Abstract
Capsule Sex ratios were determined for 11 wader species at the northern end of the East Atlantic flyway in winter. The ratio was even for six species, there were more males for four species, and more females for one species.
Aims To describe the sex ratio of adult waders in northern Scotland and examine departures from parity.
Methods Molecular sexing and biometrics were used to estimate the sex ratio (percentage male) in adult populations of 11 waders wintering on estuaries and open shores in northern Scotland (Moray Firth and Orkney), at the northern part of the East Atlantic flyway. Departures from parity were examined in relation to three possibilities: (1) that there was local variation in the distribution of the sexes; (2) that the sexes differed in their winter ranges and (3) that there was an uneven sex ratio in the entire population.
Results The percentage of males did not differ significantly from 50% for Lapwing, Ringed Plover, Dunlin, Knot, Sanderling and Turnstone. There were significant differences from parity for Oystercatcher, Redshank, Bar-tailed Godwit and Curlew at some sites, suggesting local segregation of the sexes that may be related to habitat. It was difficult to examine possibilities 2 and 3 due to the lack of data from other parts of the flyway. Only some populations of Purple Sandpipers and perhaps Bar-tailed Godwits exhibited an uneven sex ratio in favour of males across the flyway. For these species, the uneven sex ratio in favour of males was already apparent in first-year birds, showing that higher mortality amongst juvenile females, rather than higher mortality amongst breeding females probably causes the imbalance.
Conclusion Some waders showed significant deviations from parity in their sex ratio. These may be due to sex-dependent habitat selection and differential mortality rates.
The genetic process of meiosis determines that, by chance, the sex ratio should be even for birds (Ayala & Kiger Citation1980). There may also be stabilizing selection for an even sex ratio in monogamous species (Fisher Citation1930). Despite these processes, uneven sex ratios are common. In reviews of the sex ratios amongst adult birds, Mayr (Citation1939), Lack (Citation1954), Clutton-Brock (Citation1986) and Donald (Citation2007) found that males often outnumbered females, citing greater mortality amongst females, particularly associated with breeding (Trivers Citation1972, Bennett & Owens Citation2002), as the cause of the imbalance. However, Breitwisch (Citation1989) noted that higher female mortality also occurred before first breeding, indicating that higher female mortality need not be related to the stresses of reproduction or increased risk of predation during incubation.
Sexual selection (Darwin Citation1871) brought about by the different roles of the sexes during the breeding season may lead to sexual dimorphism in plumage and/or size. Such morphological differences may then determine the niche of each sex (Selander Citation1966), and this may be enhanced by character divergence through competition between the sexes (Slatkin Citation1984). Thus, we see intersexual differences in the species or size of prey taken (Summers et al. Citation1990a, Aplin & Cockburn Citation2012), the way a habitat is used (Smith & Evans Citation1973), small-scale segregation according to habitat or resource distribution (Townshend Citation1981, Alves et al. Citation2013), and geographical segregation during the non-breeding season (Ketterson & Nolan Citation1983). Therefore, sex ratio can be even within an entire population, but may vary geographically according to differential use of habitats and range.
Donald's (Citation2007) recent review of sex ratios in adult birds was biased towards Passeriformes and Ciconiiformes, which together formed 66.2% of his sample of 201 estimates. No Charadiiformes were represented. Lack of data from this order stems from the low level of sexual dimorphism in plumage, which is usually used to estimate sex ratio. It is only recently that molecular sexing techniques (Griffiths et al. Citation1998, Fridolfsson & Ellegren Citation1999) have been available to investigate the sex ratio in free-living birds with no, or little, sexual dimorphism.
In waders (Charadrii), there is often no plumage dimorphism during the non-breeding season, so that sex ratios have been described only for those with marked size dimorphism. Studies have focused on the geographical segregation by the sexes (also known as differential migration). For example, male Ruffs Philomachus pugnax and Western Sandpipers Calidris mauri tend to occur north of females during the non-breeding season (Page et al. Citation1972, Gill et al. Citation1995, OAG Münster Citation1996, Nebel et al. Citation2002). There is also some evidence of this for Least Sandpipers Calidris minutilla (Nebel Citation2006). There are three main hypotheses accounting for differential migration of the sexes; the body-size hypothesis, dominance hypothesis and arrival-time hypothesis (Cristol et al. Citation1999). In the body-size hypothesis, it is assumed that larger individuals survive the cold better than small ones because they lose less heat through a relatively smaller surface area, thereby allowing them to occupy a more northern wintering area (Ketterson & Nolan Citation1976). In the dominance hypothesis, the dominant sex acquires areas closest to the breeding grounds, forcing subordinates to more distant areas (Gauthreaux Citation1978, Townshend Citation1981). The assumptions under this hypothesis are that one sex can limit the resources of another and that there is a cost to migration. In the arrival-time hypothesis, it is assumed that by wintering close to the breeding grounds, a bird is best placed to gauge the earliest opportunity to migrate to the breeding grounds, as determined by weather conditions, and thereby increase the likelihood of attaining a territory (Myers Citation1981). Under this hypothesis, the sex that acquires a territory should winter closer to the breeding grounds.
Myers (Citation1981) reviewed the information for the above three waders (Ruff, Western Sandpiper and Least Sandpiper) and combined this with data for five other species, including two other waders; Grey Phalaropes Phalaropus fulicarius and Sanderlings Calidris alba. He found that males (even if they have a smaller body size than females) tended to winter north of females. This finding tends to falsify the body-size and dominance hypotheses and favour the arrival-time hypothesis. He concluded that males prefer to spend the non-breeding season close to the breeding grounds in order to attain the breeding grounds as soon as weather conditions allow in spring. If this was the case, one might expect this to be a general phenomenon for Arctic- and boreal-breeding waders that were territorial. However, data for the East Asian Australian flyway suggest that this is not the case. There is a tendency for a higher percentage of male Curlew Sandpipers Calidris ferruginea to occur further south: Thailand (56% males), India (54%), NW Australia (60%), SE Australia (66%) and Tasmania (77%) (Barter Citation1986, Citation1987). This contrasts with the situation in South Africa at the southern end of the East Atlantic flyway where there were more adult female than adult male (44.8%) Curlew Sandpipers (Elliott et al. Citation1976). Clearly, there is much to learn about the sex ratios of waders.
The Moray Firth in northern Scotland comprises the most northerly complex of estuaries on the East Atlantic flyway, supporting over 40 000 waders in winter (Swann & Mudge Citation1989, Kalejta-Summers Citation2006). Here, sex ratios can be observed during winter conditions close to the northern limit of many wader species. Initially, we tested whether there was an uneven sex ratio in 11 species. There was the possibility that the samples captured in one locality were not representative for the species in northern Scotland. Therefore, for those species with an uneven sex ratio, we then examined whether there was local segregation of the sexes (1), or whether there was wider geographic segregation (2). If the winter range of each sex is determined by an ability to withstand a north temperate winter (body-size hypothesis), we would expect a greater percentage of the larger sex to winter in Scotland. This is likely to be more noticeable in small species because they have a higher surface area to volume ratio, and thereby lose heat faster than large species. Likewise, if segregation is mediated through dominance, we would expect the larger sex to winter north of the smaller, if northern sites are preferred. This may be related to migration distance. However, if acquiring a territory is paramount (arrival-time hypothesis), we would expect more males and this to be more noticeable in temperate-breeding rather than Arctic-breeding species because breeding is more compressed in time for the latter. By wintering closer to their breeding areas, it will be easier for temperate-breeding birds to react to the improving spring weather than Arctic-breeding species that winter further from their breeding areas (Alerstam & Högstedt Citation1980). Finally, we reviewed the possibility that the sex ratio was uneven for the entire adult population of a species (3).
METHODS
Wader species were captured with a cannon-net at high-tide roosts at localities in the Moray Firth (58°N, 4°W) () and the Orkney Islands (59°N, 3°W), northern Scotland, in winter (November–March 1980–2012, and from 2008 for molecularly sexed samples). Sex ratios (percentage male) were based on single catches or the accumulation of data from multiple catches at given sites and it was assumed the cannon-netting provided a representative sample of the sexes that used the adjoining inter-tidal area where the birds foraged. Any differential distribution of the sexes at roosts could bias the results if only part of a roosting flock was captured. The Moray Firth comprises several smaller firths (estuaries), separated by sandy beaches or rocky shores. The Orkney samples were captured on two of the smaller islands of the archipelago; Papa Westray and Sanday. A feather was taken from adult birds of the following species for molecular sexing: Oystercatcher Haematopus ostralegus, Lapwing Vanellus vanellus, Ringed Plover Charadrius hiaticula, Redshank Tringa totanus, Curlew Numenius arquata, Dunlin Calidris alpina, Sanderling C. alba, Purple Sandpiper Calidris maritima, Knot Calidris canutus and Turnstone Arenaria interpres. All have monogamous breeding systems in which the male arrives on the breeding ground before the female or at the same time (Soikkeli Citation1967, Cramp & Simmons Citation1983, Whitfield & Brade Citation1991, Warnock & Gill Citation1996, MacWhirter et. al. Citation2002, Payne & Pierce Citation2002). Although all these species occur north of Scotland during the non-breeding season (e.g. in Iceland and/or Norway), Scotland is at the northern part of the range for most. Only the Purple Sandpiper has larger numbers north of Scotland (Cramp & Simmons Citation1983). Ageing was based on plumage characteristics; usually the wing coverts (Prater et al. Citation1977).
DNA was extracted from the feathers for molecular sex determination by applying a modified version of the Chelex-100 extraction method by Walsh et al. (Citation1991). The proximal end of the feather shaft was sliced below the barbs. Two hundred and fifty microlitres of 6% Chelex solution and 2.5 μl of 1% Proteinase K were used per sample. Molecular sex-typing was performed by amplification of the CHD-Z and CHD-W genes via polymerase chain reaction (PCR) using the 2550F/2718R primers and the PCR settings in Fridolfsson & Ellegren (Citation1999). When PCR failed to give reliable bands, the reaction was repeated, applying the P2/P8 primers and the protocol described by Griffiths et al. (Citation1998). All PCR reactions were in a volume of 10 μl using 0.09 U AmpliTaq enzyme, 1.75 μl dNTP, 1.15 μl Tween 20 (1%), 1.0 μl Taq Buffer, 0.38 μl BSA (10 mg/ml), and 0.34 μl of both 2550F (forward) and 2718R (reverse) primers. In total, 1.0 μl of DNA was added to the 9 μl PCR solution. The PCR conditions were as described by Fridolfsson & Ellegren (Citation1999). PCR products were visualized on a 1.5% agarose gel. Some samples failed so the individual could not be sexed. If a disproportionate number of one sex failed, this would bias the results. For example, there was concern that a large number of Purple Sandpipers from Papa Westray failed to be molecularly sexed () and this may have led to a bias. However, the estimate of sex ratio was consistent with the other samples of Purple Sandpipers.
Table 1. The sex ratios (% male) of adult waders in northern Scotland during winter, as determined by molecular sexing.
Curlews and Bar-tailed Godwits Limosa lapponica were sexed according to bill length (females have longer bills than males). This was possible because they are strongly sexually dimorphic in bill length (Cramp & Simmons Citation1983, Engelmoer & Roselaar Citation1998). Bill length frequency distributions were bimodal for both species, and, for the Bar-tailed Godwits, mean bill lengths and standard deviations of the sexes were derived from component lines of the cumulative percentages (Harding Citation1949, Cassie Citation1954, Lewis & Taylor Citation1967). The average of the means was used as the point of separation to sex all birds. The Curlew is not as strongly dimorphic as the Bar-tailed Godwit, so 67 birds were molecularly sexed to derive a logistic regression model that allowed calculation of the probability of each individual being a male, or female, from its bill length. In total, 95.5% of the sample of 67 was sexed correctly (Summers et al. Citation2013). For other captured samples, the probability of being male was obtained from the logistic regression equation. The percentage of birds where the probability of being male was greater or equal to 0.5 provided an initial measure of the percentage male. As some birds would have been incorrectly classified, the percentages were corrected using a linear regression between corrected and uncorrected percentages (Summers et al. Citation2013).
Where there were estimates of percentage male for the different estuaries within the Moray Firth, we used count data for each estuary (Kalejta-Summers Citation2006) to estimate the total number of each sex, and obtained a composite sex ratio for combined sites within the Moray Firth.
The Knot, Dunlin, Sanderling, Purple Sandpiper, Turnstone and Bar-tailed Godwit were classed as Arctic-breeding species, and Oystercatcher, Lapwing, Ringed Plover, Redshank and Curlew were classed as north temperate-breeding species (Swann & Etheridge Citation1996). A t-test was used to compare the mean sex ratios of Arctic- and north temperate-breeding species. Chi-squared tests were used to test if sex ratios were statistically different from parity, and to test for differences within species across sites. Unsexed birds within a sample were excluded. Where widespread sampling found a predominance of one sex, without a matching predominance of the other sex elsewhere, an uneven sex ratio within a breeding population or species was presumed.
RESULTS
For the samples sexed by molecular methods, the sex ratio for adult Lapwing, Ringed Plover, Dunlin, Knot, Sanderling and Turnstone did not deviate significantly from parity (). Uneven sex ratios were found in samples of Oystercatcher, Redshank and Purple Sandpiper (). However, the results were not consistent for Oystercatchers and Redshanks, with contrasting results from different localities. Thus, the preponderance of female Oystercatchers at a muddy site in the Inverness Firth was not seen at a sandy site in the neighbouring Cromarty Firth (Yates' corrected χ2 = 5.6, df = 1, P = 0.018). Likewise, the preponderance of male Redshanks in the two firths (both muddy sites) contrasted with the preponderance of females at Portgordon, which was a rocky shore site (χ2 = 8.8, df = 2, P = 0.012). The three samples of Purple Sandpipers showed a similar preponderance of males, which was significantly different from 50% when the Orkney samples were combined.
The bill length distribution of the adult Bar-tailed Godwits showed a marked separation of the sexes, with no overlap in the 95% ranges for the males and females (). Therefore, 90.75 mm was taken as the point of separation of the sexes. Samples from different locations around the Moray Firth showed that there was either an even sex ratio, as seen at sites in the northern estuaries, or a preponderance of males, as seen at sites in the southern estuaries ( and ). There were statistically different percentages amongst sites (χ2 = 93.6, df = 6, P < 0.001). Combining these percentages with the known winter population sizes in different areas of each firth, the overall percentage of male godwits for the Moray Firth was 69%. A preponderance of males was also apparent in first-year birds (). At most sites, the percentage of males amongst first-year birds was similar to that of the adults ().
Figure 2. Frequency histograms for bill lengths (mm) of adult and first-year Bar-tailed Godwits captured in the Moray Firth in winter. The horizontal lines show the 95% ranges (1.96 standard deviations, either side of the means) (•). The means for the adults were 80.5 mm (sd = 4 mm) for males and 101.0 mm (sd = 4.5 mm) for females, and the split between the sexes taken as 90.75 mm. The means for the first years were 80.0 mm (sd = 4 mm) for males and 100.0 mm (sd = 5 mm) for females, and the split between the sexes taken as 90.0 mm.
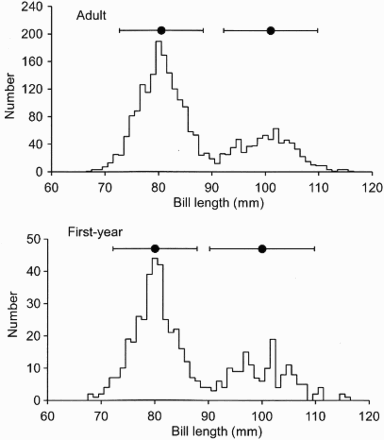
Figure 3. The percentage of adult male (black) and female (grey) Bar-tailed Godwits at different localities within the Moray Firth.
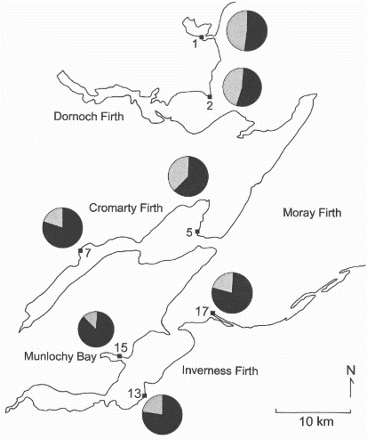
Table 2. Sex ratios (% male) of adult and first-year Bar-tailed Godwits and adult Curlews in different parts of the Moray Firth, as determined by bill length.
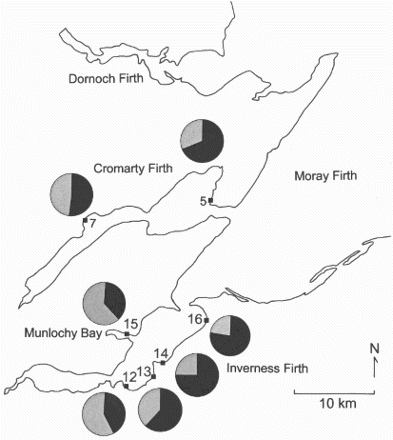
For Curlews, there was a preponderance of males in the outer parts of the firths and a preponderance of females in the inner parts ( and ). There were statistical different percentages amongst sites (χ2 = 59.3, df = 6, P < 0.001). Combining the percentages with winter population sizes in different areas, the overall percentage of males in the Moray Firth was 58%.
Figure 4. The percentage of adult male (black) and female (grey) Curlews at different localities within the Moray Firth.
There was no difference in the mean sex ratios of the Arctic-breeding waders when compared with the north temperate-breeding species (t = 0.7, df = 9, P = 0.48), and no difference when the five larger species were compared with the six smaller (t = 0.5, df = 9, P = 0.65).
DISCUSSION
Our study found that sex ratios of adult waders in northern Scotland were either not significantly different from parity (Lapwing, Ringed Plover, Knot, Sanderling, Dunlin and Turnstone), or differed from parity at different localities in the Moray Firth (Oystercatcher, Redshank, Purple Sandpiper, Bar-tailed Godwit and Curlew). Some of the samples were small, so small departures from parity may become statistically significant with larger sample sizes. Some of departures from parity may be related to habitat segregation between the sexes, as observed for Curlew (Ens Citation1979, Townshend Citation1981, Evans Citation1988). In this species, males often make more use of grassland adjoining estuaries to feed on earthworms because it becomes progressively more difficult for the shorter-billed males to capture ragworms Nereis diversicolor which lie deeper in burrows on the mudflats as the temperature drops during winter. By contrast, females can maintain their intake by reaching into the deep burrows of ragworms with their longer bills (Townshend Citation1981). The availability of grassland close to the different roosts may be affecting the sex ratio differences around the Moray Firth. For Bar-tailed Godwits, foraging differences have been found to occur at the scale of micro-habitats, with females using deeper water to forage in (Smith & Evans Citation1973). However, the differences in the percentage of males across the Moray Firth () suggest that there are perhaps other sexual differences in habitat use to be discovered.
We found that some species had a predominance of males (Redshank, Bar-tailed Godwit, Curlew and Purple Sandpiper) after pooling across sites. There is little or no information on the sex ratios of these species from localities elsewhere on the East Atlantic flyway. In a large sample of Bar-tailed Godwits (also subspecies lapponica) from the Banc d'Arguin in Mauritania, there was a preponderance of males (75.7%, n = 1047) (Altenburg et al. Citation1982, p 136). Godwits on other flyways show some differing results. Wilson et al. (Citation2007) caught more male Bar-tailed Godwits of the subspecies L. lapponica menzbieri (58.7% male, n = 3507) in northwest Australia, but similar numbers of males and females of L. lapponica baueri (49.6% male, n = 1220) in Victoria, in southern Australia. Thus, to date, no population of Bar-tailed Godwits appears to have a predominance of females, but clearly further samples are required.
There have been several studies of the sex ratio of Purple Sandpipers, showing that a preponderance of males is a feature on wintering grounds for populations that originate from Canada, Norway and Iceland (). However, at northern sites, where Russian and Greenland birds spend the winter, the sex ratio is even. The predominance of males also occurs within the first-year birds (). Thus, for Purple Sandpipers and Bar-tailed Godwits, there is some evidence that males predominate within some populations. We were, therefore, unable to find further examples of latitudinal segregation of the sexes, nor support for Myers’ (1981) observation that male waders tend to winter north of females.
Figure 5. The percentage of male Purple Sandpipers at different locations in winter for birds from different breeding populations, shown in square brackets. The horizontal lines show 95% CLs, and open symbols refer to first-year (1Y) birds alone. Circles – sexed on bill length, squares – sexed on gonads, triangles – molecularly sexed. The data were from this study, Belopolski (Citation1941), Nicoll et al. (Citation1988), Summers et al. (Citation1990b), Hake et al. (Citation1997), Mittelhauser et al. (Citation2006), Strann & Summers (Citation2006), Summers (Citation2007), Foster et al. (Citation2010), Hallgrimsson et al. (Citation2011) and Guyonnet et al. (Citation2011).
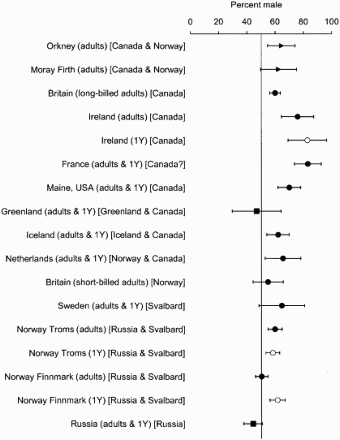
All the waders we examined are monogamous breeders (Pitelka et al. Citation1974, Cramp & Simmons Citation1983). Therefore, one might expect for those species where we found an uneven sex ratio that there will be a population elsewhere where the sex ratio is in favour of the other sex, to give a more balanced sex ratio within the species. For Ruffs and Western Sandpipers, northern wintering populations have more males and southern ones have more females (OAG Münster Citation1996, Page et al. Citation1972, Nebel et al. Citation2002). However, these sex ratios have not been applied to population sizes at different latitudes to show that there is a balanced sex ratio in the species, or a preponderance of one sex. For Purple Sandpiper and perhaps Bar-tailed Godwit, evidence suggests that there are more males throughout some geographical populations. This was apparent in Canadian Purple Sandpipers where there was a predominance of males in most samples. A preponderance of males in monogamous birds will have consequences for mate acquisition during the breeding season (Székely et al. Citation1999).
The preponderance of males in Purple Sandpipers is not due to an uneven sex ratio at hatching (Hallgrimsson et al. Citation2011). Rather, the imbalance occurs within the first six months after hatching (). Therefore, the differential mortality is not associated with higher mortality amongst breeding adult females, but with juveniles. This supports the general findings of Breitwisch (Citation1989). Female Purple Sandpiper chicks are relatively lighter than the smaller males, perhaps because they cannot acquire sufficient food to match their greater growth requirements, and this may affect their post-fledging survival (Hallgrimsson et al. Citation2011). We speculate whether a similar process may operate for the Bar-tailed Godwit, which also has a high degree of sexual size dimorphism and a sex-ratio imbalance that occurs at the juvenile stage (). If this is the case, it is possible that uneven sex ratios can be a consequence of sexual size dimorphism.
To increase our understanding of sex ratios of waders, analyses of existing data and further collection of representative data on sex ratios in different habitats across the flyway are required. Ideally, samples of over 100 birds are required to provide adequate estimates. In addition, information on habitat availability and the feeding ecology of the different sexes is required to help interpret the differences that exist.
ACKNOWLEDGEMENTS
We thank fellow members of the Highland and Orkney Ringing Groups who helped catch waders. Isabelle Louviau and Virginia Escudero Velasco assisted with molecular sexing. Nigel Clark, Jenny Gill and Natalie dos Remedios kindly commented on the drafts. Feather sampling was carried out under licence.
FUNDING
The study was funded by the Highland Ringing Group from money raised by Ronnie and Shenac Graham.
REFERENCES
- Alerstam, T. & Högstedt, G. 1980. Spring predictability and leap-frog migration. Ornis. Scand. 11: 196–200. doi: 10.2307/3676124
- Altenburg, W., Engelmoer, M., Mes, R. & Piersma, T. 1982. Wintering waders on the Banc d'Arguin Mauritania. Report of the Netherlands Ornithological Mauritanian Expedition 1980. Communication No. 6 of the Wadden Sea Working Group.
- Alves, J.A., Gunnarsson, T.G., Potts, P.M., Sutherland, W.J. & Gill, J.A. 2013. Sex-biases in distribution and resource use at different spatial scales in a migratory shorebird. Ecol. Evol. 3: 1079–1090. doi: 10.1002/ece3.503
- Aplin, L.M. & Cockburn, A. 2012. Ecological selection and sexual dimorphism in the Sooty Oystercatcher, Haematopus fuliginosus. Austral. Ecol. 37: 248–257. doi: 10.1111/j.1442-9993.2011.02263.x
- Ayala, F.J. & Kiger, J.A., Jr. 1980. Modern Genetics. Benjamin/Cummings Publishing Company, California.
- Barter, M. 1986. Sex-related differences in adult Curlew Sandpipers Calidris ferruginea caught in Victoria. Stilt 8: 2–8.
- Barter, M. 1987. Are Curlew Sandpipers sexist – and if so, why? Stilt 11: 14–17.
- Belopolski, L.O. 1941. The ecology of the wintering Purple Sandpiper Calidris maritima (Brünn.). Arch. State Nature Reserve ‘Sem’ Ostrov’ 1: 89–94.
- Bennett, P.M. & Owens, I.P.F. 2002. Evolutionary Ecology of Birds. Life Histories, Mating Systems, and Extinction. Oxford University Press, Oxford.
- Breitwisch, R. 1989. Mortality patterns, sex ratios, and parental investment in monogamous birds. Curr. Orn. 6: 1–50. doi: 10.1007/978-1-4757-9918-7_1
- Cassie, R.M. 1954. Some uses of probability paper in the analysis of size frequency distributions. Aust. J. Mar. Fresh. Res. 5: 513–522.
- Clutton-Brock, T.H. 1986. Sex ratio variation in birds. Ibis 128: 317–329. doi: 10.1111/j.1474-919X.1986.tb02682.x
- Cramp, S. & Simmons, K.E.L. (eds) 1983. The Birds of the Western Palearctic, Vol. 3. Oxford University Press, Oxford.
- Cristol, D.A., Baker, M.B. & Carbone, C. 1999. Differential migration revisited. Latitudinal segregation by age and sex class. Curr. Orn. 15: 33–88. doi: 10.1007/978-1-4757-4901-4_2
- Darwin, C. 1871. The Descent of Man, and Selection in Relation to Sex. Murray, London.
- Donald, P.F. 2007. Adult sex ratios in wild bird populations. Ibis 149: 671–692. doi: 10.1111/j.1474-919X.2007.00724.x
- Elliott, C.C.H., Waltner, M., Underhill, L.G., Pringle, J.S. & Dick, W.J.A. 1976. The migration system of the Curlew Sandpiper Calidris ferruginea in Africa. Ostrich 47: 191–213. doi: 10.1080/00306525.1976.9639558
- Engelmoer, M. & Roselaar, C.S. 1998. Geographical Variation in Waders. Kluwer, Dordrecht.
- Ens, B. 1979. Territoriality in Curlews Numenius arquata. Wader Study Group Bull 26: 28–29.
- Evans, A.D. 1988. Individual differences in foraging behaviour, habitat selection and bill morphology of wintering Curlew Numenius arquata. PhD Thesis, University of Edinburgh.
- Fisher, R.A. 1930. The Genetical Theory of Natural Selection. Clarendon Press, Oxford.
- Foster, S., Boland, H., Colhoun, K., Etheridge, B. & Summers, R. 2010. Flock composition of Purple Sandpipers Calidris maritima in the west of Ireland. Irish Birds 9: 31–34.
- Fridolfsson, A.-K. & Ellegren, H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30: 116–121. doi: 10.2307/3677252
- Gauthreaux, S.A., Jr. 1978. The ecological significance of behavioral dominance. In Bateson, P.P.G. & Klopfer, P.H. (eds.) Perspectives in Ethology: 17–54. Plenum, New York.
- Gill, J.A., Clark, J., Clark, N. & Sutherland, W.J. 1995. Sex differences in the migration, moult and wintering areas of British-ringed Ruff. Ring. Migr. 16: 159–167. doi: 10.1080/03078698.1995.9674107
- Griffiths, R., Double, M.C., Orr, K. & Dawson, R.J.G. 1998. A DNA test to sex most birds. Mol. Ecol. 7: 1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x
- Guyonnet, B., Gautier, S., Iliou, B. & Summers, R.W. 2011. The origin and population structure of Purple Sandpipers Calidris maritima in northern France. Ring. Migr. 26: 109–113. doi: 10.1080/03078698.2011.628158
- Hake, M., Blomqvist, D., Pierce, E.P., Järås, T. & Johansson, O.C. 1997. Population size, migration routes and breeding origin of Purple Sandpipers Calidris maritima wintering in Sweden. Ornis Svecica 7: 121–132.
- Hallgrimsson, G.T., Palsson, S., Summers, R.W. & Benediktsson, G.Ö. 2011. Sex ratio and sexual size dimorphism in Purple Sandpiper Calidris maritima chicks. Bird Study 58: 44–49. doi: 10.1080/00063657.2010.535807
- Harding, J.P. 1949. The use of probability paper for the graphical analysis of polymodal frequency distributions. J. Mar. Biol. Ass. UK. 28: 141–153. doi: 10.1017/S0025315400055259
- Kalejta-Summers, B. 2006. Changes in numbers and distribution of waders in the Moray Firth, 1988–2003. Scott. Birds 26: 1–16.
- Ketterson, E.D. & Nolan, V., Jr. 1976. Geographic variation and its climatic correlates in the sex ratio of eastern-wintering Dark-eyed Juncos (Junco hyemalis hyemalis). Ecology 57: 679–693.
- Ketterson, E.D. & Nolan, V., Jr. 1983. The evolution of differential bird migration. Curr. Ornithol. 1: 357–402. doi: 10.1007/978-1-4615-6781-3_12
- Lack, D. 1954. The Natural Regulation of Animal Numbers. Clarendon Press, Oxford.
- Lewis, T. & Taylor, L.R. 1967. Introduction to Experimental Ecology. Academic Press, London.
- MacWhirter, B., Austin-Smith, P., Jr. & Kroodsma, D. 2002. Sanderling (Calidris alba). In Poole, A. & Gill, F. (eds.) The Birds of North America, No. 653, 1–28. The Birds of North America, Inc., Philadelphia, PA.
- Mayr, E. 1939. The sex ratio in wild birds. Am. Nat. 73: 156–179. doi: 10.1086/280824
- Mittelhauser, G.H., Tudor, L. & Connery, B. 2006. Distribution and ecology of Purple Sandpipers wintering in the Acadia National Park Region, Maine 2001–2004. Technical Report NPS/NER/NRTR-2006/048. National Park Service, Boston, MA.
- Myers, J.P. 1981. A test of three hypotheses for latitudinal segregation of the sexes in wintering birds. Can. J. Zool. 59: 1527–1534. doi: 10.1139/z81-207
- Nebel, S. 2006. Latitudinal clines in sex ratio, bill, and wing length in Least Sandpipers. J. Field Ornithol. 77: 39–45. doi: 10.1111/j.1557-9263.2006.00021.x
- Nebel, S., Lank, D.B., O'Hara, P.D., Fernández, G., Haase, B., Delgado, F., Estela, F.A., Evans Ogden, L.J., Harrington, B., Kus, B.E., Lyons, J.E., Mercier, F., Ortego, B., Takekawa, J.Y., Warnock, N. & Warnock, S.E. 2002. Western Sandpipers (Calidris mauri) during the non breeding season: spatial segregation on a hemispheric scale. Auk 119: 922–928. doi: 10.1642/0004-8038(2002)119[0922:WSCMDT]2.0.CO;2
- Nicoll, M., Summers, R.W., Underhill, L.G., Brockie, K. & Rae, R. 1988. Regional, seasonal and annual variations in the structure of Purple Sandpiper Calidris maritima populations in Britain. Ibis 130: 221–233. doi: 10.1111/j.1474-919X.1988.tb00973.x
- OAG Münster. 1996. Do females really outnumber males in Ruff Philomachus pugnax wintering in Africa? J. Ornithol. 137: 91–100. doi: 10.1007/BF01651501
- Page, G., Fearis, B. & Jurek, R.M. 1972. Age and sex composition of Western Sandpipers on Bolinas Lagoon. Calif. Birds 3: 79–86.
- Payne, L.X. & Pierce, E.P. 2002. Purple Sandpiper (Calidris maritima). In Poole, A. & Gill, F. (eds) The Birds of North America, No. 706, 1–36. The Birds of North America, Inc., Philadelphia, PA.
- Pitelka, F.A., Holmes, R.T. & MacLean, S.F., Jr. 1974. Ecology and evolution of social organization in Arctic sandpipers. Am. Zool. 14: 185–204.
- Prater, A.J., Marchant, J.H. & Vuorinen, J. 1977. Guide to the Identification and Ageing of Holarctic Waders. BTO Guide 17. British Trust for Ornithology, Herts.
- Selander, R.K. 1966. Sexual dimorphism and differential niche utilization in birds. Condor 68: 113–151. doi: 10.2307/1365712
- Slatkin, M. 1984. Ecological causes of sexual dimorphism. Evolution 38: 622–630. doi: 10.2307/2408711
- Smith, P.C. & Evans, P.R. 1973. Studies of shorebirds at Lindisfarne, Northumberland. 1. Feeding ecology and behaviour of the Bar-tailed Godwit. Wildfowl 24: 135–139.
- Soikkeli, M. 1967. Breeding cycle and population dynamics in the Dunlin (Calidris alpina). Ann. Zool. Fenn. 4: 158–198.
- Strann, K.-B., Summers, R.W. & Rae, R. 2006. Population structure and origins of Purple Sandpipers Calidris maritima in north Norway during winter. Ring. Migr. 23: 95–100. doi: 10.1080/03078698.2006.9674351
- Summers, R.W. 2007. The origins of Purple Sandpipers wintering in Greenland. Wader Study Group Bull. 112: 65–67.
- Summers, R.W., Smith, S., Nicoll, M. & Atkinson, N.K. 1990a. Tidal and sexual differences in the diet of Purple Sandpipers Calidris maritima in Scotland. Bird Study 37: 187–194. doi: 10.1080/00063659009477056
- Summers, R.W., Strann, K.-B., Rae, R. & Heggås, J. 1990b. Wintering Purple Sandpipers Calidris maritima in Troms county, northern Norway. Ornis. Scand. 21: 248–254. doi: 10.2307/3676388
- Summers, R.W., Pálsson, S., Etheridge, B., Foster, S. & Swann, R.L. 2013. Using biometrics to sex adult Eurasian Curlews Numenius a. arquata. Wader Study Group Bull. 120: 71–74.
- Swann, R.L. & Etheridge, B. 1996. Movements of waders to and from the Moray Firth. Ring. Migr. 17: 111–121. doi: 10.1080/03078698.1996.9674126
- Swann, R.L. & Mudge, G.P. 1989. Moray Basin wader populations. Scott. Birds 15: 97–105.
- Székely, T., Cuthill, I.C. & Kis, J. 1999. Brood desertion in Kentish Plover: Sex differences in remating opportunities. Behav. Ecol. 10: 185–190. doi: 10.1093/beheco/10.2.185
- Townshend, D.J. 1981. The importance of field feeding to the survival of wintering male and female Curlews Numenius arquata on the Tees estuary. In Jones, N.V. & Wolff, W.J. (eds) Feeding and Survival Strategies of Estuarine Organisms: 261–273. Plenum Press, New York.
- Trivers, R.L. 1972. Parental investment and sexual selection. In Campbell, B. (ed.) Sexual Selection and the Descent of Man, 1871–1971: 136–179. Aldine, Chicago, IL.
- Walsh, P.S., Metzger, D.A. & Higuchi, R. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10: 506–513.
- Warnock, N.D. & Gill, R.E. 1996. Dunlin (Calidris alpina). In Poole, A. & Gill, F. (eds) The Birds of North America, No. 203, 1–24. The Academy of Natural Sciences, Philadelphia, PA, and The American Ornithologists’ Union, Washington, DC.
- Whitfield, D.P. & Brade, J.J. 1991. The breeding behaviour of the Knot Calidris canutus. Ibis 133: 246–255. doi: 10.1111/j.1474-919X.1991.tb04566.x
- Wilson, J.R., Nebel, S. & Minton, C.D.T. 2007. Migration ecology and morphometrics of two Bar-tailed Godwit populations in Australia. Emu 107: 262–274. doi: 10.1071/MU07026