habitat requirements of Whitethroats Sylvia communis breeding in an alluvial plain. Bird Study 49: 297–299. doi: 10.1080/00063650209461278
- Hedenström, A. & Åkesson, S. 1991. Notes on the breeding biology of the Barred Warbler Sylvia nisoria at Ottenby, Sweden. Ornis Svecica 1: 57–59.
- Hinsley, S.A. & Bellamy, P.E. 2000. The influence of hedge structure, management and landscape context on the value of hedgerows to birds: a review. J. Environ. Manage. 60: 33–49. doi: 10.1006/jema.2000.0360
- Lack, P. 1992. Birds on Lowland Farms. HMSO, London.
- Lepš, J. & Šmilauer, P. 2003. Multivariate Analyzes of Ecological Data Using CANOCO. Cambridge University Press, Cambridge.
- MacDonald, D. 1979. Notes on the Whitethroat in Sutherland. Scottish Birds 10: 296–305.
- Mason, C.F. 1976. Breeding biology of the Sylvia Warblers. Bird Study 23: 213–232. doi: 10.1080/00063657609476506
- Newton, I. 1998. Population Limitation in Birds. Academic Press, London.
- O'Connor, R.J. 1987. Environmental interests of field margins for birds. In Way, J.M. & Greig-Smith, P.J. (eds) Field Margins. Monograph No. 35: 35–48. British Crop Protection Council, Thornton Heath, Surrey, London.
- Osborne, P. 1984. Bird numbers and habitat characteristics in farmland hedgerows. J. Appl. Ecol. 21: 63–82. doi: 10.2307/2403037
- Parish, T., Lakhani, K.H. & Sparks, T.H. 1995. Modelling the relationship between bird population variables and hedgerow and other field margin attributes. II. Abundance of individual species and of groups of similar species. J. Appl. Ecol. 32: 362–371. doi: 10.2307/2405102
- Persson, B. 1971. Habitat selection and nesting of a south Swedish Whitethroat Sylvia communis Lath. population. Ornis Scand. 2: 119–126. doi: 10.2307/3676211
- Polak, M. 2012. Habitat preferences of the sympatric Barred Warbler Sylvia nisoria and the Red-Backed Shrike Lanius collurio breeding in central Poland. Ann. Zool. Fenn. 49: 355–363. doi: 10.5735/086.049.0509
- Sparks, T.H., Parish, T. & Hinsley, S.A. 1996. Breeding birds in field boundaries in an agricultural landscape. Agric. Ecosyst. Environ. 60: 1–8. doi: 10.1016/S0167-8809(96)01067-5
- Stoate, C. & Szczur, J. 2001. Whitethroat Sylvia communis and Yellowhammer Emberiza citrinella nesting success and breeding distribution in relation to field boundary vegetation. Bird Study 48: 229–235. doi: 10.1080/00063650109461222
- Szymański, P. 2010. Liczebność i rozmieszczenie cierniówki Sylvia communis w krajobrazie rolniczym południowo-zachodniej Wielkopolski w latach 1998–2008. Ornis Polonica 51: 14–20 (in Polish).
- Tattersall, F.H., Macdonald, D.W., Hart, B.J., Johnson, P., Manley, W. & Feber, R. 2002. Is habitat linearity important for small mammal communities on farmland? J. Appl. Ecol. 39: 643–652. doi: 10.1046/j.1365-2664.2002.00741.x
- Tomiałojć, L. 1980. The combined version of the mapping method. In Oelke, H. (ed.) Bird Census Work and Nature Conservation, 92–106. Proc.VI Intern. Conf. Bird Census and Atlas Work, Göttingen.
- Tryjanowski, P., Kuz´niak, S., Kujawa, K. & Jerzak, L. 2009. Ekologia ptaków krajobrazu rolniczego. Bogucki Wyd. Naukowe, Poznań (in Polish).
- Tryjanowski, P., Hartel, T., Báldi, A., Szymański, P., Tobolka, M., Herzon, I., Goławski, A., Konvička, M., Hromada, M., Jerzak, L., Kujawa, K., Lenda, M., Orłowski, M., Panek, M., Skórka, P., Sparks, T.H., Tworek, S., Wuczyński, A. & Żmihorski, M. 2011. Conservation of farmland birds faces different challenges in Western and Central-Eastern Europe. Acta Ornithol. 46: 1–12. doi: 10.3161/000164511X589857
- Tscharntke, T., Klein, A.M., Kruess, A., Steffan-Dewenter, I. & Thies, C. 2005. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 8: 857–874. doi: 10.1111/j.1461-0248.2005.00782.x
- Tsiakiris, R., Stara, K., Pantis, J.D. & Sgardelis, S.P. 2009. Microhabitat selection by three common bird species of montane farmlands in northern Greece. Environ. Manage. 44: 874–887. doi: 10.1007/s00267-009-9359-8
- Turrian, F. & Jenni, L. 1989. Etude de trois espèces de fauvettes en période de migration postnuptiale à Verbois, Genève: phénologie du passage et utilisation du milieu. Alauda 57: 133–154 (in French).
- Vickery, J., Rowcliffe, M., Cresswell, W., Jones, P. & Holt, S. 1999. Habitat selection by Whitethroats Sylvia communis during spring passage in the Sahel zone of northern Nigeria. Bird Study 46: 348–355. doi: 10.1080/00063659909461149
- Waldenström, J., Rhönnstad, P. & Hasselquist, D. 2004. Habitat preferences and population trends in the Barred Warbler Sylvia nisoria in the Ottenby area, southeast Sweden. Ornis Svecica 14: 107–116.
- Wuczyński, A., Kujawa, K., Dajdok, Z. & Grzesiak, W. 2011. Species richness and composition of bird communities in various field margins of Poland. Agric. Ecosyst. Environ. 141: 202–209. doi: 10.1016/j.agee.2011.02.031