Abstract
Capsule The highest densities of Meadow Pipits in Central Europe are found in lowland and upland wet meadows.
Aims To create a large-scale predictive model of Meadow Pipit density.
Methods We analysed factors affecting the density of the Meadows Pipit in Poland using data from 777 × 1 km study plots and a set of 22 environmental variables, including agriculture intensification and habitat-specific plant species as classifiers of meadow types. Predictors were selected using variation inflation factor, then related to species density data using generalized additive models.
Results The best-supported model included 11 variables and was clearly better (Akaike information criterion weight = 0.47) than other models. The density of the Meadow Pipit reaches its highest levels on large areas of extensively used wet meadows as well as pastures where livestock graze and which show high photosynthetic activity in April.
Conclusion Some aspects of the environment that were not identified from remote sensing data were vital for determining relatively high density. Conservation efforts for preserving Meadow Pipit populations should focus on maintaining wet meadows and extensively grazed pastures. Given the results, the Meadows Pipit may be classified as a good indicator of traditional agriculture.
Changes in agricultural land use patterns, driven by the Common Agricultural Policy, have been identified as primary factors affecting species distribution at a regional and local scale (Pereira et al. Citation2004, Holzkämper & Seppelt Citation2007, Giordano et al. Citation2010). European grassland birds have already suffered long-term population decline as a result of changes in land use. Further declines are forecast, because agricultural intensification is the simplest way to increase efficiency in agricultural production (Newton Citation2004, Butler et al. Citation2010, Setchfield et al. Citation2012). As part of this process, conservationists pay a lot of attention to developing methods for collecting and analysing data from both small and large areas so they can monitor the population health of many species (Franco & Sutherland Citation2004, Posillico et al. Citation2004, Balbotín Citation2005, Gregory et al. Citation2007, Franklin Citation2010). Despite numerous techniques for predictive mapping of species distribution (Carpenter et al. Citation1993, Elith et al. Citation2006, Araújo & New Citation2007, Giordano et al. Citation2010), e.g. maxent (Phillips Citation2005); bioclim (Austin et al. Citation1994); saga (http://www.saga-gis.org/en/index.html); garp (Anderson et al. Citation2002); and enfa (Hirzel et al. Citation2002), procedures tend to be similar, i.e. extrapolating data from a small- or meso-geographical scale to a large geographical scale. This technique has been used for predicting species distribution on different scales, for example, wetland amphibians in South Africa (Tarrant & Armstrong Citation2013), gastropods in South Australia (Fordham et al. Citation2013), mammals in North America (Badgley & Fox Citation2000), and bird species richness in Central Europe (Kosicki & Chylarecki Citation2012a).
In many cases, data from geographic information systems (gis) alone are used as predictors, such as corine land cover (CLC), ndvi dataset, wordclim, etc. (Guisan & Zimmermann Citation2000, Giordano et al. Citation2010, Kosicki & Chylarecki Citation2012a, Citation2012b). Such data are only suitable for some species that have habitat requirements with sharp boundaries in the landscape, e.g. the Common Quail Coturnix coturnix (Sardà-Palomera & Vieites Citation2011) and the Ortolan Bunting Emberiza hortulana (Kosicki & Chylarecki Citation2012b). However, other species may respond to more detailed habitats, which are not defined in remote sensing data (Akçakaya Citation2001, Giordano et al. Citation2010). This may be the case of the Meadow Pipit Anthus pratensis, a declining grassland bird (Pearce-Higgins & Grant Citation2006, Chylarecki & Jawińska Citation2007) that inhabits large areas of moorland and wet meadows (Moss et al. Citation1979, Cramp Citation1988, Brown & Stillman Citation1993).
The Meadow Pipit is a migratory bird that winters in Southern Europe and North Africa and breeds across Eurasia. Its population in Poland has decreased by 20% in the last ten years (Chylarecki & Jawińska Citation2007), and a similar tendency has been recorded in other European countries, including Scotland, England, and France (Birdlife International 2013).
Previous studies on habitat selection conducted on a small spatial scale showed a connection between the density of Meadow Pipits and lowland open grassy areas with dense low vegetation cover, such as meadows, wet marshy meadows, and extensive meadows (Cramp Citation1988, Vanhinsbergh & Chamberlain Citation2001), whereas high grazing pressure in upland negatively affected egg size and the species’ abundance (Evans et al. Citation2005).
Here, we construct habitat suitability models for Meadow Pipit density in Poland using remote sensing data, the atlas of vascular plants (Zając & Zając Citation2001) and data from the ‘Common Bird Monitoring Scheme’ (Gregory et al. Citation2007, Kosicki & Chylarecki Citation2012a, Citation2012b). The habitats identified by remote sensing were reclassified into finer categories according to where they were relative to plant atlas records, i.e. extensively and intensively used meadows, dry meadows and peat meadows as well as upland and lowland pastures, etc. In this way, we were able to capture more detailed relationships between Meadow Pipit occurrence and environmental conditions on a large spatial scale (McPherson & Jetz Citation2007). Our models help to identify areas critical not only for Meadow Pipit populations but also for other endangered grassland species with similar habitat requirements, such as the Lapwing (Sabatier et al. Citation2012) and the White stork (Kosicki Citation2010), the numbers of which have drastically decreased in recent years.
METHODS
Bird data
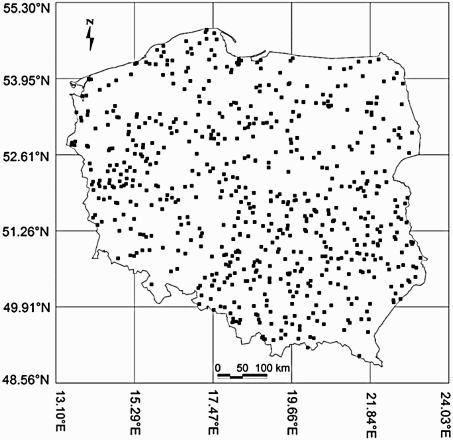
The data were derived from the Common Breeding Birds Monitoring Scheme (Chylarecki & Jawińska Citation2007) and gathered in Poland from 2000 to 2010 in 777 grid cells measuring 1 km2 (). Each cell was chosen at random out of 311 664 squares, and it was surveyed twice a year by volunteers. During the eleven-year period, each square was inspected on average (± sd) 5.4 ± 3.0 times. The first visit took place between 10 April and 15 May and the second between 16 May and 30 June, between 06:00 hours and 07:00 hours. The route of the bird census consisted of two parallel 1 km × 1 km sections (transects), along an east–west or north–south axis. Each transect was divided into five 200 m sections, in which birds were noted in three distance categories (< 25 m, 25–100 m and > 100 m).
Environmental data
All remote sensing data (Supplementary Table A1) were converted into grass gis file format (Neteler & Mitasova Citation2008) and re-projected to a coordinate system EPSG4284 projection (http://spatialreference.org/ref/epsg/4284/). Altitude data, which come from the digital evaluation model dataset (GTOPO30), were originally provided by the US Geological Survey's EROS Data Center (http://eros.usgs.gov). Climate data were derived from the worldclim database (www.worldclim.org), which is a set of global climate layers (climate grids) with spatial resolution of 1 km2. All grid cells were characterized by six variables, such as Annual Mean Temperature (temperature), Mean Temperature of Warmest Quarter (mtwaq), Mean Temperature of Coldest Quarter (mtcq), Annual Precipitation (precipitation), Precipitation of Warmest Quarter (pwaq), and Precipitation of Coldest Quarter (pcq).
Thirty-six land cover types are recognized in the clc database (based on remote sensing with basic spatial units of 100 m × 100 m), created in 1986–96, 2000, 2003, and 2006 from Landsat TM and SPOT satellites (http://www.eea.europa.eu/publications/COR0-landcover). In Poland, ten types of habitats were distinguished on average in each grid cell. The most frequent were environments classified as arable fields, forests (including coniferous, deciduous, and mixed forest), meadows, complex cultivation patterns, agricultural areas with natural vegetation, urban areas, shrubs, water bodies, and inland marshes. Urban areas were excluded from the analysis because more accurate data were available. We used images of lights at night (human) instead of urban land cover because they measure the distribution of urban cover more accurately. This kind of data is highly correlated with industrial activities (Small et al. Citation2005, Doll et al. Citation2007) (Supplementary Table A1).
Meadows (Supplementary Table A1) were categorized on the basis of the Distribution Atlas of Vascular Plants in Poland (Zając & Zając Citation2001). Maps showing the occurrence of 20 plant species were digitized and arranged in layers. The sum of habitat-specific plant species at the same point reflected the type of meadow. According to plant species specific to a given habitat, six meadow types were distinguished: (1) extensively used lowland meadows, which were very wet in spring and dry in summer, mowed once a year, and not fertilized. This type was chosen on the basis of four plant species, such as Lathyrus palustris, Dactylorhiza majalis, Trollius europeus, Ostericum palustre, Viola persicifolia, Gentiana pneumonanthe, Silaum silaus, and Iris sibirica; (2) dry meadows, characterized by a low level of ground water and no flooding, included Dianthus arenarius, Pulsatilla pratensis, Aster amellus, Adonis vernalis, and Orchis militaris; (3) mountain meadows were classified on the basis of Phyteum orbiculare and Poa chaixa; (4) meadows adjacent to pine forests, which were unpredictable with regards to water conditions and likely to be flooded, included plants such as Nardus stricta and Pedicularis sylvatica; (5) intensively used meadows characterized by a high level of fertilizing and frequent mowing included Poa trivialis and Festuca pratensis and (6) peat meadows, which were very damp, mainly due to rainfall, included Scheuchzeria palustris as the most typical species.
We obtained the normalized differences vegetation index (ndvi, original resolution of 1 km2). The data were derived from the spot dataset (http://free.vgt.vito.be/). ndvi is an index of green vegetation, expressed as a mean monthly value (from March to June), calculated from three measurements taken every ten days.
Data on farming intensification were provided by the Agricultural Census 2002 (http://www.stat.gov.pl/bdl/app/strona.html?p_name=indeks). The variables combine the number of tractors (tractors) and number of cows (cows) per farm. However, the spatial resolution of these data is inconsistent with other class data, because the data are collected within larger administrative units. Therefore, in each of the 16 administrative units of Poland, tractors and cows were expressed as the number of pieces of agricultural equipment per hectare of an open habitat in each grid cell (1 km2) (i.e. sum of the areas: arable fields, meadows, complex cultivation patterns, agricultural areas with natural vegetation), and the number of cows per meadow area in each grid cell, respectively.
Data processing and analysis
All statistical analyses were performed using the statistical package r (R Development Core Team 2010). The mean density of Meadows Pipit as the number of birds in each grid cell was 4.5 individuals/km² (95% confidence limits (CL) = 3.4–5.7, n = 777). However, due to the fact that species density in cells depends on the transect's length and the bird's distance from the observer (Krebs Citation1999), the Hayne estimator of species density was calculated for each cell according to the following equation (Hayne Citation1949, Krebs Citation1999):where DH is Hayne's estimator of density, n is the number of animals seen, L is the length of transect, and ri is the sighting distance to each animal i.
The Pearson correlation coefficients and the variation inflation factor (VIF, using the HH library in r; Heiberger 2013) were used to assess the relationship between all predictors (Supplementary Table A2). A VIF of 5 and above indicated a multi-collinearity problem (O'Brien Citation2007). In the present study, the VIF ranged from 1.00 to 7.79, therefore predictors with a VIF ≥ 5 were excluded from the analysis (Supplementary Table A2).
We used generalized additive models (GAMs) to fit resource selection functions (Hastie & Tibshirani Citation1990). The response was the Hayne estimator of Meadow Pipit density. Twenty-two variables selected by their VIF were used as predictors (Supplementary Table A2).The most parsimonious model was selected using Akaike information criterion (AIC) (mgcv library in r; Wood Citation2013) with the lowest AIC and consequently the highest Akaike weight (Burnham & Anderson Citation2002). We analysed all possible models (2n , where n is the number of variables), using MuMIn library in r (Bartoń Citation2013, Hastie & Tibshirani Citation1990). Although this approach is criticized, the analysis of all possible models is often used when there is not enough a priori information to develop a small set of models (Whittingham et al. Citation2005, Reino et al. Citation2010). To reduce the possibility of finding spurious models, we used only dominant uncorrelated habitat variables selected by their VIF from the initial large set of variables (see also Reino et al. Citation2010). The probability of including a variable in the best parsimonious model was estimated as relative importance (RI) by summing the Akaike weights of all candidate models in which the variable was included (Burnham & Anderson, Citation2002, Reino et al. Citation2010). In order to allow some complexity in the functions, while avoiding over-fitting the data, we defined the basic dimension, i.e. k = 4 (Santana et al. Citation2012). Furthermore, scatter plots and regression diagnostics were used to inspect the shape of the fitted curves. This procedure identifies problems resulting from outliers and influential points (Legendre & Legendre Citation1998, Santana et al. Citation2012). A Gaussian distribution of errors and the identity link function were used. As a measure of deviance reduction, we used the D2 coefficient, which is equivalent to R2, well known from least-squares estimation (Weisberg Citation1980). We then created a predictive distribution map of the densities of Meadow Pipits from these models. The correlation coefficient between the predicted versus the observed densities (log-transformed) was used as a measure of error prediction (Hastie et al. Citation2008, Kuczyński et al. Citation2009).
RESULTS
Population size
Breeding populations of the Meadow Pipit were recorded in 39.6% of grid cells. The mean density expressed as a Hayne estimator was 1.4 (95% CL: 0.7–1.8) individuals/km2, while the mean Hayne estimator on occupied plots was 4.2 (95% CL: 3.9–5.5) individuals/km2.
Habitat use
Out of all analysed models, only six gained support using information-theoretic criteria, showing AIC weights > 0 (). The most parsimonious GAM () fit to the Hayne estimator of Meadow Pipit density included a smooth GAM fit to extensively used lowland meadows, intensively used meadows, precipitation and latitude and a linear fit to mountain meadows, peat meadows, arable fields, forests, cows, tractors, and ndvi in April. The D2 coefficient of this model was 0.64 and it was more than two times better for describing the variation in Meadow Pipit density than the second best model (evidence ratio 2.27) in our candidate set. The most important predictor based on RI was extensively used lowland meadows (RI = 1, F = 4.7, P < 0.001, a), which had a non-linear function and showed that the highest density of Meadow Pipits depended on meadows which were wet in spring and dry in summer, mowed once a year, and not fertilized. The second ariable, with a linear function showed that the highest density of the Meadow Pipit was also found in mountain meadows (RI = 1, F = 3.6, P < 0.001, b). The third significant variable showed that the highest density was in cells with large areas of peat meadows (RI = 0.939, F = 2.3, P < 0.01 c). The fourth variable was arable fields (RI = 0.928, F = 8.2, P < 0.0001, d) with a linear function, showing that Meadow Pipits avoided large areas of arable fields. Forest was yet another important predictor (RI = 0.841, F = 7.3, P < 0.0001, e), also with a linear function, which showed that the species avoided large areas of all types of forests (deciduous, coniferous, and mixed). An equally important variable (RI = 0.841, F = 4.0, P < 0.001, f) was intensively used meadows proving that the species also avoided meadows which were fertilized and frequently mowed. The seventh major variable was precipitation, showing that the highest density depended on a high level of rainfall (RI = 0.767, F = 3.7, P < 0.001, g). The next two factors included in the top model were cows (RI = 0.707, F = 3.2, P < 0.001, h) and tractors (RI = 0.707, F = 3.0, P < 0.001, i). Both were characterized by a linear shape of the response function revealing that Meadow Pipits avoid areas with a high level of farmland mechanization and at the same time preferred areas with a high number of livestock. The ninth variable was ndvi in April (RI = 0.701, F = 5.2, P < 0.0001, j) with the highest density of Meadow Pipits noted in areas with a high level of green vegetation at the beginning of spring. The final variable was latitude (RI = 0.690, F = 3.8, P < 0.001, k), demonstrating that the density of Meadow Pipits increased from the south to the north of Poland.
Figure 2. GAM fits for Meadow Pipit density. Each plot represents a variable's response shape, independent of the other variables, in relation to Meadows Pipit density. The y-axis in each case represents Meadow Pipit density for a smoothed environmental variable and the estimated degrees of freedom are given in parentheses. The shaded areas represent standard errors of the estimated curves. The values fitted are partial residuals. (a) extensive.m – extensively used lowland meadows, (b) mountaim.m – mountain meadows, (c) peat.m – peat meadows, (d) arable fields – area of fields according with clc, (e) forests – area of three types of forest (coniferous, deciduous and mixed) according with clc, (f) intense.m – intensively used meadows, (g) precipitation – annual precipitation according with WorldClim database, (h) cows – number of cows per meadow area, (i) tractors – agricultural equipment per hectare of an open habitat, (j) ndvi.april – green vegetation in April, and (k) latitude – geographical gradient from south (low value) to north (high value).
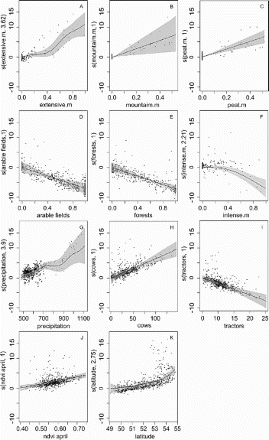
Table 1. A comparison of general additive models (null and six top-ranked models, where AIC weight >0).
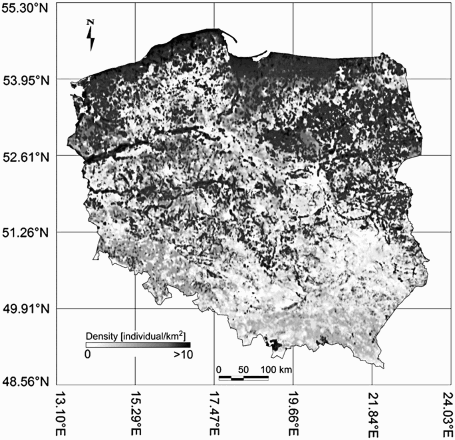
On the basis of the GAMs, we created predictive maps of the occurrence of Meadow Pipit density (). The correlation coefficient between the predicted versus the observed density was 0.84 (df = 777, P < 0.0001).
DISCUSSION
On the basis of data from the Polish Common Breeding Birds Monitoring Scheme, Atlas of Vascular Plants (Zając & Zając Citation2001) and remote sensing data, we created predictive maps of Meadow Pipit density. Our analyses focused on detailed explanations of relationships between species density, habitat, vegetation variables, and climate conditions on a meso-geographical scale (Heikkinen et al. Citation2004), as well as their extrapolation to a large geographical scale. In general, our results were consistent with other studies (Vanhinsbergh & Chamberlain Citation2001, Pearce-Higgins & Grant Citation2006) but several patterns have not been described before. Meadow Pipits reach the highest density on large areas of extensively used meadows (i.e. with a low number of tractors per hectare), peat meadows, and lowland and upland pastures (i.e. with high density of cows), provided that these areas are located in regions with a relatively high level of rainfall. At the same time, Meadow Pipits avoid dry areas as well as pastures, meadows, and arable fields that are intensively used (i.e. with a high number of tractors per hectare).
The preferred habitats reflect the foraging requirements of Meadow Pipits (Douglas et al. Citation2008). Extensively used wet meadows and pastures, i.e. areas with traditional farming, have high vegetation productivity, seed and insect abundance, and thus provide food resources for many birds (Marone Citation1992, Verner & Purcell Citation1999). Meadow Pipits feed on a wide range of invertebrates, particularly tipulidae larvae (Walton Citation1979, Cramp Citation1988, Douglas et al. Citation2008), which in turn rely on wet and stable hydrological conditions such as bogs and wet meadows. On the other hand, in upland pastures, where tipulid density is lower, cattle grazing can lead to a greater diversity and abundance of arthropods that are attracted to the dung (e.g. Diptera spp.). This additional factor may positively affect density in pastures. Some larvae of Tipulid species, such as Tipula oleracea, are plant pests, and are controlled by draining meadows or the use of pesticides (Boczek Citation2001). Such anthropogenic endeavours, as well as summer droughts, may contribute to the decline of the Meadow Pipit population in intensively used meadows (Renwick et al. Citation2012).
As far as climate components are concerned, only precipitation affected the high density of the species. Its effect was linear and positive: in areas with higher levels of rainfall, a higher density of Meadows Pipits was recorded. Many studies show that open habitat birds are more sensitive to changes in moisture conditions than species that reside in forests (Marone Citation1992, Verner & Purcell Citation1999, Kosicki & Chylarecki Citation2012a). We suspect that the influence of rainfall on Meadow Pipit density is indirect. Apart from extreme climatic situations, rainfall provides uniform humidity in ‘wet meadows’, e.g. in late spring, when the ground water level is low, thus providing a diversity of potential food.
Our findings also show that Meadow Pipits choose areas with high levels of green vegetation (ndvi) in April, although this has no effect in May and June. Green vegetation levels recorded in April reflect both the condition of the meadows after the winter and water retention in the meadows and/or pastures. The index's high value at the beginning of spring indicates meadows/pastures where flooding is absent and where grass growth has already started. These conditions provide optimal nesting habitats for early spring migrant species (Tryjanowski et al. Citation2005). In the months that follow, i.e. May and June, grass vegetation is higher, so food is not as easily accessible and detectable for Meadow Pipits (Butler & Gillings Citation2004, Douglas et al. Citation2008, Vandenberghe et al. Citation2009), and foraging mobility is restricted (Devereux et al. Citation2006, Douglas et al. Citation2008). We suspect that lowland meadows, which are mowed once or twice per year, provide low vegetation; in lowland and upland pastures, low vegetation is also achieved by grazing. However, the latter relationship is not linear, because severe grazing pressure, especially with regards to sheep, is blamed for being deleterious for vegetation and birds in many upland regions of the UK (Fuller & Gough Citation1999). Unfortunately, we were unable to conduct a detailed investigation of the effects of grazing intensity, and the richness of specific groups of invertebrate species, because no data were available on a Poland-wide scale.
We also observed that the geographical gradient of Meadow Pipit density in Poland depends on latitude. The highest density occurs in the northern and north-east parts of the country, while the lowest occurs in the southern and south-east parts. A similar pattern can be found in the Polish Breeding Birds Atlas (Sikora et al. Citation2007), where a heuristic comparison of species occurrence maps shows that this species prefers areas in the northern and eastern parts of Poland and avoids the southeast and central regions. We show that this geographical density gradient of the Meadow Pipit can be attributed to habitat distribution and differences in weather conditions. The high density of Meadow Pipits in lowlands depends on stable or predictable hydrological conditions in meadows, while in the uplands, extensively used pastures tend to play an important role (Buchanan et al. Citation2006). Alternatively, the geographical gradient could be attributed to habitat modification that was not explored in this study (e.g. fertilizer use, water pollution, wind farms, etc). An earlier study showed that farmland in the western and central parts of Poland is much more intensive (the highest percentage of fertilizer and herbicide use) than in the northern and eastern parts, where traditional farming still occurs (Kosicki & Chylarecki Citation2012a).
Our model of species density, which is based on habitat, vegetation, and climate variables, is yet another step in predicting species distribution according to different environmental scenarios. By employing remote sensing data and maps of vascular plant distribution, we have not limited the study to climate and habitat (Sardà-Palomera & Vieites Citation2011, Kosicki & Chylarecki Citation2012b). Meadow Pipit density relies strongly on an adequate, wet meadow habitat, which in turn depends on precipitation and non-natural processes, i.e. farmland use intensity. Our findings suggest that drainage, and the consequent changes in the vegetation structure caused by changing water conditions, may have a direct effect on this species. Having demonstrated associations between ‘wet meadows’, bird abundance and detailed measures of vegetation, the results of this study have succeeded in providing a quantitative basis for assessing how recent habitat changes may have affected bird abundance.
SUPPLEMENTAL DATA
Supplementary online Tables A1 and A2 can be accessed at http://dx.doi.org/10.1080/00063657.2013.849656.
Supplementary material
Download MS Word (200 KB)ACKNOWLEDGEMENTS
We wish to express our gratitude to observers who collected data in the field. The full list of their names can be found at http://main3.amu.edu.pl/~kubako/tsr/. We want to thank Justyna Grześkowiak and Sylwia Wilcox for their linguistic assistance and two anonymous reviewers for comments, which improved the manuscript.
FUNDING
The study was supported by MNiSW [grant N N304 025936].
REFERENCES
- Akçakaya, H.R. 2001. Linking population-level risk assessment landscape with and habitat models. Sci. Total Environ. 274: 283–291. doi: 10.1016/S0048-9697(01)00750-1
- Anderson, R.P., Gómez-Laverde, M. & Peterson, A.T. 2002. Geographical distributions of spiny pocket mice in South America: insights from predictive models. Global Ecol. Biogeogr. 11: 131–141. doi: 10.1046/j.1466-822X.2002.00275.x
- Araújo, M.B. & New, M. 2007. Ensemble forecasting of species distributions. Trends Ecol. Evol. 22: 42–47. doi: 10.1016/j.tree.2006.09.010
- Austin, M.P., Nicholls, A.O., Doherty, M.D. & Meyers, J.A. 1994. Determining species response functions to an environmental gradient by means of a b-function. J. Veg. Sci. 5: 215–228. doi: 10.2307/3236154
- Badgley, C. & Fox, G.L. 2000. Ecological biogeography of North American mammals: species diversity and ecological structure in relation to environmental gradients. J. Biogeogr. 27: 1437–1467. doi: 10.1046/j.1365-2699.2000.00498.x
- Balbotín, J. 2005. Identifying suitable habitat for dispersal in Bonelli's eagle: an important issue in halting its decline in Europe. Biol. Conserv. 126: 74–83. doi: 10.1016/j.biocon.2005.04.023
- Bartoń, K. 2013. MuMIn: multi-model inference. R package version 1.9.0. Available from: http://CRAN.R-project.org/package=MuMIn.
- Boczek, J. 2001. Nauka o szkodnikach roślin uprawnych. Wydawnictwo SGGW, Warszawa.
- Brown, A.F. & Stillman, R.A. 1993. Bird-habitat associations in the eastern Highlands of Scotland. J. App. Ecol. 30: 1–42. doi: 10.2307/2404265
- Buchanan, G.M., Pearce-Higgins, J.W. & Grant, M.C. 2006. Observer variation in estimates of meadow pipit and skylark abundance on moorland. Bird Study 53: 92–95. doi: 10.1080/00063650609461421
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: a practical information theoretic approach – Second edition. Springer, New York.
- Butler, S.J. & Gillings, S. 2004. Quantifying the effects of habitat structure on prey detectability and accessibility to farmland birds. Ibis 146: 123–130. doi: 10.1111/j.1474-919X.2004.00352.x
- Butler, S.J., Boccaccio, L., Gregory, R.D., Vorisek, P. & Norris, K. 2010. Quantifying the impact of land-use change to European farmland bird populations. Agri. Ecosyst. Environ. 137: 348–357. doi: 10.1016/j.agee.2010.03.005
- Carpenter, G., Gillison, A.N. & Winter, J. 1993. DOMAIN: a flexible modelling procedure for mapping potential distributions of plants, animals. Biodivers. Conserv. 2: 667–680. doi: 10.1007/BF00051966
- Chylarecki, P. & Jawińska, D. 2007. Monitoring Pospolitych Ptaków Lęgowych. OTOP, Warszawa, Raport z lat 2005–2006.
- Cramp, S. (eds) 1988. The Birds of the Western Palearctic, Volume V, Tyrant Flycatchers to Thrushes. Oxford University Press, Oxford.
- Devereux, C.L., Vickery, J.A., Fernàndez-Juricic, E., Krebs, J.R. & Whittingham, M.J. 2006. Does sward density affect prey availability for grassland birds? Agri. Ecosyst. Environ. 117: 57–62. doi: 10.1016/j.agee.2006.03.007
- Doll, C.N.H., Muller. J.-P. & Morley, J.G. 2007. Mapping regional economic activity from night-time light satellite imagery. Ecol. Econ. 57: 75–92.
- Douglas, D.J.T., Evans, D.M. & Redpath, S.M. 2008. Selection of foraging habitat and nestling diet by Meadow pipits Anthus pratensis breeding on intensively grazed moorland. Bird Study 55: 290–296. doi: 10.1080/00063650809461534
- Elith, J., Graham, C.H., Anderson, R.P., Dudík, M., Ferrier, S., Guisan, A., Hijmans, R.J., Huettmann, F., Leathwick, J.R., Lehmann, A., Li, J., Lohmann, L.G., Loiselle, B.A., Manion, G., Moritz, C., Nakamura, M., Nakazawa, Y., McC, J., Overton, A., Peterson, A.T., Phillips, S.J., Richardson, K., Scachetti-Pereira, R., Schapire, R.E., Soberón, J., Williams, S., Wisz, M.S. & Zimmermann, N.E. 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151. doi: 10.1111/j.2006.0906-7590.04596.x
- Evans, D.M., Redpath, S.M., Evans, S.A., Elston, D.A. & Dennis, P. 2005. Livestock grazing affects the egg size of an insectivorous passerine. Biol. Lett. 1: 322–325. doi: 10.1098/rsbl.2005.0335
- Franco, A.M.A. & Sutherland, W.J. 2004. Modelling the foraging habitat selection of the lesser kestrels: conservation implications of European Agricultural Policies. Biol. Conserv. 120: 63–74. doi: 10.1016/j.biocon.2004.01.026
- Franklin, J. 2010. Mapping Species Distributions. Spatial Inference and Prediction. Cambridge University Press, Cambridge.
- Fordham, D.A., Brook, B.W., Caley, M.J., Bradshaw, C.J.A. & Mellin, C. 2013. Conservation management and sustainable harvest quotas are sensitive to choice of climate modelling approach for two marine gastropods. Diversity Distrib. 19: 1299–1312. doi: 10.1111/ddi.12092
- Fuller, R.J. & Gough, S.J. 1999. Changes in sheep numbers in Britain: implications for bird populations. Biol. Conserv. 91: 73–89. doi: 10.1016/S0006-3207(99)00039-7
- Giordano, P.F., Navarro, J.L. & Martella, M.B. 2010. Building large-scale spatially explicit models to predict the distribution of suitable habitat patches for the Greater rhea (Rhea Americana), a near-threatened species. Biol. Conserv. 143: 357–365. doi: 10.1016/j.biocon.2009.10.022
- Gregory, D.R., Vorisek, P., Van Strien, A., Gemelig-Meyling, A.W., Jiguet, F., Fornasari, L., Reif, J., Chylarecki, P. & Burfield, I.J. 2007. Population trends of widespread woodland birds in Europe. Ibis 149: 78–97. doi: 10.1111/j.1474-919X.2007.00698.x
- Guisan, A. & Zimmermann, N.E. 2000. Predictive habitat distribution models in ecology. Ecol. Model. 135: 147–186. doi: 10.1016/S0304-3800(00)00354-9
- Hastie, T. & Tibshirani, R. 1990. Generalized Additive Models. Chapman and Hall, London.
- Hastie, T., Tibshirani, R.J. & Friedman, J. 2008. The Elements of Statistical Learning (2nd edition). Springer, New York.
- Hayne, D.W. 1949. An examination of the strip census method for estimation animal populations. J. Wildl. Manage. 13: 145–157. doi: 10.2307/3796084
- Heikkinen, R.K., Luoto, M., Virkkala, R. & Rainio, K. 2004. Effect of habitat cover, landscape structure and spatial variables on the abundance of birds in agricultural-forest mosaic. J. Appl. Ecol. 41: 824–835. doi: 10.1111/j.0021-8901.2004.00938.x
- Hirzel, A.H., Hausser, J., Chessel, D. & Perrin, N. 2002. Ecological-Niche factor analysis: how to compute habitat suitability maps without absence data? Ecology 83: 2027–2036. doi: 10.1890/0012-9658(2002)083[2027:ENFAHT]2.0.CO;2
- Holzkämper, A. & Seppelt, R. 2007. Evaluating cost-effectiveness of conservation management actions in an agricultural landscape on a regional scale. Biol. Conserv. 136: 117–127. doi: 10.1016/j.biocon.2006.11.011
- Kosicki, J.Z. 2010. Reproductive success of the White Stork Ciconia ciconia population in intensively cultivated farmlands in western Poland. Ardeola 57: 243–255.
- Kosicki, J.Z. & Chylarecki, P. 2012a. Effect of climate, topography and habitat on species-richness of breeding birds in Poland. Basic Appl. Ecol. 13: 475–483. doi: 10.1016/j.baae.2012.07.007
- Kosicki, J.Z. & Chylarecki, P. 2012b. Habitat selection of the Ortolan Bunting Emberiza hortulana in Poland: predictions from large-scale habitat elements. Ecol. Res. 27: 347–355. doi: 10.1007/s11284-011-0906-4
- Krebs, CH.J. 1999. Ecological Methodology. Addison Wesley Longman, New York.
- Kuczyński, L., Rzępała, M., Goławski, A. & Tryjanowski, P. 2009. The wintering distribution of great grey shrike Lanius excubitor in Poland: predictions from a large-scale historical survey. Acta Ornithol. 44: 159–166. doi: 10.3161/000164509X482731
- Legendre, P. & Legendre, L. 1998. Numerical Ecology, 2nd edn. Elsevier, Amsterdam.
- Marone, L. 1992. Seasonal and year-to-year fluctuations in bird populations and guilds in the Monte Desert, Argentina. J. Field Ornithol. 63: 294–308.
- McPherson, J.M. & Jetz, W. 2007. Effect of species` ecology on the accuracy of distribution models. Ecography 30: 135–151.
- Moss, D., Taylor, P.N. & Easterbee, N. 1979. The effects on song-bird populations of upland afforestation with spruce. Forestry 52: 129–147. doi: 10.1093/forestry/52.2.129
- Neteler, M. & Mitasova, H. 2008. Open Source GIS: A GRASS GIS Approach, 3rd edn. Springer, New York.
- Newton, I. 2004. The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146: 579–600. doi: 10.1111/j.1474-919X.2004.00375.x
- O'Brien, R.M. 2007. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual. Quant. 41: 673–690.
- Pearce-Higgins, J.W. & Grant, M.C. 2006. Relationships between bird abundance and the composition and structure of moorland vegetation. Bird Study 53: 112–125. doi: 10.1080/00063650609461424
- Pereira, H.M., Gretchen, C.D. & Roughgarden, J. 2004. A framework for assessing the relative vulnerability of species to land-use change. Ecol. Appl. 14: 730–742. doi: 10.1890/02-5405
- Phillips, S.J. 2005. Maxent software for species distribution modelling. Available from: http://www.cs.princeton.edu/schapire/maxent/.
- Posillico, M., Meriggi, A., Pagnin, E., Lovari, S. & Russo, L. 2004. A habitat model for brown bear conservation and land use planning in the central Apennines. Biol. Conserv. 118: 141–150. doi: 10.1016/j.biocon.2003.07.017
- Reino, L., Portod, M., Morgado, R., Carvalhof, F., Miraf, A. & Bejac, P. 2010. Does afforestation increase bird nest predation risk in surrounding farmland? Forest Ecol. Manag. 260: 1359–1366. doi: 10.1016/j.foreco.2010.07.032
- Renwick, A.R., Massimino, D., Newson, S.E., Chamberlain, D.E., Pearce-Higgins. J.W. & Johnston, A. 2012. Modelling changes in species' abundance in response to projected climate change. Diversity Distrib. 18: 121–132.
- Sabatier, R., Doyen, L. & Tichit, M. 2012. Action versus result-oriented schemes in a Grassland agroecosystem: a dynamic modelling approach. PLoS ONE 7: e33257.
- Santana, J., Porto, M., Gordinho, L., Reino, L. & Beja, P. 2012. Long-term responses of Mediterranean birds to forest fuel management. J. Appl. Ecol. 49: 632–643.
- Sardà-Palomera, F. & Vieites, D.R. 2011. Modelling species’ climatic distributions under habitat constraints: a case study with Coturnix coturnix. Ann. Zol. Fenn. 4: 147–160. doi: 10.5735/086.048.0303
- Setchfield, R.P., Mucklow, C., Davey, A., Bradter, U. & Anderson, G.Q.A. 2012. An agri-environment option boosts productivity of Corn Buntings Emberiza calandra in the UK. Ibis 154: 235–247. doi: 10.1111/j.1474-919X.2011.01207.x
- Sikora, A., Rohde, Z., Gromadzki, M., Neubauer, G. & Chylarecki, P. 2007. Polski Atlas Ornitologiczny. Bogucki Wydawnictwo Naukowe, Poznań.
- Small, C., Pozzi, F. & Elvidge, C.D. 2005. Spatial analysis of global urban extent from DMSP-OLS night time lights. Remote Sens. Environ. 96: 277–291.
- Tarrant, J. & Armstrong, A.J. 2013. Using predictive modelling to guide the conservation of a critically endangered coastal wetland amphibian. J. Nat. Conserv. 21: 369–381. doi: 10.1016/j.jnc.2013.03.006
- Tryjanowski, P., Jerzak, L. & Radkiewicz, J. 2005. Effect of water level and livestock on the productivity and numbers of breeding White Storks. Waterbirds 28: 378–382. doi: 10.1675/1524-4695(2005)028[0378:EOWLAL]2.0.CO;2
- Vandenberghe, Vh., Prior, G., Littewood, N.A., Brooker, R. & Pakeman, I. 2009. Influence of livestock grazing on meadow pipit foraging behaviour in upland grassland. Basic Appl. Ecol. 10: 662–670. doi: 10.1016/j.baae.2009.03.009
- Vanhinsbergh, D.P. & Chamberlain, D.E. 2001. Habitat associations of breeding Meadows Pipits Anthus pratensis in the British uplands. Bird Study 48: 159–172. doi: 10.1080/00063650109461214
- Verner, J. & Purcell, K.L. 1999. Fluctuating populations of House Wrens and Bewick's Wrens in foothills of the western Sierra Nevada of California. Condor 101: 219–229. doi: 10.2307/1369985
- Walton, K.C. 1979. Diet of meadow pipits Anthus pratensis on mountain grassland in Snowdonia. Ibis 121: 325–329. doi: 10.1111/j.1474-919X.1979.tb06850.x
- Weisberg, S. 1980. Applied Linear Regression. Wiley, New York.
- Whittingham, M.J., Swetnam, R.D., Wilson, J.D., Chamberlain, D.E. & Freckleton, R.P. 2005. Habitat selection by yellowhammers Emberiza citrinella on lowland farmland at two spatial scales: implications for conservation management. J. Appl. Ecol. 42: 270–280. doi: 10.1111/j.1365-2664.2005.01007.x
- Wood, S. 2013. mgcv: Mixed GAM Computation Vehicle with GCV/AIC/REML smoothness estimation. R package version 1.7–22. Available from: http://cran.rproject.org/web/packages/mgcv.
- Zając, A. & Zając, M. 2001. Distribution Atlas of Vascular Plants in Poland. Institute of Botany, Jagiellonian University, Kraków.