Abstract
Capsule Apparent survival rates of Yellow Wagtails breeding in abandoned fields in Russia are determined by previous breeding success.
Aims To examine apparent survival and its link to previous breeding success in Yellow Wagtails breeding in abandoned fields in the Vologda region, northern European Russia.
Methods We ringed and measured apparent survival of Yellow Wagtails at two abandoned agricultural sites over eight years (2005–2012). We modelled the impact of age, nest stage, and time of season on daily nest survival rates.
Results Predation was the main cause of nest failure. Nest daily survival rate was highest at the beginning of the breeding season. Overall nest survival probability was 0.40 ± 0.02. Adult apparent survival after successful breeding was 0.42 ± 0.06 and after unsuccessful breeding this was 0.13 ± 0.06.
Conclusion Reproductive success can be regarded as the crucial demographic parameter of the local Yellow Wagtail population in northern European Russia. Apparent survival after successful breeding is significantly higher than after unsuccessful breeding, because unsuccessful breeders probably move to new breeding sites the following year. High adult survival may be particularly important to Yellow Wagtail population dynamics in the study region, because second breeding attempts are apparently unusual.
Over the last few decades, many species of farmland birds have declined in Western Europe. This has been caused mainly by a decrease in the diversification of the landscape linked to the elimination of uncultivated areas and agricultural intensification (Smart et al. Citation2000, Orłowski Citation2005, Donald et al. Citation2006, Wretenberg et al. Citation2007). In contrast, in Eastern Europe, political and economic transformation has often caused the degradation of agriculture and the appearance of a large number of abandoned fields. These processes affect birds breeding on farmland with some species positively affected and others negatively affected (Dombrowski & Golawski Citation2002; Orłowski Citation2005, Tryjanowski et al. Citation2011).
In order to identify the environmental changes responsible for variable population trends in farmland, it is useful first to determine the demographic mechanisms through which they may have acted (Siriwardena et al. Citation1998). Demographic parameters responsible for changes in population size are fecundity, survival probability, and dispersal probability (Cilimburg et al. Citation2002, Anders & Marshall Citation2005). Because breeding dispersal is difficult to document, permanent emigration and mortality are usually not distinguished in estimates of passerine survival (Cilimburg et al. Citation2002, Gilroy et al. Citation2012). In lieu of a workable solution to this problem, it has become usual to report survival estimates using the term ‘apparent survival’, so incorporating a simple acknowledgment of the uncertainty associated with permanent emigration. Apparent survival probability is defined as the probability that an individual alive at time t survives to time t + 1 and does not permanently emigrate from the study area between time t and t + 1 (Lebreton et al. Citation1992). Thus, variation in factors influencing dispersal decisions also influence variation in apparent survival estimates (Doligez & Pärt Citation2008). Breeding dispersal, survival, and future reproduction may all depend on current reproduction. For example, in passerine birds, unsuccessful breeders disperse more frequently than successful ones (Haas Citation1998, Sedgwick Citation2004, Pasinelli et al. Citation2007). Hence, reproductive success plays a central role in the dynamics of a local population, both directly and indirectly (Schaub & Von Hirschheydt Citation2009).
In this study, we explored the relationship between reproductive success and apparent survival of Yellow Wagtails Motacilla flava in the Vologda region of northern European Russia. The Yellow Wagtail is one of the species which has declined rapidly in Western Europe (Tryjanowski & Bajczyk Citation1999, Wilson & Vickery Citation2005, Kirby et al. Citation2012). The causes of its decline remain unclear, although changes in the management of grassland breeding habitats may be important (Tryjanowski & Bajczyk Citation1999, Wilson & Vickery 2004), as well as the effects of soil degradation on prey availability (Gilroy et al. Citation2008). In England, there has probably been a significant shift in habitat use by Yellow Wagtails, with a decreasing proportion breeding in grassland and higher proportion on arable land (Wilson & Vickery Citation2005, Gilroy et al. Citation2010). In the north of European Russia, until the end of last century, Yellow Wagtails bred mainly in humid meadows and mossy bogs, and cases of breeding in arable land were infrequent (Malchevsky & Pukinsky Citation1983, Shitikov Citation2000). However, recent degradation of agriculture in the region has caused the appearance of a large number of abandoned fields, which have become used by Yellow Wagtails.
The demographic parameters of the Yellow Wagtail's life history on arable land in Western Europe have been extensively studied. There is extensive information on its breeding success on different types of grasslands (Paulsen Citation1993, Flyckt Citation1999, Bradbury & Bradter Citation2004) and arable land (Stiebel Citation1997, Morris & Gilroy Citation2008, Gilroy et al. Citation2010, Kirby et al. Citation2012), but there are few data on the survival rate of adult birds. True survival of adult Yellow Wagtails in Great Britain, according to ring recovery data, is 0.53 ± 0.03 (Siriwardena et al. Citation1998).
We ringed and measured the apparent survival rate of Yellow Wagtails in abandoned agricultural fields at two sites over eight years (2005–2012).We constructed a multistate model in which the states reflect different classes of reproductive success. This model allowed testing of whether apparent survival was affected by current reproductive success. We also tested whether this relationship differed between sex classes and study sites. For one of the study sites, where sample size was greatest, we modelled the impact of age and time of season on daily nest survival rates. Finally, we described the main factors determining the reproductive success of Yellow Wagtails on abandoned fields.
METHODS
Study sites
The research was carried out in the ‘Russky Sever’ National Park, located in the north-west of the European part of Russia in the Kirillov district of the Vologda region in 2005–2012. The study was carried out at two sites: Topornya (N 59°46′, E 38°22′) and Chistii Dor (N 60°09′; E 38°22′), located 43 km from each other. The study plot in Chistii Dor is a 20-ha humid grass–forb meadow overgrown with osier bushes (Salix sp.). Before the degradation of agriculture in the mid-1990s, this meadow was used for mowing and grazing. On the one side, the study plot borders coniferous and small-leaved forest, and on the other side, it is surrounded by fields of spring crops and flax with a total area of about 160 ha. Grassland occurs only in small isolated patches within 0.1–1 km of the study plot, with a total area of 4–5 ha. In Chistii Dor, Yellow Wagtails inhabited only meadows (Shitikov et al. Citation2012b). Spring sown crops (wheat, oats) and perennial grasses (Cock's-foot Dactylis glomerata and Meadow Fescue Festuca pratensis) were dominant at the Topornya study site (400 ha) before the beginning of our research. After 2005, abandoned fields occupied more than 90% of the study site. Abandoned fields were covered with ruderal vegetation (Corn Sow Thistle Sonchus arvensis, Mugwort Artemisia vulgaris, Field Thistle Cirsium setosum, Cow Parsley Anthriscus sylvestris, Musk Thistle Carduus nutans, and Couch Grass Elytrigia repens). In Topornya, Yellow Wagtails bred throughout the study site but most of the nests (up to 90% in some years) were concentrated in one of the abandoned fields (80 ha).
Field methods
Each year, field work started in Topornya on the 15–25th of May and lasted until the 20–21st of July. In Chistii Dor, field work was carried out in three time periods (Shitikov et al. Citation2012b). The first period was one to two days between the end of May and the beginning of June, the second period was five to seven days in mid-June, and the third period was one to three days in the beginning of July.
Nests were located by observing the behaviour of adult birds and systematically searching all suitable nesting habitat throughout the season. In total, 112 nests of Yellow Wagtail were found. Since 2007 in Topornya, nests were visited every two to three days except when near to the expected hatching and fledging date, when they were checked on alternate days. During each nest check, we recorded the presence or absence of adults, the number of eggs or nestlings, and, if appropriate, the developmental stage of the nestlings. Nestlings were ringed with an aluminium ring on one tarsus and a plastic colour ring on the other at an age of approximately five to nine days. All nestlings of a given year were ringed using the same colour. In Topornya before 2007, and in Chistii Dor, we monitored the fates of nests only. We considered a nest as successful if it produced at least one fledgling. Observations of individually marked young and adult birds were used to detect the fates of nests at both study sites. If a nest was found empty around the expected time of fledging, we confirmed successful breeding by locating the fledglings and observing parents carrying food and engaged in defensive behaviour. The nest was considered unsuccessful if its contents disappeared or adult birds did not appear near the nest for two or more control visits in a row. For unsuccessful nests, we noted any probable cause of destruction based on characteristic signs (an abandoned nest, unsuccessful because of weather, destroyed by predators, etc.).
Adults were captured with automatic spring traps or mist nets set near nests and were ringed with aluminium rings and individual combinations of colour plastic rings. Spring traps were used only on nests with nestlings aged five days or more. Adult birds returning the year after ringing were recognized by individual combinations of colour plastic rings with 12× binoculars. In Chistii Dor, colour marked birds were found by searching the 20-ha study plot and other small (4 ha or less) meadows within a radius of 0.5 km around the study plot. In Topornya, ringed Wagtails were observed throughout the study site (400 ha). Juveniles that returned to breed were caught again and given individual colour combinations. Some adults were recaptured and re-ringed too if it was necessary (i.e. where plastic rings were discoloured or lost).
For the nests found after clutch completion, first-egg dates (FEDs) were calculated from hatching date or nestling age, using the formula: FED = hatch date − 13 − clutch size + 1. Then, we calculated the mean nest initiation date. This estimate may be biased if not adjusted for nests that failed before they were found. Therefore, we used the Horvitz–Thompson estimator described by Dinsmore et al. (Citation2002). This method uses the top nest survival model (see below) to calculate the probability of nest survival. By dividing the observed frequency of each nest type by this probability, we can estimate how many other nests might have been initiated on the same day but failed before they were found. We applied this approach to each nest in the sample and used the expected number of nest initiations as our corrected estimate of mean initiation date. We took the 17th day after the first egg was laid as the hatching date.
Nest survival analysis
Potential causes of variation in nest survival were examined with the program mark 7.0 (Dinsmore et al. Citation2002, Dinsmore & Dinsmore Citation2007). This approach requires the following assumptions: (1) nest ages are correctly determined, (2) nest fates are known with certainty, (3) investigator disturbance does not influence nest survival, and (4) nest fates are independent. For nests with uncertain fates we only used nest information up to the last date the nest was confirmed active and then denoted the nest as successful over that period. If there was uncertainty in nest age or if survival was influenced by human disturbance, then the nest was not used in the analysis. To calculate daily survival rates (DSRs), we used data from 73 nests of known age that were found in 2007–2012 at the Topornya study site. Nests were monitored over 803 exposure days.
We expected DSRs for nests of Yellow Wagtails to vary with respect to nest age, nest stage (incubating or nestling), or date. We used information-theoretic methods (Anderson et al. Citation1994) to evaluate candidate models that explained variation in daily nest survival. In addition to a model that assumed constant daily survival, we considered models that included (1) a linear, quadratic, or cubic effect of age; (2) either a linear or quadratic effect of date; (3) a categorical year effect; and (4) all combinations with age, date, and year effects (Grant et al. Citation2005). We used a logit link function for all models. There is currently no suitable goodness-of-fit test for nest survival models in mark (Dinsmore & Dinsmore Citation2007), and therefore, we have not used one here. We used Akaike's information criterion for small samples (AICc) to rank candidate models. We constructed a confidence set of the models and considered models with δAICc ≤ 2 to be well supported by the data. Although model averaging can be useful to represent variable uncertainty, we chose not to use it here because it would have meant averaging across models with quadratic and cubic terms.
We used the logistic regression equation from the best model to estimate nest survival. To compute overall nest survival, we calculated the survival rate for each nest initiation date for the whole nest period as a product of 27 (assuming 4 days egg laying, 13 days incubation, and 10 days nestling period) consecutive daily nest survival rates (Shaffer & Thompson Citation2007). Then, we calculated survival of all nests as a weighted average of the individual period survival rates. We used the expected number of nest initiations for each date obtained by a Horvitz–Thompson estimator (Dinsmore et al. Citation2002) as a weighting variable (Shaffer & Thompson Citation2007).
Adult apparent survival analysis
We used multistate mark–recapture models in program mark to estimate adult survival probabilities. We used a multistate framework because it permits the estimation of survival probabilities specific to states which may change for individuals from one year to the next and, thus, cannot be incorporated within a traditional Cormack–Jolly–Seber encounter history (White et al. Citation2006). In our study, individuals were assigned to one of two states (successful or unsuccessful breeders). No ringed birds were seen that had not been ringed or seen in the previous year and the recapture probability was constant in all models. First, we examined support for various components of the transition parameter (the probability that an adult that reproduced successfully in year t, reproduces successfully in year t + 1), given that it is alive and in the study area in year t + 1, when the recapture probability was constant and survival probability varied by sex, site, and breeding success. In a second step, we modelled the survival probability using the constant recapture probability and the transition probability obtained from the first step. We performed a goodness-of-fit test for global multistate models (Pradel et al. Citation2003) with program u-care (Choquet et al. Citation2009): this was not significant (χ2 = 2.7, df = 15, P = 0.97) indicating that the model fitted the data adequately.
RESULTS
Abundance
The numbers of breeding Yellow Wagtails at our study sites were not high and varied from 5 to 23 pairs in Topornya and from 1 to 7 pairs in Chistii Dor (). In Topornya, numbers increased during the period of our research (Kendal τ = 0.78, P < 0.01). In Chistii Dor, abundance was constant until 2008, but Yellow Wagtails disappeared after unsuccessful breeding in that year (Shitikov et al. Citation2012b) and did not reappear until 2012.
Table 1. Numbers of breeding Yellow Wagtails at the two study sites.
Breeding performance
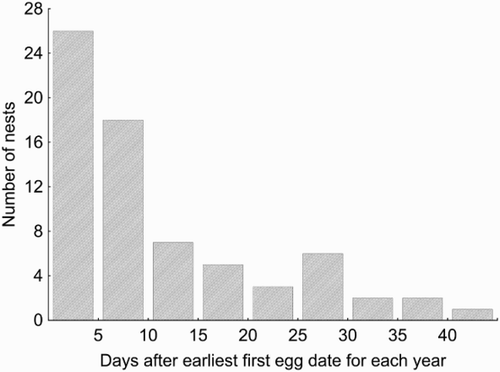
The earliest FED was the 13th of May and the latest, the 26th of June (adjusted mean date was the 1st of June). Every year most clutches were started during the ten days after the very first nests were initiated (). There were from 4 to 7 eggs in a complete clutch, 5.65 ± 0.08 on average (n = 57, not including replacement broods). Clutch size did not vary significantly over the study period (Kruskal–Wallis test: H = 6.5, P = 0.26). All Wagtails at our study sites had only one successful nest attempt per year. We observed 21 unsuccessful pairs of Yellow Wagtails in which at least one adult was ringed. Replacement clutches were found for eight of them. Replacement clutch size was 4.88 ± 0.23 (n = 8). Note that we could have overlooked some cases of renesting because most adults from nests depredated at the incubation stage nests were unringed.
Nest survival
From 2005 to 2012, we observed the fate of 103 nests in total, 57 of them were successful and 46 were unsuccessful. In 29 cases the reason for failure was depredation, 6 nests were abandoned without visible reasons, 5 nests perished because of ploughing for fire breaks, and 6 nests were abandoned after capture and ringing of adults. In most cases we could not identify predators, but we observed the destruction of some nests by Hen Harrier Circus cyaneus, Short-eared Owl Asio flammeus, and Common European Adder Vipera berus.
DSR analysis included data from 73 nests of Yellow Wagtail at the Topornya study site, which were observed during 803 days in total (see Methods). The best model included nest stage (eggs or nestlings) and a quadratic function of the day of the season providing strong evidence that these variables were influential in determining nest survival in this population (). DSR was highest at the beginning of the breeding season, when most of the nests were at the egg-laying stage or at the beginning of incubation. Then DSR decreased reaching the lowest level by the last week of June before increasing again at the beginning of July (). The overall nest survival probability estimated from the best nest survival model was 0.40 ± 0.02.
Figure 2. Changes in daily nest survival across breeding season estimated from the model Stage + T2. Dashed lines represent 95% confidence intervals for the DSR.
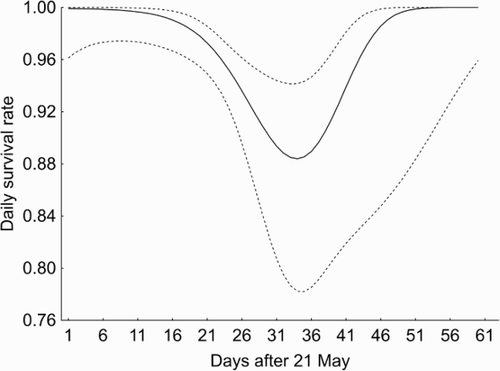
Table 2. Summary of model selection results for nest survival of Yellow Wagtails in 2007–2012.
Juvenile and adult apparent survival
Only 4 males returned the following year from 264 ringed nestlings (194 in Topornya and 70 in Chistii Dor). A single female, ringed in 2008 at Chistii Dor as a nestling, was accidentally found 14 km east of its birthplace (Shitikov et al. Citation2012b). From 67 marked adult Yellow Wagtails (35 males and 32 females), 11 males and 8 females returned the following years, 12 of them returned only the following year, 5 birds returned two years in a row, 1 female returned three years in a row, and 1 male returned four years in a row.
Model selection showed that adult apparent survival differed between individuals with successful and unsuccessful breeding the previous year. Selection probability of the effect of breeding success (total AICc weight) was 0.98 (). Using the best model, apparent survival after successful breeding was 0.42 ± 0.06 (95% CI 0.31, 0.53) but for unsuccessful breeders apparent survival rate was only 0.13 ± 0.06 (95% CI 0.05, 0.29). Apparent survival rate depended on study site (AIC weight = 0.40). Apparent survival of successful breeders was higher in Chistii Dor (0.50 ± 0.1, 95% CI 0.32, 0.68) than in Topornya (0.37 ± 0.07, 95% CI 0.24, 0.52). In contrast, apparent survival of unsuccessful breeders in Topornya was 0.16 ± 0.07 (95% CI 0.06, 0.36) but in Chistii Dor this was zero (i.e. unsuccessful breeders did not return to the study site at all). Sex of Yellow Wagtails also slightly influenced apparent survival (AIC weight = 0.19). Among unsuccessful breeders, male survival was less (0.07 ± 0.06, 95% CI 0, 0.37) than female survival (0.17 ± 0.09, 95% CI 0.05, 0.40). Recapture probability was equal to one at both study sites. The estimated probability to reproduce successfully (the transition probability) was constant for both classes of reproductive success, both sexes and both sites (0.45 ± 0.09, 95% CI 0.3, 0.62; ).
Table 3. Summary of model selection results for adult apparent survival of Yellow Wagtails in 2007–2012.
Table 4. Summary of model selection results for the transition probability.
DISCUSSION
Clutch size and number of nesting attempts
Our research highlights some important differences from previous studies, and in particular an important feature of Yellow Wagtails breeding on abandoned fields in the ‘Russky Sever’ National Park is only one successful brood per year. Two successive nesting attempts in a season is one of the key adaptations of the Yellow Wagtail for nesting on arable lands in Western Europe (Gilroy et al. Citation2010, Kirby et al. Citation2012), and this is associated with a mid-season nesting habitat shift (Stiebel Citation1997, Gilroy et al. Citation2010, Kragten Citation2011), where initial and late broods are made in different habitats.
One brood per year may be partly compensated for by the size of clutch. Mean clutch size in our study was larger than in Western and Central Europe (Mason & Lyczynski Citation1980, Kirby et al. Citation2012) and comparable with data from Scandinavia (Paulsen Citation1993, Flyckt Citation1999). Bell (Citation1996) described a cline of increasing clutch size for Yellow Wagtails across the species range from south to north, with an increase of about one egg per 19° of latitude through Europe. The mean clutch size in our region (5.67) was larger than that predicted by Bell's (Citation1996) model from Western Asia/Eastern Europe (predicted 5.28) and less than that predicted by a model from Western Europe (predicted 6.08).
Nest survival
Predation is the main cause of nesting failure for most songbirds (Ricklefs Citation1969, Martin Citation1992). The potential positive effects of agricultural land abandonment on populations may be obscured by increasing risk of predation (Tryjanowski et al. Citation2011). At our study site ground-situated nests could be destroyed by a wide range of predators (Shitikov et al. Citation2012a). Besides those predator species which were seen directly at a Yellow Wagtail nest (Hen Harrier, Short-eared Owl, and Common Viper), crows (Hooded Crow Corvus cornix, Jackdaw Corvus monedula, Chough Corvus frugilegus, and Magpie Pica pica) and carnivorous mammals (domestic dogs and cats, mustelids) may also play an important role in nest destruction.
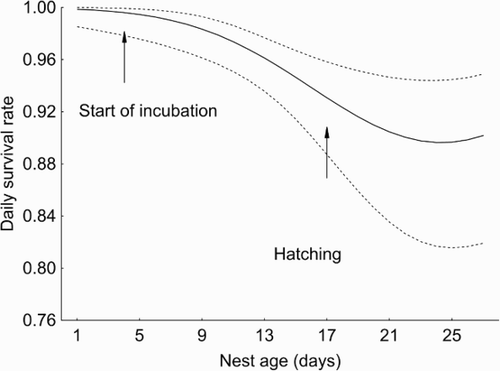
Our results showed that DSRs of Yellow Wagtail nests were strongly dependent on nest stage: nests with nestlings were depredated more often than nests with eggs (). The differences in DSRs for nests at different periods of the breeding season, as a rule, can probably be explained by their different detectability to predators: during the nestling period, nests may attract greater attention because of begging nestlings and frequent movements to and from the nest by foraging adults (Martin et al. Citation2000). DSR was high at the beginning of breeding season, and then it declined towards the end of June and returned to initial values in July. Fledglings successfully left either early nests in the first half of June or late nests: nests in the middle of the breeding season were usually depredated. In contrast, there were no inter-annual fluctuations in breeding success for Yellow Wagtail. Overall reproductive success in our region was slightly less than that reported by Kirby et al. (Citation2012) for the Yellow Wagtails breeding in wheat fields in Great Britain (49% for early nests and 85% for late ones) and much more than the success on arable lands in Germany (Stiebel Citation1997) and Great Britain (Gilroy et al. Citation2010).
Figure 3. Changes in daily nest survival across the 27-day nest cycle (for nests initiated on the 1st of June) estimated from the model Stage + T2. Dashed lines represent 95% confidence intervals for the DSR.
Current reproductive success may be independent of previous reproductive success, or it may depend on it. The latter is to be expected if there are strong individual differences in the reproductive performance or if breeding success changes with age. In contrast, random changes among classes of reproductive success can be expected if reproductive success is mainly determined by environmental effects (Schaub & Von Hirschheydt Citation2009). In our study, the transition probability for classes of reproductive success obtained from a multistate capture–recapture model was 0.45 ± 0.09. Therefore, current reproductive success of Yellow Wagtail was not likely to be dependent on previous reproductive success.
Apparent survival
Our results show that the reproductive success of Yellow Wagtails has a strong impact on their adult apparent survival. This result is similar to that obtained for many passerines (Haas Citation1998, Sedgwick Citation2004, Schaub & Von Hirschheydt Citation2009, Schaub et al. Citation2011). Apparent survival of successful breeders in Chistii Dor (0.5 ± 0.1) was slightly higher than that in Topornya (0.37 ± 0.07). Differences in apparent survival at our sites may be explained by the isolated position of the study site in Chistii Dor (Shitikov et al. Citation2012b). The estimates of apparent survival we obtained for successful breeders in Chistii Dor are similar to the estimates of true survival of adult Yellow Wagtails in arable fields in Great Britain (0.53 ± 0.03) (Siriwardena et al. Citation1998). The isolated position of the Chistii Dor study site may limit breeding dispersal and so the estimates of apparent survival for successful breeders on this study site may indicate the true survival rate estimate. In Topornya, the local population of Yellow Wagtail is not as isolated and apparent survival estimates may be more influenced by emigration. Unsuccessful breeders emigrated from both study sites with a high probability that is reflected in low apparent survival. In Chistii Dor, unsuccessful breeders did not return at all (apparent survival rate was zero). After unsuccessful breeding in 2008, the local population in Chistii Dor virtually disappeared (Shitikov et al. Citation2012b).
Apparent survival rate for males was slightly less than that for females for both classes of reproductive success. This is counter to female-biased dispersal that has been observed in some passerines after unsuccessful breeding (Bollinger & Gavin Citation1989, Schaub & Von Hirschheydt Citation2009). Drost's (Citation1948) five-year ringing study on Helgoland, Germany, showed that females of Yellow Wagtails returned in nearly equal proportions to males. Renner & McCaffery (Citation2008) at Cape Romanzof, Alaska, had high apparent survival of adult males of the Eastern Yellow Wagtail Motacilla tschutschensis but adult females did not return to the study area (i.e. apparent survival was zero). Thus, sex-biased dispersal and survival of Yellow Wagtails is variable and requires further investigation.
Apparent survival of juveniles was extremely low: less than 2% of Wagtails ringed as nestlings returned to both study sites. Clearly, a population with such low juvenile annual survival rate would be destined for rapid extinction and so a better explanation of low juvenile apparent survival may be high rates of natal dispersal.
Thus, apparent survival rates of Yellow Wagtails using abandoned fields of the north of European Russia are determined by previous breeding success and so their tendency to change breeding sites between years. Apparent survival after successful breeding was significantly higher than after unsuccessful breeding. Therefore, predation was the main factor limiting two important life history traits – reproductive success and site fidelity (dispersal). Overall high adult survival may be important in the study region for population persistence, where second breeding attempts are apparently unusual.
ACKNOWLEDGEMENTS
The work in the ‘Russky Sever’ National Park was made possible through the support of the administration of the Park, particularly A. L. Kuznetsov and L. V. Kuznetsova. The students and graduates of the Department of Zoology and Ecology, Moscow Pedagogical State University (T. Bulkina, T. Vaytina, D. Fedchuk, S. Makushina, N. Miklin, and K. Starostina) were involved in the field work.
FUNDING
Our investigations have received financial support from the Russian Fund of Basic Researches (13-04-00745-a).
REFERENCES
- Anders, A.D. & Marshall, M.R. 2005. Increasing the accuracy of productivity and survival estimates in assessing landbird population status. Conserv. Biol. 19: 66–74. doi: 10.1111/j.1523-1739.2005.00543.x
- Anderson, D.R., Burnham, K.P. & White, G.C. 1994. AIC model selection in overdispersed capture–recapture data. Ecology 75: 1780–1793. doi: 10.2307/1939637
- Bell, C.P. 1996. The relationship between geographic variation in clutch size and migration pattern in the Yellow Wagtail. Bird Study 43: 333–341. doi: 10.1080/00063659609461026
- Bollinger, E.K. & Gavin, T.A. 1989. The effects of site quality on breeding-site fidelity in Bobolinks. Auk 106: 584–594.
- Bradbury, R.B. & Bradter, U. 2004. Habitat associations of Yellow Wagtails Motacilla flava flavissima on lowland wet grassland. Ibis 146: 241–246. doi: 10.1111/j.1474-919x.2003.00251.x
- Choquet, R., Lebreton, J.-D., Gimenez, O., Reboulet, A.-M. & Pradel, R. 2009. U-CARE: utilities for performing goodness of fit tests and manipulating CApture–REcapture data. Ecography 32: 1071–1074. doi: 10.1111/j.1600-0587.2009.05968.x
- Cilimburg, A.B., Lindberg, M.S., Tewksbury, J.J., Hejl, S.J. & Thompson Iii, F. 2002. Effects of dispersal on survival probability of adult Yellow Warblers (Dendroica petechia). Auk 119: 778–789. doi: 10.1642/0004-8038(2002)119[0778:EODOSP]2.0.CO;2
- Dinsmore, S.J. & Dinsmore, J.J. 2007. Modeling avian nest survival in program MARK. Stud. Avian Biol. 34: 73–83.
- Dinsmore, S.J., White, G.C. & Knopf, F.L. 2002. Advanced techniques for modeling avian nest survival. Ecology 83: 3476–3488. doi: 10.1890/0012-9658(2002)083[3476:ATFMAN]2.0.CO;2
- Doligez, B. & Pärt, T. 2008. Estimating fitness consequences of dispersal: a road to ‘know-where’? Non-random dispersal and the underestimation of dispersers’ fitness. J. Anim. Ecol. 77: 1199–1211. doi: 10.1111/j.1365-2656.2008.01446.x
- Dombrowski, A. & Golawski, A. 2002. Changes in numbers of breeding birds in an agricultural landscape of east-central Poland. Vogelwelt 123: 79–88.
- Donald, P.F., Sanderson, F.J., Burfield, I.J. & van Bommel, F.P.J. 2006. Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agr. Ecosyst. Environ. 116: 189–196. doi: 10.1016/j.agee.2006.02.007
- Drost, V.R. 1948. Populationsstudien an der Englischen Schafstelze, Motacilla flava flavissima Blyth, auf Helgoland. Vogelwarte 1: 18–28.
- Flyckt, G. 1999. Yellow Wagtails Motacilla f. flava nesting biology in southern Swedenj. Ornis Svecica 9: 217–223.
- Gilroy, J.J., Anderson, G.Q.A., Grice, P.V., Vickery, J.A., Bray, I., Watts, N.P. & Sutherland, W.J. 2008. Could soil degradation contribute to farmland bird declines? Links between soil penetrability and the abundance of Yellow Wagtails Motacilla flava in arable fields. Biol. Conserv. 141: 3116–3126. doi: 10.1016/j.biocon.2008.09.019
- Gilroy, J.J., Anderson, G.Q.A., Grice, P.V., Vickery, J.A. & Sutherland, W.J. 2010. Mid-season shifts in the habitat associations of Yellow Wagtails Motacilla flava breeding in arable farmland. Ibis 152: 90–104. doi: 10.1111/j.1474-919X.2009.00988.x
- Gilroy, J.J., Virzi, T., Boulton, R.L. & Lockwood, J.L. 2012. A new approach to the ‘apparent survival’ problem: estimating true survival rates from mark–recapture studies. Ecology 93: 1509–1516. doi: 10.1890/12-0124.1
- Grant, T.A., Shaffer, T.L., Madden, E.M. & Pietz, P.J. 2005. Time-specific variation in passerine nest survival: new insights into old questions. Auk 122: 661–672. doi: 10.1642/0004-8038(2005)122[0661:TVIPNS]2.0.CO;2
- Haas, C.A. 1998. Effects of prior nesting success on site fidelity and breeding dispersal: an experimental approach. Auk 115: 929–936. doi: 10.2307/4089511
- Kirby, W.B., Anderson, G.Q.A., Grice, P.V., Soanes, L., Thompson, C. & Peach, W.J. 2012. Breeding ecology of Yellow Wagtails Motacilla flava in an arable landscape dominated by autumn-sown crops. Bird Study 59: 383–393. doi: 10.1080/00063657.2012.715136
- Kragten, S. 2011. Shift in crop preference during the breeding season by Yellow Wagtails Motacilla flava flava on arable farms in the Netherlands. J. Ornithol. 152: 751–757. doi: 10.1007/s10336-011-0655-8
- Lebreton, J.-D., Burnham, K.P., Clobert, J. & Anderson, D.R. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62: 67–118. doi: 10.2307/2937171
- Malchevsky, A.S. & Pukinsky, Y.V. 1983. [The birds of Leningrad region and adjacent areas], Vol. 2. Leningrad: Leningrad State University publishers. pp. 1–504. (In Russian).
- Martin, T.E. 1992. Interaction of nest predation and food limitation in reproductive strategies. Curr. Ornithol. 9: 163–197. doi: 10.1007/978-1-4757-9921-7_5
- Martin, T.E., Scott, J. & Menges, C. 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. Lond. B 267: 2287–2293. doi: 10.1098/rspb.2000.1281
- Mason, C.F. & Lyczynski, F. 1980. Breeding biology of the Pied and Yellow Wagtails. Bird Study 27: 1–10. doi: 10.1080/00063658009476650
- Morris, A.J. & Gilroy, J.J. 2008. Close to the edge: predation risks for two declining farmland passerines. Ibis 150: 168–177. doi: 10.1111/j.1474-919X.2008.00857.x
- Orłowski, G. 2005. Endangered and declining bird species of abandoned farmland in south-western Poland. Agr. Ecosyst. Environ. 111: 231–236. doi: 10.1016/j.agee.2005.06.012
- Pasinelli, G., Müller, M., Schaub, M. & Jenni, L. 2007. Possible causes and consequences of philopatry and breeding dispersal in Red-backed Shrikes Lanius collurio. Behav. Ecol. Sociobiol. 61: 1061–1074. doi: 10.1007/s00265-006-0339-1
- Paulsen, B. 1993. Breeding biology of the Yellow Wagtail Motacilla flava flavissima in SW Norway. Fauna Norvegica C 16: 31–35.
- Pradel, R., Wintrebert, C. M. A. & Gimenez, O. 2003. A proposal for a goodness-of-fit test to the Arnason-Schwarz multisite capture-recapture model. Biometrics 59: 3–53. doi: 10.1111/1541-0420.00006
- Renner, H.M. & McCaffery, B.J. 2008. Demography of Eastern Yellow Wagtails at Cape Romanzof, Alaska. Wilson J. Ornithol. 120: 85–91. doi: 10.1676/06-122.1
- Ricklefs, R.E. 1969. An analysis of nesting mortality in birds. Smithson. Contrib. Zool. 9: 1–48. doi: 10.5479/si.00810282.9
- Schaub, M. & Von Hirschheydt, J. 2009. Effect of current reproduction on apparent survival, breeding dispersal, and future reproduction in barn swallows assessed by multistate capture–recapture models. J. Anim. Ecol. 78: 625–635. doi: 10.1111/j.1365-2656.2008.01508.x
- Schaub, M., Jakober, H. & Stauber, W. 2011. Demographic response to environmental variation in breeding, stopover and non-breeding areas in a migratory passerine. Oecologia 167: 445–459. doi: 10.1007/s00442-011-1999-8
- Sedgwick, J.A. 2004. Site fidelity, territory fidelity, and natal philopatry in Willow Flycatchers (Empidonax traillii). Auk 121: 1103–1121.
- Shaffer, T.L. & Thompson, F.R. 2007. Making meaningful estimates of nest survival with model-based methods. Stud. Avian Biol. 34: 84–95.
- Shitikov, D.A. 2000. [Space-time structure of fauna and bird population of the European agricultural lands of northern Russia]. PhD Thesis, Moscow Pedagogical State University, Moscow: 1–16 (in Russian).
- Shitikov, D.A., Fedotova, S.E. & Gagieva, V.A. 2012a. Nest survival, predators and breeding performance of Booted Warblers Iduna caligata in the abandoned fields of the North of European Russia. Acta Ornithol. 47: 137–146. doi: 10.3161/000164512X662241
- Shitikov, D., Fedotova, S., Gagieva, V., Fedchuk, D., Dubkova, E. & Vaytina, T. 2012b. Breeding-site fidelity and dispersal in isolated populations of three migratory passerines. Ornis Fenn. 89: 53–62.
- Siriwardena, G.M., Baillie, S.R. & Wilson, J.D. 1998. Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study 45: 276–292. doi: 10.1080/00063659809461099
- Smart, S.M., Firbank, L.G., Bunce, R.G.H. & Watkins, J.W. 2000. Quantifying changes in abundance of food plants for butterfly larvae and farmland birds. J. Appl. Ecol. 37: 398–414. doi: 10.1046/j.1365-2664.2000.00508.x
- Stiebel, H. 1997. Habitatwahl, Habitatnutzung Und Bruterfolg Der Schafstelze Motacilla flava in Einer Agrarlandschaft. Vogelwelt 118: 257–268.
- Tryjanowski, P. & Bajczyk, R. 1999. Population decline of the Yellow Wagtail Motacilla flava in intensively used farmland of Western Poland. Vogelwelt 120: 205–207.
- Tryjanowski, P., Hartel, T., Báldi, A., Szymański, P., Tobolka, M., Herzon, I., Goławski, A., Konvička, M., Hromada, M., Jerzak, L., Kujawa, K., Lenda, M., Orłowski, G., Panek, M., Skórka, P., Sparks, T.H., Tworek, S., Wuczyński, A. & Żmihorski, M. 2011. Conservation of farmland birds faces different challenges in Western and Central-Eastern Europe. Acta Ornithol. 46: 1–12. doi: 10.3161/000164511X589857
- White, G.C., Kendall, W.L. & Barker, R.J. 2006. Multistate survival models and their extensions in program MARK. J. Wildlife Manage. 70: 1521–1529. doi: 10.2193/0022-541X(2006)70[1521:MSMATE]2.0.CO;2
- Wilson, A.M. & Vickery, J.A. 2005. Decline in Yellow Wagtail Motacilla flava flavissima breeding on lowland wet grassland in England and Wales between 1982 and 2002. Bird Study 52: 88–92. doi: 10.1080/00063650509461377
- Wretenberg, J., Lindström, Å., Svensson, S. & Pärt, T. 2007. Linking agricultural policies to population trends of Swedish farmland birds in different agricultural regions. J. Appl. Ecol. 44: 933–941. doi: 10.1111/j.1365-2664.2007.01349.x