Abstract
Capsule Low clutch size (CS) in the Atlas Pied Flycatcher breeding in evergreen Mediterranean forest was compensated for by relatively high overall reproductive success.
Aims To describe the breeding ecology of the Atlas Pied Flycatcher Ficedula speculigera in detail for the first time, in an old oak Quercus suber forest.
Methods A total of 102 nests were monitored during 2010–2012. Breeding phenology, population density, clutch and brood sizes, egg biometrics, breeding losses and breeding success were accurately determined.
Results The species arrived in the breeding area in late April. Population density was very high with 4.87 (±1.02) pairs/ha. Mean egg laying date (LD) was 19 May and CS averaged 4.92 eggs. Hatching and fledging success were 88.7% and 83.5%, respectively. The number of fledged young averaged 3.8 (±1.66) and decreased with LD (4.2 ± 1.45 chicks fledged per nest at the start of the season versus 2.8 ± 1.56 at the end).
Conclusion Lower CS compared to Ficedula hypoleuca populations was compensated by relatively high fledging success, thereby ensuring overall reproductive success of this species. Moreover, the Atlas Pied Flycatchers seem to benefit from the lower seasonality in their food in the evergreen habitat.
The Atlas Pied Flycatcher Ficedula speculigera is a migratory passerine that breeds in north-west Africa between 36°N and 33°N (Heim de Balsac & Mayaud Citation1962). It is part of the black-and-white flycatcher species complex that also includes the European Pied, Collared and Semicollared Flycatchers (Sætre et al. Citation2001a, Citation2001b). It has formerly been considered to be a sub-species of European Pied Flycatcher Ficedula hypoleuca, but has been recently recognized as a distinct species (based on morphological and molecular evidence; Sætre et al. Citation2001a, Citation2001b).
The Atlas Pied Flycatcher has its breeding grounds in the Atlas Mountains at 1200–1800 m in forests of cedar Cedrus, oak Quercus suber and Quercus Ilex, and Aleppo pine Pinus halepensis (Snow Citation1952). However, the limits of its distribution are not well defined. In Algeria, some populations have been located in Cedrus forests in the mountains of Kabylie (Isenmann & Moali Citation1987) and in Cork Oak Q. suber wood in the north-east of country (Benyacoub & Chabi Citation2000). In Morocco, the species is present in mountainous areas of the Rif and in Middle and High Atlas where it breeds from 1000 to 2250 m (Thévenot et al. Citation2003). Population status of Atlas Pied Flycatcher is not known in detail but may be declining. The species lives in a limited geographic area, mainly consisting of small fragmented patches of forest. A significant increase in degradation of oak and cedar forest attributed to a significant increase in grazing animals (Ciani et al. Citation2005) and fire (Benyacoub & Chabi Citation2000) has been observed. This degradation may represent loss of habitat, and is a potential threat to the Atlas Pied Flycatcher.
Available information on the breeding ecology of the species is very scarce and scattered and is mostly in handbooks and guides based on studies with very small sample sizes (Heim de Balsac & Mayaud Citation1962, Etchecopar & Hüe Citation1964, Isenmann & Moali Citation1987, Thévenot et al. Citation2003). Many important life history traits, such as hatching, fledging and reproductive success have not been published previously. More data are therefore needed to fully understand the reproductive biology of the species. We aim here to describe the breeding ecology of a population breeding in nest-boxes located in an old oak Q. suber forest in northeastern Algeria. Our objectives were to: (1) characterize timing of egg laying and its consequences for other life history traits; (2) obtain reliable data on population density, clutch size (CS), egg characteristics, hatching and fledging success; (3) investigate whether breeding success is related to other life history traits and (4) any inter-annual variation in timing of breeding and reproductive performance.
METHODS
The study was conducted during three breeding seasons (between 2010 and 2012) in a mature evergreen Q. suber oak wood without undergrowth on the Djebel Ghorra (36°32′N, 8°20′E, elevation 600–800 m a.s.l.) 50 km south of El Kala City, northeastern Algeria. In the study sites, the trees of Q. suber have a homogeneous spatial distribution; their area is contiguous with that of Zeen Oak Quercus faginea located at higher altitude. The trees have an average height of 10.4 m and a ground cover of 65% on average. The almost total absence of the undergrowth is due to the action of cattle grazing. This could eventually prevent the regeneration of the forest cover (Benyacoub & Chabi Citation2000). The herbaceous layer is moderately developed and is represented by opportunistic species such as, Asphodelus microcarpus, Urginea maritima and some grasses. The climate is Mediterranean with dry and hot summers and wet and mild winters. During May and June the mean temperature is 19.4°C (10–29°C) and the annual rainfall slightly exceeds 1000 mm.
We erected 182 nest-boxes during the entire study period (57 in 2010, 67 in 2011 and 58 in 2012; 125 × 117 mm bottom area) just before Flycatchers arrived in spring (to avoid occupation by competing species, mainly titmice). The size of the area covered by boxes was 6.5 ha in 2010, 7.5 ha in 2011 and 6.8 ha in 2012. Nest-boxes were placed at a height of approximately 4 m above ground on tree trunks and the distance between adjacent boxes was about 30–35 m. The area was visited at two- to three-day intervals from 10 April in each year, and daily after observation of the first male, to determine the arrival date of the first arriving 10–15 male Atlas Pied Flycatchers. Arrival dates of males were determined by recording the daily numbers of individuals heard or seen in the area. Female arrival was determined when nest material was observed in the nest-box.
We checked all nest-boxes every three to five days from late April until mid-July to determine the beginning of nest building. Active nests were then visited every one to two days to determine breeding parameters accurately. Flycatcher density was expressed as the number of occupied nest-boxes per hectare. For each nest-box we recorded the onset of nest building and egg laying, CS, incubation period, brood size at hatching, nestling mortality and number of fledglings. Finally, it was possible to calculate the hatching success (% of eggs that hatched successfully), fledging success (number of fledglings/numbers of hatched eggs), chick mortality rate (% of chicks that died in the nest) and breeding success (number of fledglings/numbers of eggs laid). The nest building period was determined as the time that elapsed from the day the female began nest building to the day that she laid her first egg. The incubation period was determined from the last egg laid till the first egg hatched. Male assistance during incubation was investigated in 30 breeding pairs. We recorded male provisioning by filming nest-boxes with video cameras (Sony DCR-HC36) placed in front of the box entrance at a distance of 4–6 m (using optical zooms up to 20×) and hidden by vegetation as much as possible. Recordings were only made in dry weather and took place between 08:00 and 12:00 hour. Each nest was filmed for 63 minutes and the total observation time was 31 hour 30 minutes. Male visits to their nest were analysed from the films and expressed as an hourly rate. We collected data on hatching asynchrony by checked nest-boxes daily from two days before the eggs were expected to hatch until hatching and between 08:00 and 14:00 hour (the same nests were visited at the same time of the day). In our study we defined asynchronous clutches as those in which nestlings were hatching for more than 24 hours. Nests where egg laying was initiated, whether successfully or not, were considered as occupied nests. A breeding attempt was considered successful if at least one young fledged. Eggs were weighed, one or two days after the start of incubation, to the nearest 0.05 g using a Pesola spring balance (10 g) and their lengths (L) and breadths (B) were measured to the nearest 0.1 mm using vernier callipers. Egg volume (V) was obtained with Hoyt's (Citation1979) formula: V = 0.000507 × L × B2. All characteristics related to egg dimensions are presented as means per clutch to avoid the non-independence of egg traits within clutches (Bańbura & Zieliński Citation1998).
The data were analysed by standard parametric and non-parametric methods (Sokal & Rohlf Citation1995). A χ2 analysis was used to compare annual differences in proportion of nest-boxes occupied. We compared reproductive traits between years using one-way anova. Linear regression was carried out to examine the influence of laying date (LD) on different reproductive characteristics and for all analyses we give the equations of the regression Y = a + bX (where Y = dependent variable, b = slope of the regression coefficient and a = constant value). Multiple regression was used to analyse the effects of several reproductive parameters simultaneously on egg volume, brood size at hatching, fledging success and mortality rate. The effect of LD, CS or egg volume on hatchability (the proportion of eggs hatched per nest, the dependent variable) was analysed with a logistic regression using generalized linear models (logit link function, binomial distribution). Values are presented as means and standard deviations (sd). Tests were considered significant when P ≤ 0.05. All statistical analyses were performed using STATISTICA 10 package (StatSoft, Inc. Citation2011).
RESULTS
Atlas Pied Flycatchers arrived at their breeding grounds by the third week in April. The first male was observed in our study area on 16 April 2012 and the first female on 20 April 2011. Numbers of Atlas Pied Flycatcher increased in the following weeks until the beginning of May. Early males arrived 7.8 (±1.48) days earlier than early females.
On average, the occupancy rate of nest-boxes was 56% (ranging from 42% in the first year, 60% in the second and 65% in the third year). The other species occupying the boxes were Blue Tit Cyanistes caeruleus and Great Tit Parus major. There was a significant difference in the percentage of occupation by Atlas Pied Flycatcher among years (χ2 test, χ2 = 12.9, df = 2, P = 0.015). Average Atlas Pied Flycatchers density in the study area was 4.87 (±1.02) pairs/ha and ranged from 3.7 pairs/ha in 2010, 5.33 pairs/ha in 2011 and 5.58 pairs/ha in 2012.
A total of 102 nests were monitored during the entire study period. However, the number of monitored nests differed among years, 24 in 2010, 40 in 2011 and 38 in 2012. Courtship and nest building activity was highest during the first and second week of May. Nest construction averaged 8.5 (±1.76) days, n = 72 and ranged from 5 to 11 days. In five nests, we observed male contribution to nest building. The principal materials used for nest construction were dry grasses, moss, leaves of Q. suber with animal hair and sheep wool occasionally placed within the cup of the nest.
Egg laying was spread from early May (first egg laid on 5 May 2012) through late June (first egg of last clutch laid on 26 June 2010) (). Half of the females started laying during the second ten days of May with a median LD of 14 May (). We could not confirm whether second clutches were laid after completion or failure of the first nesting attempt in a breeding season. No variation in arrival dates or dates of laying was observed between years (anova: early males arrival dates, F2,36 = 1.2, P = 0.30, n = 39; early females arrival dates, F2,69 = 1.1, P = 0.34, n = 72; LD, F2,99 = 1.4, P = 0.24, n = 102; ).
Figure 1. Temporal distribution of the date of laying the first egg by the Atlas Pied Flycatcher. The time of clutch initiation is arranged in five-day periods. The arrow indicates median LD for all years.
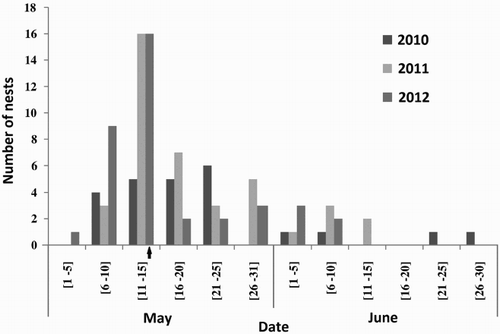
Table 1. Breeding parameters for Atlas Pied Flycatchers in the study area.
CS ranged from two to six eggs, with a mode of five representing 54.9% of all clutches (). CS decreased with LD (linear regression: slope = –0.044 ± 0.0054, F1,100 = 65.2, P < 0.0001, r2 = 0.39, n = 102; a). Eggs averaged 17.7 ± 0.67 mm in length (range 15.1–20.0 mm, n = 82 nests), 13.2 ± 0.37 mm in width (range 11.9–14.4 mm, n = 83) and 1.6 ± 0.14 g in weight (range 1.1–2.0 g, n = 83). Mean egg volume was 1.6 ± 0.12 cm3 (range 1.20–1.90 cm3, n = 83). A multiple regression analysis showed that there was no effect of CS or LD on egg volume (F2,80 = 0.9, P = 0.45, r² = 0.02, n = 83).
Figure 2. Relationship between LD and both CS (a) and number of young fledged (b) in the Atlas Pied Flycatcher in 2010–2012. The regression equations are (a) CS = 5.76–0.044 LD and (b) number of young fledged = 5.29–0.0753 LD.
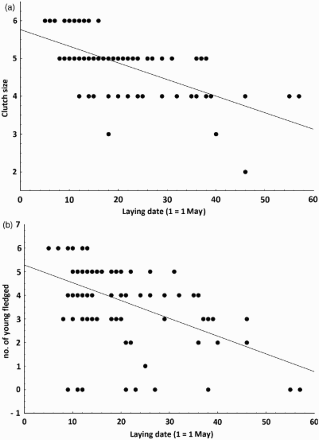
Table 2. CS variations of the Atlas Pied Flycatcher in the study area.
Only females incubated the eggs and males were observed feeding their mate in 27 (90%) of nests observed. Over the observational period, we recorded males with and without food entering the nest in which the female was sitting with a visit rate averaging 4.4 (±1.07) visits/hour. The incubation period lasted 12–16 days and the mean incubation period was 13.2 (±0.82) days, n = 96. Clutches started later had shorter incubation duration (linear regression: incubation duration = 13.6–0.0214 LD, F1,94 = 8.7, P = 0.004, r2 = 0.08, n = 96) whereas no influence of CS or egg volume on incubation duration was recorded (multiple regression: F2,77 = 1.7, P = 0.19, r² = 0.04, n = 80).
Eggs hatched asynchronously in 33 (32.3%) nests with a mean hatch interval estimated at 1.48 ± 0.51 days (ranged from one to two days, n = 33). Asynchronous broods had later mean LDs compared to synchronous broods (23.6 ± 11.9 May versus 17.5 ± 10.1 May, t = 2.6, P = 0.012, df = 88). Of the 497 eggs laid in 101 clutches, 441 eggs hatched and thus gave a mean hatching success of 88.7% (). Five nests (5.0%) failed to hatch at least one egg, with four of these occurring in 2012. This failure was caused probably by nest desertion or death of the female. If these clutches are excluded from the analyses, hatching success was 93.2%. In five nests, females may have reduced their CSs by removing or displacing an egg onto the rim of the nest. We found no effect of LD, CS or egg volume on egg hatchability (logistic regression: LD: Wald = 0.2, df = 1, P = 0.68; CS: Wald = 0.7, df = 1, P = 0.41; egg volume: Wald = 3.3, df = 1, P = 0.06). Brood size at hatching ranged from zero to six nestlings, with brood size negatively correlated to LD and positively to CS (multiple regression: brood size at hatching = −0.067 + 0.955 CS − 0.002 LD, F2,93 = 60.7, P < 0.0001, r2 = 0.57, n = 96).
Nestlings fledged 14–18 days after hatching and the mean was 16.3 (±0.91) days, n = 84. Nestling period increased with the advancement of the season (linear regression: nestling period = 15.7 + 0.03 LD, F1.82 = 12.8, P = 0.001, r2 = 0.13, n = 84). A total of 355 nestlings fledged which yielded a mean fledging success of 83.5% (n = 93; ). Pairs that started laying eggs later had significantly fewer fledglings (linear regression: slope = −0.075 ± 0.013, F1.91 = 30.8, P < 0.0001, r2 = 0.25, n = 93; b). CS and brood size at hatching positively influenced fledging success (multiple regression: number of fledglings = −1.39 + 0.29 CS + 0.87 brood size at hatching, F2,86 = 42.5, P < 0.0001, r² = 0.49, n = 89). However, fledging success was not influenced by egg volume (linear regression: F1,67 = 3.7, P = 0.058, r² = 0.05, n = 69).
More than 82% of the nests were successful () of which 100% success was found in 46% of nests. Successful and non-successful broods did not differ in the date of laying, CS or egg volumes (Mann–Whitney U-test: all P > 0.11). Of the 18 nests that failed during the three years, five nests failed due to abandonment. All cases of abandonment occurred before hatching. Three nests were lost to predation: one by an Ocellated Lizard Timon pater and two nests after attack by red ants: all predation events were recorded in 2012. In two nests in 2010, chicks left the nest 9–11 days after hatching despite the nests being intact. Three nest-boxes were dislodged by strong winds on a single night (14 June 2010) and one was completely destroyed by people. In four nests, all nestlings were found dead. Chick mortality rate increased seasonally (linear regression: chick mortality = −0.165 + 0.035 LD, F1.85 = 12.9, P = 0.001, r2 = 0.13, n = 87). However, no relationship was found between mortality rate and CS or brood size at hatching (multiple regression: F2,84 = 0.4, P = 0.64, r² = 0.01, n = 87). Mortality rate was higher in asynchronous broods compared to synchronous ones (Mann–Whitney U-test: Z = 2.1, P = 0.01). No between-year differences in CS, hatching success or egg traits (length, width, weight and volume) were found (one-way anova: all P > 0.19; ). However, both chick mortality and fledging success varied significantly among the three years of the study (anova: chick mortality, F2,85 = 6.6, P = 0.0021, n = 88; fledging success, F2,90 = 6.7, P = 0.002, n = 93): 2010 was the worst breeding season with only 64.9% of nestlings fledged ().
DISCUSSION
This study highlights part of the breeding biology of a rare and still poorly known species: the Atlas Pied Flycatcher, studied over three years. Our data showed that the species arrived in the breeding area from late April, started laying between early May and late June with a distinct peak in mid-May and had high breeding density exceeding 4.5 pairs/ha. The Atlas Pied Flycatcher had small clutches, but appeared nevertheless to have relatively high productivity with a fledging success exceeding 83%. Some reproductive parameters showed intra-seasonal variation, whereas inter-annual differences were much less marked. We discuss the results of the present study with respect to data available for this species in north-west Africa where possible and mainly from the European Pied Flycatcher F. hypoleuca.
Arrival dates of the study population (from 16 April) were almost similar to the values reported in Morocco (present on Jbel Bou-Iblane, altitude 1500–1600 m, in Eastern Middle Atlas from 25 April; Brosset Citation1961), but are much earlier than those reported in northwestern Tunisia (altitude about 500–1000 m, 16–22 May; Lundberg & Alatalo Citation1992). Lundberg & Alatalo (Citation1992) reported that no bird had arrived between 19 and 24 April 1985 near Ifrane 33°N, 5°W in Western Middle Atlas, at about 1500 m altitude. However, these early values are based on studies with smaller sample sizes. Arrival dates of the Atlas Pied Flycatcher were similar to that reported from a Spanish population of Pied Flycatcher (Potti Citation1998a), but later than that reported from several populations in western Europe between 50°N and 55°N (Both Citation2010). Male Atlas Pied Flycatchers arrived on the breeding ground approximately one week before females, a common pattern in several species (Francis & Cooke Citation1986). Flycatcher males arrive earlier to establish and defend a breeding territory (Potti Citation1998a).
The population density recorded in the study area (487 pairs/km2) was clearly higher than the values obtained from woodland (9.5 pairs/km2) and humid forest (38 pairs/km2) in Morocco (Thévenot Citation1982). This high density is within the range of that observed in European Pied Flycatcher populations in an area provided with nest-boxes (Lundberg & Alatalo Citation1992). High occupancy rate in our population indicates that number of nesting cavities was an important limiting factor for the Atlas Pied Flycatcher. In our study area, the nesting cavities were occupied first of all by other hole-nesting species mainly blue tits (which have a high density and mean LD about two weeks earlier than reported here for the Atlas Pied flycatchers; Chabi & Isenmann Citation1997). Atlas Pied Flycatchers arrived at the breeding area mostly in late April and occupied empty boxes (recently installed) which yielded a high occupancy rate. Another reason why high breeding densities can be attained is that the Atlas Pied Flycatcher, like the European Pied Flycatcher, defends a rather small territory and as a result population density is restricted by the number of available nest sites rather than by territorial behaviour (Von Haartman Citation1956). An annual increase in breeding density has occurred as a result of the installation of nest-boxes between 2010 and 2012.
The beginning of the Atlas Pied Flycatcher breeding season was less synchronized compared to central and northern European populations of Pied Flycatcher (Järvinen & Lindén Citation1980, Skwarska et al. Citation2012). Our study area is evergreen forest, which is more characterized with a broad but low peak in caterpillar abundance (Ziane et al. Citation2006). This may make it less important to highly synchronize, and may also explain differences with European populations that mostly breed in deciduous forests characterized by a narrow and high peak in caterpillar abundance in early spring (Visser et al. Citation2006). The onset of laying in our population in early May was similar compared to data reported by Heim de Balsac & Mayaud (Citation1962) where eggs were present first week of May and Thévenot et al. (Citation2003) in Morocco where eggs were present from early May to late June. The timing of laying in the study population is similar to the values reported from Spanish populations of Pied Flycatcher (Sanz et al. Citation2003). Mean LD of the Atlas Pied Flycatchers (19 May) is later by about two weeks compared to western European populations of Pied Flycatcher which have a mean LD around 7 May (Both et al. Citation2004, Both Citation2010). Timing of laying is mostly related to arrival from wintering areas and to spring temperatures. This trend was found in the Western European part of the Pied Flycatcher geographic area (Both et al. Citation2004, Both et al. Citation2005). In southern areas, European Pied Flycatchers and other bird species showed an earlier LD because of the earlier onset of spring and production of food that occurs in these latitudes (Järvinen Citation1989). Isenmann et al. (Citation1990) suggested that early laying Blue Tits in southern Spain might behave this way because they have to finish their breeding cycle by the time when high temperatures occur there in early May. Our data showed that the timing of breeding in our study area did not differ from year to year, and this stability may be explained by stable weather conditions, during the pre-laying period, that prevailed in those years. Similarly, no variation in arrival dates was observed. Atlas Pied Flycatchers probably produced only one brood per year and this is similar to the European Pied Flycatcher (Lundberg & Alatalo Citation1992).
The average CS in the present study (4.92 ± 0.77 eggs) is similar to that obtained in North Africa (5.15 eggs, n = 20, Heim de Balsac & Mayaud Citation1962; 5.0 eggs, n = 6, Etchecopar & Hüe Citation1964), but slightly lower than the mean CS of 5.83 eggs (n = 6) reported in Tizi Ouzou (36°35′N/4°10′E) in Algeria by Isenmann & Moali (Citation1987). This difference might be explained by differences in habitat quality and food abundance between the two areas (Q. suber forests versus Cedrus forests). The mean CS in our study population was lower than for Pied Flycatcher in different European areas which can reach an average of 6.92 eggs (Sanz Citation1997). A five egg clutch appears to be typical in Atlas Pied Flycatcher in this latitude. Our population live in evergreen forests where there are fewer food resources available during the breeding period than in the deciduous forests (Chabi & Isenmann Citation1997, Ziane et al. Citation2006) possibly leading to a reduction in CS. Sanz (Citation1997) reported that the average CSs of European Pied Flycatcher in the Palearctic, including North Africa (before separation of the two species), displayed a geographic pattern characterized by increasing CS with increasing latitude, which supports Lack's (Citation1947) generalization derived from many earlier observations on different avian species. Different hypotheses have been presented to explain this increase of CS with latitude in passerines. The most widely accepted hypotheses to explain smaller CS of Atlas Pied Flycatcher in southern areas has been day-length and food diversity together, as proposed by Von Haartman (Citation1973) and Owen (Citation1979). Shorter spring days in northern Algeria (the reduction is two to three hours per day) compared to Central Europe probably reduce habitat quality (Lack Citation1966). The southern and peripheral position of northern Africa within the breeding range probably leads to a lower variation in the seasonality of food resources and poorer conditions for reproduction (Järvinen Citation1986). In our population, the decline in the number of eggs through the season (−0.044 eggs/day) was lower than that observed in European Pied Flycatchers breeding in deciduous forest: e.g. −0.13 eggs/day (varies between −0.07 and −0.19) in the Netherlands (Both & Visser Citation2005), and about −0.08 eggs/day in Finland (Järvinen & Lindén Citation1980). Again, a likely reason is the seasonality of the habitat: evergreen versus deciduous forest. Decline of CS with LD might reflect a mechanism of proximate control of CS that is linked with the absolute date (the ‘calendar effect’; Von Haartman Citation1967). Moreover, seasonal decline in CS is usually related to a difference in the quality of individuals breeding earlier versus later in the season, or to a degradation of environmental conditions, with poorer breeding conditions later in the season (Crick et al. Citation1993).
Females alone incubated the eggs and males fed their mates during incubation. This has also been reported for European Pied Flycatchers (Lundberg & Alatalo Citation1992, Sanz Citation1996). The mean incubation period recorded in this study (13.2 days) is less than that reported for European Pied Flycatchers breeding in temperate areas (from 13.9 to 14.7 in seven sites; Järvinen Citation1990). No comparative data are available on duration of incubation in the Atlas Pied Flycatcher. Sanz (Citation1996) concluded that female European Pied Flycatchers supplied with food at the nest-box during incubation had significantly shorter incubation periods. In our study area, we observed males feeding their mate in the majority of nests observed. This may lead to a reduction in the incubation period. We also observed seasonal declines in incubation periods according to Slagsvold (Citation1986). The reason why early clutches take longer to hatch could be that they are larger than late clutches (Moreno & Carlson Citation1989). However, we did not find any correlation between length of incubation and CS. It is also possible that this resulted from more efficient incubation with increased temperature with the season. Another reason is that the early clutches were being delayed (i.e. adults not incubating initially) to match a late season food peak as in great tits (Cresswell & McCleery Citation2003).
Hatching success in our study was high with 88% of 497 eggs hatching. Analogous values from European Pied Flycatcher for different areas of Europe were slightly higher (Lundberg & Alatalo Citation1992). Common causes of hatching failure are inbreeding depression (Van Noordwijk et al. Citation1981), bad weather during the incubation stage (Järvinen & Väisänen Citation1984) or poor condition of incubating parents, usually females, leading to abandonment of nests (Wiggins et al. Citation1994). Cases of nest desertion in our study area that occurred in 2012 were caused by an intense human disturbance due to forest management after a snowy winter. Similar to Spanish population of Pied Flycatcher (Potti & Merino Citation1996), hatchability in Atlas Pied Flycatcher was unrelated to LDs. On the other hand, Potti & Merino (Citation1996) reported that hatchability decreased in small eggs and with increasing CS which was not found in our study.
Asynchronous hatching observed in our population (33%) is less than that reported for European Pied Flycatchers breeding in deciduous forest in central Spain (between 36% and 89%; Potti Citation1998b) and for Blue Tits in Pedunculate Oak in England (more than 70%; Stenning Citation2008). The proportion of asynchronous hatching can vary by habitat quality and weather (Slagsvold Citation1986), and with the quality of the female (Potti Citation1998b). Slagsvold & Lifjeld (Citation1989) suggested that hatching asynchrony was greater in deciduous (better habitat) than in coniferous (poor habitat) woodland. Stenning (Citation2008) reported that Blue Tits start incubation before laying the last egg in order to avoid missing the short-term seasonal abundance of caterpillars in deciduous forests, leading to a high proportion of asynchronous broods. Nestling mortality increased in our asynchronous broods, a result similar to the one reported in the European Pied Flycatcher (Slagsvold Citation1986). Here mortality was caused by smaller chicks' inability to swallow large food objects brought by the parents for senior nestlings prevailing in the brood (Slagsvold & Wiebe Citation2007).
Fledging success found in our study area (83.5%) was relatively high and within the range reported for the European Pied Flycatcher from many areas in Europe (60–99.8%, Lundberg & Alatalo Citation1992; see Sanz Citation1997). The number of fledglings in European Pied Flycatcher is affected by habitat, with lower values in coniferous than in deciduous forests (Sanz Citation1995) and/or with bad weather during the breeding season (Lundberg & Alatalo Citation1992). Low fledgling success observed at the end of the season in our study resulted from natural mortality. In the absence of high predation, food quality and availability, degradation of environmental conditions and parental quality (older females versus yearlings) may affect nestling survival. Another reason for the increase in chick mortality rate is the increase in asynchronous hatching occurring later in the season which is predicted to make brood reduction more efficient (Stenning Citation2008). The dry weather of Mediterranean habitats has been suggested to cause poor conditions for raising young at the end of the season (Belda Citation1991). This was also suggested by Blondel et al. (Citation1987) to explain the end of the breeding season in Corsica. Low fledging success observed in 2010, when chick mortality rate was high, was caused by strong winds that occurred during one night during the nesting period, and also possibly because the laying period was prolonged compared to later breeding seasons (49 days in 2010 compared to 35 in 2012). Fledging success was positively related to CS, a result which has not been reported very often (Williams Citation1994). A possible explanation may be that Atlas Pied Flycatcher pairs of high quality, possibly older birds, arrived early and were good at selecting the best territories and produced larger clutches and effectively more fledglings. Such effects have been described in European Pied Flycatcher and some avian species (Askenmo Citation1982, Goodburn Citation1991).
In conclusion, the results of the present study suggest that lower CS for the Atlas Pied Flycatcher in Q. suber forest was compensated for by relatively high fledging success, thereby ensuring overall reproductive success of this species. The smaller CS observed in Atlas Pied Flycatchers can be explained by adaptation to the lower food density available in evergreen forests. However, lower seasonality in the evergreen habitat (a broad but low peak in caterpillar abundance) explains the longer laying period, the small seasonal decline in CS and the low proportion of broods with asynchronous hatching.
Our results suggest that Atlas Pied Flycatchers breeding in evergreen habitats may benefit from the lower seasonality in their food, so making LD effects less strong and so they may have less need to adjust timing of breeding to be in synchrony with the caterpillar availability, contrary to European Pied Flycatchers in deciduous habitats (Burger et al. Citation2012). Nevertheless, our results warrant further research mainly on diet composition, parental care and synchronization between timing of breeding and food abundance to fully understand the breeding ecology and phenology of the Atlas Pied Flycatcher.
ACKNOWLEDGEMENTS
We are grateful to Ahcen Berjam and the authorities of the National Park of El-Kala for accommodation. We thank Pierre-Alain Ravussin and Michel Thévenot for supplying some items of literature. I am greatly indebted to El Aichar Mehdi for his assistance in the field. We thank Philippe Christe for his critical comments and remarks on the manuscript. Also special thanks to Will Cresswell and two anonymous reviewers for their comments on an earlier version of the manuscript.
REFERENCES
- Askenmo, C. 1982. Clutch size flexibility in the Pied Flycatcher (Ficedula hypoleuca). Ardea 70: 189–196.
- Bańbura, J. & Zieliński, P. 1998. An analysis of egg-size repeatability in Barn Swallows (Hirundo rustica). Ardeola 45: 183–192.
- Belda, E.J. 1991. Estudio de las causas que inciden sobre el éxito reproductor del verdecillo (Serinus serinus) al final de la estación reproductora. PhD Thesis, University of Valencia.
- Benyacoub, S. & Chabi, Y. 2000. Diagnose écologique de l'avifaune du Parc National d'El-Kala. Synthese Publication, Université d'Annaba Algérie, Annaba (in French).
- Blondel, J., Clamens, A., Cramm, P., Gaubert, H. & Isenmann, P. 1987. Population studies on tits in the Mediterranean region. Ardea 75: 21–34.
- Both, C. 2010. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Curr. Biol. 20: 243–248. doi: 10.1016/j.cub.2009.11.074
- Both, C. & Visser, M.E. 2005. The effect of climate change on the correlation between avian life-history traits. Global Change Biol. 11: 1606–1613. doi: 10.1111/j.1365-2486.2005.01038.x
- Both, C., Artemyev, A.V., Blaauw, B., Cowie, R.J., Dekhuijzen, A.J., Eeva, T., Enemar, A., Gustafsson, L., Ivankina, E.V., Jarvinen, A., Metcalfe, N.B., Nyholm, N.E.I., Potti, J., Ravussin, P.A., Sanz, J.J., Silverin, B., Slater, F.M., Sokolov, L.V., Torok, J., Winkel, W., Wright, J., Zang, H. & Visser, M.E. 2004. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. Lond. B 271: 1657–1662. doi: 10.1098/rspb.2004.2770
- Both, C., Bijlsma, R.G. & Visser, M.E. 2005. Climatic effects on timing of spring migration and breeding in a long-distance migrant, the Pied Flycatcher (Ficedula hypoleuca). J. Avian Biol. 36: 368–373. doi: 10.1111/j.0908-8857.2005.03484.x
- Brosset, A. 1961. Ecologie des oiseaux du Maroc oriental, Numéro 22 de Travaux: Série zoologie, Institut scientific chérifien, Rabat.
- Burger, C., Belskii, E., Eeva, T., Laaksonen, T., Mägi, M., Mänd, R., Qvarnström, A., Slagsvold, T., Veen, T., Visser, M.E., Wiebe, K.L., Wiley, C., Wright, J. & Both, C. 2012. Climate change, breeding date and nestling diet: how temperature differentially affects seasonal changes in pied flycatcher diet depending on habitat variation. J. Anim. Ecol. 81: 926–936. doi: 10.1111/j.1365-2656.2012.01968.x
- Chabi, Y. & Isenmann, P. 1997. La reproduction de la Mésange bleue (Parus caeruleus ultramarinus) dans des subéraies (Quercus suber) á trois différentes altitudes en Algérie. Alauda 65: 13–18 (in French).
- Ciani, A.C., Palentini, L., Arahou, M., Martinoli, L., Capiluppi, C. & Mouna, M. 2005. Population decline of (Macaca sylvans) in the Middle Atlas of Morocco. Biol. Conserv. 121: 635–641. doi: 10.1016/j.biocon.2004.06.009
- Cresswell, W. & McCleery, R.H. 2003. How great tits maintain synchronization of their hatch date with food supply to long-term variability in temperature. J. Anim. Ecol. 72: 356–366. doi: 10.1046/j.1365-2656.2003.00701.x
- Crick, H.Q.P., Gibbons, D.W. & Magrath, R.D. 1993. Seasonal changes in clutch size in British birds. J. Anim. Ecol. 62: 263–273. doi: 10.2307/5357
- Etchecopar, R.D. & Hüe, F. 1964. Les Oiseaux du Nord de 1’Afrique. Boubée, Paris.
- Francis, C.M. & Cooke, E. 1986. Differential timing of spring migration in wood warblers. Auk 103: 548–556.
- Goodburn, S.F. 1991. Territory quality or bird quality? Factors determining breeding success in the magpie (Pica pica). Ibis 133: 85–90. doi: 10.1111/j.1474-919X.1991.tb04815.x
- Heim De Balsac, H. & Mayaud, N. 1962. Les Oiseaux du Nord-Ouest de l'Afrique. Le chevalier, Paris.
- Hoyt, D.F. 1979. Practical method of estimating volume and fresh weight of bird eggs. Auk 96: 73–77.
- Isenmann, P. & Moali, A. 1987. Clutch-size reduction in the Pied Flycatcher (Ficedula hypoleuca) in North-West Africa. Ornis Fennica 64: 119.
- Isenmann, P., Ales, E. & Moreno, O. 1990. The timing of breeding and clutch size of Blue Tist (Parus caeruleus) in an evergreen holm oak habitat in southern Spain. Rev. Ecol. (Terre et Vie) 45: 177–181.
- Järvinen, A. 1986. Clutch size of passerines in harsh environments. Oikos 46: 365–371. doi: 10.2307/3565836
- Järvinen, A. 1989. Clutch-size variation in the Pied Flycatcher (Ficedula hypoleuca). Ibis 131: 572–577. doi: 10.1111/j.1474-919X.1989.tb04792.x
- Järvinen, A. 1990. Incubation and nestling periods in hole-nesting passerines in Finnish Lapland. Ornis Fennica 67: 65–72.
- Järvinen, A. & Lindén, H. 1980. Timing of breeding and the clutch size in the Pied Flycatcher Ficedula hypoleuca in Finnish Lapland. Ornis Fennica 57: 112–116.
- Järvinen, A. & Väisänen, R.A. 1984. Reproduction of Pied Flycatchers (Ficedula hypoleuca) in good and bad breeding seasons in a northern marginal area. Auk 101: 439–450.
- Lack, D. 1947. Darwin's Finches. Cambridge University Press, Cambridge.
- Lack, D. 1966. Population Studies of Birds. Clarendon Press, Oxford.
- Lundberg, A. & Alatalo, R.V. 1992. The Pied Flycatcher. T. & A. D. Poyser, London.
- Moreno, J. & Carlson, A. 1989. Clutch size and the costs of incubation in the Pied Flycatcher (Ficedula hypoleuca). Ornis Scand. 20: 123–128. doi: 10.2307/3676879
- Owen, D.F. 1979. Latitudinal gradients in clutch size: an extension of David Lack's theory. In Stonehouse, B. & Perrins. C.M. (eds) Evolutionary Ecology, 171–179. MacMillan, London.
- Potti, J. 1998a. Arrival time from spring migration in male Pied Flycatchers (Ficedula hypoleuca): individual consistency and familial resemblance. Condor 100: 702–708. doi: 10.2307/1369752
- Potti, J. 1998b. Variation in the onset of incubation in the Pied Flycatcher (Ficedula hypoleuca): fitness consequences and constraints. J. Zool. 245: 335–344. doi: 10.1111/j.1469-7998.1998.tb00108.x
- Potti, J. & Merino, S. 1996. Causes of hatching failure in the Pied Flycatcher. Condor 98: 328–336. doi: 10.2307/1369151
- Sætre, G.P., Borge, T., Lindell, J., Moum, T., Primmer, C.R., Sheldon, B.C., Haavie, J., Johnsen, A. & Ellegren, H. 2001a. Speciation, introgressive hybridization and nonlinear rate of molecular evolution in flycatchers. Mol. Ecol. 10: 737–749. doi: 10.1046/j.1365-294x.2001.01208.x
- Sætre, G.P., Borge, T. & Moum, T. 2001b. A new bird species? The taxonomic status of ‘the Atlas Flycatcher’ assessed from DNA sequence analysis. Ibis 143: 494–497. doi: 10.1111/j.1474-919X.2001.tb04951.x
- Sanz, J.J. 1995. Environmental restrictions on reproduction in the Pied Flycatcher (Ficedula hypoleuca). Ardea 83: 421–430.
- Sanz, J.J. 1996. Effect of food availability on incubation period in the Pied Flycatcher (Ficedula hypoleuca). Auk 113: 249–253. doi: 10.2307/4088958
- Sanz, J.J. 1997. Geographic variation in breeding parameters of the Pied Flycatcher (Ficedula hypoleuca). Ibis 139: 107–114. doi: 10.1111/j.1474-919X.1997.tb04509.x
- Sanz, J.J., Potti, J., Moreno, J., Merino, S. & Frias, O. 2003. Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Global Change Biol. 9: 461–472. doi: 10.1046/j.1365-2486.2003.00575.x
- Skwarska, J., Kalinski, A., Wawrzyniak, J., Markowski, M., Mikus, W., Banbura, M., Gladalski, M., Zielinski, P. & Banbura, J. 2012. Long-term variation in laying date and clutch size of Pied Flycatchers Ficedula hypoleuca in central Poland. Pol. J. Ecol. 60: 187–192.
- Slagsvold, T. 1986. Asynchronous versus synchronous hatching in birds – experiments with the pied flycatcher. J. Anim. Ecol. 55: 1115–1134. doi: 10.2307/4437
- Slagsvold, T. & Lifjeld, J.T. 1989. Constraints on hatching asynchrony and egg size in Pied Flycatchers. J. Anim. Ecol. 58: 837–849. doi: 10.2307/5127
- Slagsvold, T. & Wiebe, K. 2007. Hatching asynchrony and early nestling mortality: the feeding constraint hypothesis. Anim. Behav. 73: 691–700. doi: 10.1016/j.anbehav.2006.05.021
- Snow, D. 1952. A contribution to the ornithology of North-West Africa. Ibis 94: 473–498. doi: 10.1111/j.1474-919X.1952.tb01846.x
- Sokal, R.R. & Rohlf, F.J. 1995. Biometry, 3rd edn. Freeman, New York.
- Statsoft INC. 2011. STATISTICA (Data Analysis Software System), Version 10. StatSoft, Inc., Tulsa, OK.
- Stenning, M.J. 2008. Hatching asynchrony and brood reduction in Blue Tits Cyanistes caeruleus may be a plastic response to local oak Quercus robur bud burst and caterpillar emergence. Acta Ornithol. 43: 97–106. doi: 10.3161/000164508X345383
- Thévenot, M. 1982. Contribution à l'étude écologique des Passereaux forestiers du Plateau Central et de la corniche du Moyen Atlas (Maroc). Oiseau Rev. Fr. Ornithol. 52: 21–86, 97–144.
- Thévenot, M., Vernon, J.D.R. & Bergier, P. 2003. The Birds of Morocco. BOU Checklist Series: 20. British Ornithologists' Union & British Ornithologists' Club, Tring.
- Van Noordwijk, A., Van Balen, J.H. & Scharloo, W. 1981. Genetic variation in the timing of reproduction in the Great tit. Oecologia 49: 158–166. doi: 10.1007/BF00349183
- Visser, M.E., Holleman, L.J.M. & Gienapp, P. 2006. Shifts in caterpillar bio-mass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147: 164–172. doi: 10.1007/s00442-005-0299-6
- Von Haartman, L. 1956. Territory in the pied flycatcher Muscicapa hypoleuca. Ibis 98: 460–475. doi: 10.1111/j.1474-919X.1956.tb01431.x
- Von Haartman, L. 1967. Clutch-size in the Pied Flycatcher. Proc. Int. Ornithol. Congr. 14: 155–164.
- Von Haartman, L. 1973. Talgmespopulationen på Lemsjöholm. Lintumies 8: 7–9.
- Wiggins, D.A., Pärt, T. & Gustafsson, L. 1994. Correlates of clutch desertion by female Collared Flycatchers (Ficedula albicollis). J. Avian Biol. 25: 93–97. doi: 10.2307/3677025
- Williams, T.D. 1994. Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol. Rev. 68: 35–59. doi: 10.1111/j.1469-185X.1994.tb01485.x
- Ziane, N., Chabi, Y. & Lambrechts, M.M. 2006. Breeding performance of Blue Tits Cyanistes caeruleus ultramarinus in relation to habitat richness of oak forest patches in north-eastern Algeria. Acta Ornithol. 41: 163–169. doi: 10.3161/068.041.0201
