Abstract
Capsule Male bearded vultures tended to disperse closer to natal territories than females with a preferred eastward natal dispersal direction. In general, birds settled for the first time in breeding territories at an average age of 7.6 years, first breeding attempt occurred at 10.0 years and first successful breeding at 10.4 years. Measures aimed at favouring the settlement of new pairs in the edge of their current distribution area would allow natural range expansion of this endangered species.
Natal dispersal, age of recruitment and age of first breeding are key events in organisms' life history with important effects at an ecological and evolutionary scale (Clobert et al. Citation2001). These events play a critical role in demography of birds, influencing population dynamics and potential range expansion (Newton Citation1998). Therefore, detailed knowledge about the spatial scale and timing at which they occur is of the utmost importance for conservation (Kokko & López-Sepulcre Citation2006).
The bearded vulture Gypaetus barbatus is a cliff-nesting raptor that feeds primarily on bones and other parts of the carcasses of wild ungulates and livestock casualties (Ferguson-Lees & Christie Citation2001). Its breeding range extends throughout the mountains of Asia, Europe and Africa. The species is globally listed as Least Concern due to its extremely large range and slow decline during the 20th century (BirdLife International Citation2013). The Spanish population constitutes an outstanding exception to this worldwide declining trend, gradually increasing since 1986 (Heredia Citation2005). The species is currently listed as a ‘priority species’ in the conservation strategies of the European Union (Annex I of the Birds Directive) and is listed as ‘endangered’ in Spain (Antor et al. Citation2005).
Bearded vultures are characterized by a long lifespan, delayed maturity, deferred age of first reproduction, high parental investment, low fecundity, low mortality rate and a high offspring survival rate (Ferguson-Lees & Christie Citation2001, Margalida & Heredia Citation2005). However, whereas most studies have focused on particular aspects of its biology, mainly reproduction (Margalida et al. Citation2003) and conservation (Hirzel et al. Citation2004, Margalida & Heredia Citation2005, Oro et al. Citation2008, Schaub et al. Citation2009), other topics such as natal dispersal, philopatry and age of recruitment remain poorly understood. To date, the only information available about bearded vultures' life-history events and their age of first breeding comes from a study by Antor et al. (Citation2007) carried out in the Pyrenees. Therefore, our study provides an update of life-history parameters beyond those reported in Antor et al. (Citation2007) and we also provide novel detailed information about natal dispersal distance. Taking into account the endangered conservation status of the bearded vulture in some parts of its range, a detailed knowledge about its life-history events can be of practical help for conservation.
To this end, 88 bearded vultures of different ages (49 nestlings, 20 immatures, 14 subadults and 5 adults) and both sexes (33 males, 44 females, 11 unknown sex) were trapped and tagged with plastic wing-tags in the southern slope of the Pyrenees (Huesca province, Aragón region, Spain) from 1987 to 2004. Tags included an alphanumeric code allowing individual identification at distance with spotting scopes. This work took place as a part of a long-term conservation and research programme conducted by the Foundation for the Conservation of the Bearded Vulture (FCQ) in central Pyrenees (Gil et al. Citation2010). Systematic field surveys consisting of a minimum of three to four visits per territory were conducted annually throughout the Pyrenees to detect the settlement of individuals in breeding territories, and to record breeding behaviour and performance.
Nestlings were tagged with vinyl wing-tags at age of ca. 90–100 days. The rest of birds were captured at supplementary feeding stations by means of a cannon-projected net (López-López et al. Citation2011). Bearded vultures were aged according to plumage characteristics as follows: nestlings (< 120 days), immatures (from ca. 4 months–3 years), subadults (4–6 years) and adults (> 6 years; Lacasa Citation2012). Adult birds (> 6 years), for which we did not know whether they had been recruited or were breeding before trapping, were excluded from the analyses. Sex was determined by means of genetic analysis of blood samples (García et al. Citation2009).
Of the 88 bearded vultures marked in this study, we recorded information on life-history events from 38 individuals (16 males and 22 females). Considering the age class at capture, we recorded information of 17 nestlings, 11 immatures and 10 subadults. Of the 88 birds, 26 individuals (29.6%) were still alive in 2012, 23 (26.1%) died during the study period and 39 (44.3%) were missing.
We estimated the following parameters: (i) recruitment age (i.e. age of first settlement in a territory), (ii) age of first breeding attempt, (iii) age of first successful breeding, (iv) natal dispersal distance and (v) natal dispersal direction. According to Greenwood & Harvey (Citation1982), natal dispersal distance was computed as the Euclidean linear distance between the place of birth and the first breeding site (i.e. the first territory where individuals or their mates laid an egg). Therefore, natal dispersal distance and direction were only calculated for birds marked as nestlings. Distances and directions were computed in ArcMap 10.0 (www.esri.com) and sex differences in life-history events and dispersal directions were evaluated by means of non-parametric Mann–Whitney tests using Statistica version 10.0 (www.statsoft.com). Summary statistics and the preference for particular dispersal directions were computed by means of the Rayleigh test of uniformity with specified mean direction (‘v0.test’ script in CircStats R-Package, Agostinelli Citation2009). This test evaluates the significance of the uniformity of mean resultant directions (i.e. the null hypothesis) against the alternative hypothesis of a unimodal distribution with a specified mean direction (i.e. in our case an eastward direction of 90°).
In order to analyse philopatric behaviour, we followed the study of Serrano et al. (Citation2008) on dispersal patterns in Lesser Kestrel Falco naumanni. To this end, we used Monte Carlo simulations to evaluate the probability that observed natal dispersal distances could have occurred by chance and thus to test whether dispersal patterns were constrained by the spatial distribution of breeding territories or not. This method has several advantages because it allows the generation of frequency distributions and hypothesis testing based on real field data and not theoretical distributions. In addition, it allows taking into account how dispersal patterns are constrained by the spatial distribution of dispersal options (i.e. available territories for nesting; Serrano et al. Citation2008).
First, we calculated observed natal dispersal distances from natal nests to the territory of first breeding for each individual. Next, we calculated a matrix of distances among all territories (i.e. existing and historical) in the study area. Then, to obtain the expected natal dispersal distances, we resampled an equal number of observed natal dispersal distances from the raw matrix of potential distances using the ‘shuffle’ function implemented in PopTools version 3.2.5 for Excel (http://www.poptools.org). This procedure was repeated 1000 times. Dispersal distances were analysed using all nestlings pooled (n = 10), and using males (n = 6) and females (n = 4) separately. Six nestlings were marked in six different territories and four nestlings were marked in the same territory but in different years. Taking into account that bearded vultures only produce one chick per year (in good years), the complex mating system of the species (including polyandrous and polygynic trios and quartets), the turnover rate of breeding individuals in the territories and that birds of the same territory were marked in different years, it is very unlikely that birds marked in the same territory were siblings, suggesting that the potential for pseudo-replication is low in these analyses. Then, we calculated the median natal dispersal distance for the observed data and for each of the 1000 subsets of expected natal dispersal distances. Monte Carlo simulations were run under two different scenarios: (i) using a ‘conservative’ approach which considered expected dispersal distances only from the natal territories where birds were marked to the rest of territories in central Pyrenees; and (ii) a ‘general’ approach in which expected natal dispersal distances were obtained from the raw matrix of all possible distances from all territories in the study area. This procedure allowed us to test whether observed natal dispersal distances were constrained by the natal territories where birds were trapped or dispersal patterns were consistent regardless of the natal territory. Finally, critical values of significance were generated by counting the number of randomization cases that resulted in an equal or larger/smaller value than the observed median dispersal distance divided by the number of randomizations (Serrano et al. Citation2008). Tests were two-tailed and significance level was set at α = 0.05. Descriptive values are presented as mean ± standard deviation.
Detailed life-history events are shown in . No statistically significant differences were found between sexes either considering individuals of all ages or considering only those birds marked as nestlings (). In general, the life-history parameters we report here were quite similar to those reported by Antor et al. (Citation2007). Importantly, the main difference between our results and those reported by Antor et al. (Citation2007) are likely because of the higher sample size and the longer study period in our case. Consequently, we probably sampled a greater proportion of older recruiting birds and thus our data provide a revision upwards of previous estimates. For example, our data showed that bearded vultures recruited and bred later () than the figures reported in Antor et al. (Citation2007; 6.5 ± 1.6 years, n = 23 and 8.1 ± 1.8 years, n = 10, respectively). Age of first successful breeding was lower than the 11.4 ± 3.9 years (n = 5) reported in Antor et al. (Citation2007). Males may breed successfully earlier than females () although no statistical differences were found between sexes in our study.
Table 1. Descriptive statistics of life-history events of bearded vultures tagged with plastic wing-tags in central Pyrenees (Spain).
Bearded vultures showed delayed reproduction, ranging extensively during their juvenile dispersal period until settlement in a breeding territory (Urios et al. Citation2010, authors' unpubl. data) which usually occurs when full adult plumage was attained. This pattern differs from that reported in other raptors, where subadult birds usually form a breeding pair with an adult (Ferrer et al. Citation2003, López-López et al. Citation2007). In our case, one subadult attempted to breed for the first time at the age of six years, although unsuccessfully. Similar results of subadult birds attempting to breed have been reported in the small population of Crete (Xirouchakis & Grivas Citation2002) and Corsica (Antor et al. Citation2007, unpubl. data).
In relation to mating composition, of 38 birds, nine birds (23.7%) settled for the first time in territories replacing one adult of a the pair; five birds (13.2%) in territories occupied by trios; 16 birds (42.1%) formed new territories of which 13 were occupied by pairs, one by a trio and one unknown; one bird (2.6%) settled in an old vacant territory; and seven birds (18.4%) in territories where the mating composition was unknown. Of 23 first breeding attempts recorded, 60.9% occurred in territories occupied by pairs, 13.0% in territories occupied by trios and 26.1% in territories were mating composition was unknown.
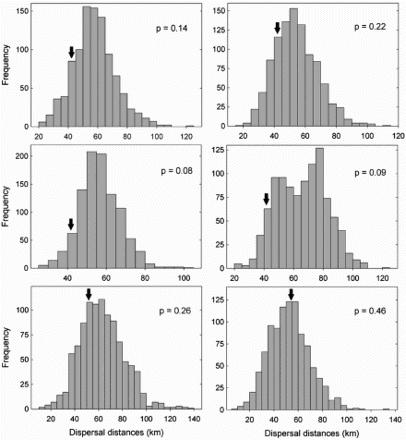
Average natal dispersal distance was 47.1 ± 26.5 km (median = 43.1 km; range: 13.1–96.3 km; n = 10). Dispersal distances did not differ between sexes (males: 47.3 ± 29.5 km; median = 43.1 km; range = 13.1–96.3 km; n = 6; females: 46.7 ± 25.6 km; median = 50.5 km; range = 17.6–68.4 km; n = 4; Mann–Whitney, U4,6 = 11.0, Z = 0.21, P = 0.83). In general, using data of males and females pooled, Monte Carlo random models for expected median dispersal distances did not deviate from a random dispersal pattern (). However, when sexes were examined separately, natal dispersal distances tended to be lower than expected for males but not for females (). Although results should be interpreted cautiously due to limited sample size, the output of Monte Carlo simulations over standard parametric analyses was preferred because they do not require the assumption that the data are sampled from a specific theoretical probability distribution (Gotelli & Ellison Citation2004, Manly Citation2006, Serrano et al. Citation2008). In fact, spatially explicit information including the location of territories was used to generate an appropriate null distribution of potential natal dispersal distances for this study. In addition, another advantage of the Monte Carlo method is that it makes clear and explicit the underlying assumptions and the structure of the null hypothesis (Gotelli & Ellison Citation2004). Interestingly, similar sex-differences in dispersal behaviour were obtained under the two different scenarios considered in Monte Carlo simulations, which possibly suggests that natal dispersal patterns were not constrained by the natal territories where birds were trapped ().
Figure 1. Frequency distributions of expected median dispersal distances for bearded vultures using null models and Monte Carlo simulations under two different scenarios: (i) expected distances from natal territories of marked individuals (left column), and (ii) expected distances among all territories (right column). Simulations were run separately for all individuals (top), males (centre) and females (bottom). The black arrow indicates observed median dispersal distances. We used Monte Carlo methods to evaluate the probability that observed natal dispersal distances could have occurred by chance by generating our own null distribution of potential natal dispersal distances. This null distribution was generated using spatially explicit information including the exact location of territories in the study area. P-values were obtained by counting the number of randomized cases that resulted in an equal or larger/smaller value than the observed frequency of dispersal distances, and then divided by the total number of randomizations (i.e. 1000 in our case).
Dispersal has many advantages such as range expansion and avoidance of potential inbreeding depression (Clobert et al. Citation2001, Newton Citation2003). Sex differences in natal dispersal have been reported in a wide range of species, including passerines, raptors, owls, waders and colonial seabirds (Newton Citation2003). In birds, females tend to disperse farther than males (Greenwood Citation1980). In addition, philopatry favours the evolution of cooperative traits between members of the sedentary sex (Greenwood Citation1980). In agreement with this, the bearded vulture may follow all these general rules, with the males likely being both the philopatric and the cooperative sex, although dispersal distances in this study only showed a tendency to be lower for males (but with limited power because of small sample sizes). The existence of polyandrous trios and even polyandrous quartets has been widely reported for bearded vultures (Heredia & Donázar Citation1990, Margalida et al. Citation1997), outnumbering cooperative polygynic cases (Fasce & Fasce Citation2012).
In general, natal dispersal distances were quite similar to those previously reported in Margalida et al. (Citation2005; 48.0 ± 28.9 km; range: 18.2–87.3; n = 5). No individuals were known to settle out of the Pyrenees and thus, at this scale, bearded vultures showed consistent phylopatric behaviour. This result is reliable in terms of detectability taking into account that field identification is easy and there are many field ornithologists in the potential dispersal areas of the species that could report the observation of new pairs outside the Pyrenees. Similar results were found in the Alps, where birds also showed high philopatric behaviour (Zink & Izquierdo Citation2011).
Finally, mean dispersal direction was 73.7° ± 24.2° (males: 80.8° ± 38.3°, n = 6; females: 9.8° ± 7.9°, n = 4), with no significant differences between sexes (Mann–Whitney test, U4,6 = 7.0, Z = 1.07, P = 0.29). When data were analysed separately, males showed a preferred eastward natal dispersal direction (Rayleigh test, r = 0.66, P = 0.009) and females did not differ from a uniform distribution (Rayleigh test, r = 0.02, P = 0.48). Although these results should be considered cautiously because of limited sample size, this has several important consequences from the conservation point of view and might help to understand the asymmetric observed pattern of eastward range expansion exhibited by the bearded vulture in the Pyrenees in the previous decades. From a conservation perspective, possible management actions aimed at favouring westwards range expansion could include indirect measures such as the establishment of supplementary feeding areas in the western Pyrenees and neighbouring mountain ranges (e.g. Navarre-Basque-Cantabric Mountains), and direct measures such as hacking of juveniles (preferably males, the most likely philopatric sex) in the territories located in the western part of the current distribution area.
ACKNOWLEDGEMENTS
We are indebted to regional and national governments of Spain and France, EU (LIFE and INTERREG programs), Guardia Civil, Forest Rangers, Wildlife Rehabilitation Centre at La Alfranca, WWF/Spain, SEO-BirdLife, Ecologistas en Acción, GREFA, LPO, LVFS, IREC-CSIC, EBD-CSIC, ‘Aragón’ Ringing Group and all field naturalists that helped in fieldwork. Special thanks are due to J. Guiral, F. Hernández and to all members of the FCQ. W. Cresswell and two anonymous referees made valuable suggestions to improve the original manuscript. The authors declare that they have no conflict of interest. This paper complies with the current laws in Spain.
FUNDING
P. López-López is supported by a ‘Juan de la Cierva’ postdoctoral grant of the Spanish Ministry of Economy and Competitiveness [reference JCI-2011-09588].
References
- Agostinelli, C. 2009. CircStats: circular statistics, from “Topics in Circular Statistics” (2001) [online]. R package version 0.2-4. Available from: http://CRAN.R-project.org/package=CircStats
- Antor, R.J., Margalida, A. & Heredia, R. 2005. Quebrantahuesos. Gypaetus barbatus. In Madroño, A., González, C. & Atienza, J.C. (eds.) Libro rojo de las aves de España. Dirección General para la Biodiversidad-SEO/BirdLife, 125–129. Madrid (in Spanish).
- Antor, R.J., Margalida, A., Frey, H., Heredia, R., Lorente, L. & Sesé, J.A. 2007. First breeding age in captive and wild bearded vultures Gypaetus barbatus. Acta Ornithol. 42: 114–118. doi: 10.3161/068.042.0106
- BirdLife International. 2013. Species factsheet: Gypaetus barbatus [online]. Available from: http://www.birdlife.org [Accessed 2 September 2013].
- Clobert, J., Danchin, E., Dhondt, A.A. & Nichols, J.D. 2001. Dispersal. Oxford University Press.
- Fasce, P. & Fasce, L. 2012. First polygynous trio of bearded vultures (Gypaetus barbatus). J. Raptor Res. 46: 216–219. doi: 10.3356/JRR-11-62.1
- Ferguson-Lees, J. & Christie, D.A. 2001. Raptors: Birds of Prey of the World. A & C Black, London.
- Ferrer, M., Penteriani, V., Balbontín, J. & Pandolfi, M. 2003. The proportion of immature breeders as a reliable early warning signal of population decline: evidence from the Spanish imperial eagle in Doñana. Biol. Conserv. 114: 463–466. doi: 10.1016/S0006-3207(03)00085-5
- García, C.B., Insausti, J.A., Gil, J.A., De Frutos, Á., Alcántara, M., González, J., Cortés, M.R., Bonafonte, J.I. & Arruga, M.V. 2009. Comparison of different procedures of DNA analysis for sex identification in the endangered bearded vulture (Gypaetus barbatus). Eur. J. Wildlife Res. 55: 309–312. doi: 10.1007/s10344-008-0239-y
- Gil, J.A., Díez, O., Báguena, G., Lorente, L., Pérez, C., Losada, J.A. & Alcántara, M. 2010. Juvenile Dispersal of the Bearded Vulture (Gypaetus barbatus) in the Pyrenees (Spain-France). Fundación para la Conservación del Quebrantahuesos (FCQ), Zaragoza.
- Gotelli, N.J. & Ellison, A.M. 2004. A Primer of Ecological Statistics. Sinauer Associates, Sunderland, MA.
- Greenwood, P.J. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28: 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
- Greenwood, P.J. & Harvey, P.H. 1982. The natal and breeding dispersal of birds. Ann. Rev. Ecol. Syst. 13: 1–12. doi: 10.1146/annurev.es.13.110182.000245
- Heredia, R. 2005. Status y distribución del quebrantahuesos en España y diagnóstico de la situación de la población en la UE. In Margalida, A. & Heredia, R. (eds.) Biología de la conservación del Quebrantahuesos (Gypaetus barbatus) en España. Naturaleza y Parques Nacionales, Serie técnica, Organismo Autónomo Parques Nacionales, 21–37. Madrid (in Spanish).
- Heredia, R. & Donázar, J.A. 1990. High frequency of polyandrous trios in an endangered population of Lammergeiers Gypaetus barbatus in Northern Spain. Biol. Conserv. 53: 163–171. doi: 10.1016/0006-3207(90)90083-2
- Hirzel, A.H., Posse, B., Oggier, P.A., Crettenand, Y., Glenz, C., & Arlettaz, R. 2004. Ecological requirements of reintroduced species and the implications for release policy: the case of the bearded vulture. J. Appl. Ecol. 41: 1103–1116. doi: 10.1111/j.0021-8901.2004.00980.x
- Kokko, H. & López-Sepulcre, A. 2006. From individual dispersal to species ranges: perspectives for a changing world. Science 313: 789–791. doi: 10.1126/science.1128566
- Lacasa, M. 2012. The Book of Raptors. Photodigiscoping, Sabadell.
- López-López, P., García-Ripollés, C. & Urios, V. 2007. Population size, breeding performance and territory quality of Bonellís eagle (Hieraaetus fasciatus) in eastern Spain. Bird Study 54: 335–342. doi: 10.1080/00063650709461493
- López-López, P., Gil, J.A. & Alcántara, M. 2011. Morphometrics and sex determination in the endangered bearded vulture (Gypaetus barbatus). J. Raptor Res. 45: 361–366. doi: 10.3356/JRR-11-32.1
- Manly, B.F.J. 2006. Randomization, Bootstrap and Monte Carlo Methods in Biology. Chapman & Hall/CRC.
- Margalida, A. & Heredia, R. (eds). 2005. Biología de la Conservación del Quebrantahuesos (Gypaetus barbatus) en España. Serie técnica, Organismo Autónomo Parques Nacionales, Ministerio de Medio Ambiente, Madrid (in Spanish).
- Margalida, A., García, D. & Bertran, J. 1997. A possible case of a polyandrous quartet in the bearded vulture (Gypaetus barbatus). Ardeola 44: 109–111.
- Margalida, A., García, D., Bertran, J. & Heredia, R. 2003. Breeding biology and success of the bearded vulture Gypaetus barbatus in the eastern Pyrenees. Ibis 145: 244–252. doi: 10.1046/j.1474-919X.2003.00148.x
- Margalida, A., García, D., Bertran, J. & Heredia, R. 2005. Biología de la reproducción del quebrantahuesos en los Pirineos. In Margalida, A. & Heredia, R. (eds.) Biología de la Conservación del Quebrantahuesos (Gypaetus barbatus) en España, 279–304. Serie técnica, Organismo Autónomo Parques Nacionales, Ministerio de Medio Ambiente, Madrid (in Spanish).
- Newton, I. 1998. Population Limitation in Birds. Academic Press, London.
- Newton, I. 2003. The Speciation and Biogeography of Birds. Academic Press, London.
- Oro, D., Margalida, A., Carrete, M., Heredia, R. & Donázar, J.A. 2008. Testing the goodness of supplementary feeding to enhance population viability in an endangered vulture. PLOS ONE 3: e4084. doi: 10.1371/journal.pone.0004084
- Schaub, M., Zink, R., Beissmann, H., Sarrazin, F., & Arlettaz, R. 2009. When to end releases in reintroduction programmes: demographic rates and population viability analysis of bearded vultures in the Alps. J. Appl. Ecol. 46: 92–100. doi: 10.1111/j.1365-2664.2008.01585.x
- Serrano, D., Carrete, M. & Tella, J.L. 2008. Describing dispersal under habitat constraints: a randomization approach in lesser kestrels. Basic Appl. Ecol. 9: 771–778. doi: 10.1016/j.baae.2007.08.013
- Urios, V., López-López, P., Limiñana, R. & Godino, A. 2010. Ranging behaviour of a juvenile bearded vulture (Gypaetus barbatus meridionalis) in South Africa revealed by GPS satellite telemetry. Ornis Fennica 87: 114–118.
- Xirouchakis, S. & Grivas, C. 2002. Age at first breeding of Lammergeier Gypaetus barbatus. Sandgrouse 24: 130–134.
- Zink, R. & Izquierdo, D. 2011. Annual Report 2010. International Bearded vulture Monitoring (IBM). Hohe Tauern National Park/Owl- and Raptor Centre Haringsee, Austria.