Abstract
Capsule The onset of incubation relative to clutch completion is highly variable in Great Tits Parus major, and has important consequences for the duration of the incubation and hatching periods.
Aim To investigate when incubation starts relative to clutch completion, its effects on the length of the incubation and hatching periods, and which proximate factors affect all of these traits.
Methods We used data from a Great Tit population in Eastern Spain collected over 15 years. Periodic visits to the nests (daily at some stages) allowed the determination of breeding parameters of interest. General linear models were used for analyses.
Results On average, incubation started the day of laying of the last egg and lasted 13.2 days. The hatching period lasted 1.7 days. Incubation started earlier relative to clutch completion as temperatures during the laying period were higher and as clutch size increased. The incubation period was shorter if incubation started later relative to clutch completion. Hatching asynchrony increased as incubation started earlier relative to clutch completion and as the incubation period decreased.
Conclusions The onset of incubation relative to clutch completion is highly variable, and has important consequences for the duration of the incubation and hatching periods. Starting incubation before or after clutch completion could, respectively, advance or delay the hatching date, although advancement would be at the cost of increasing the degree of hatching asynchrony.
The incubation period is the time needed for the development of an embryo, assuming regular and constant attendance by the parents (Drent Citation1975). In passerines, incubation can start from several days before, to several days after clutch completion (Gibb Citation1950, Monrós et al. Citation1998, Ardia et al. Citation2006, Stenning Citation2008). However, the proximate causes of starting incubation sooner or later relative to clutch completion have been the subject of very few studies. From these, we know that incubation tends to start earlier relative to clutch completion if temperatures during the laying period are higher (Ardia et al. Citation2006, Vedder Citation2012; but see Wang & Beissinger Citation2009) and in larger clutches (Potti Citation1998, Wang & Beissinger Citation2009). Increasing temperatures as the season progresses would suggest that incubation should start earlier relative to clutch completion later in the season, but we are unaware of studies directly reporting this trend – although hatching patterns (see below) suggest that this is true.
The duration of the incubation period varies between individuals within populations. This within-population variability is most probably caused by environmental factors, because the duration of the incubation period has a low heritability (Husby et al. Citation2012). For example, the duration of the incubation period is shorter when temperatures during the incubation period are higher (Hepp et al. Citation2006, Ardia et al. Citation2010), and in part because of it, also late in the season (Wesołowski Citation2000, García-Navas & Sanz Citation2011). This likely because females are able to allocate more time to incubation in favourable environments, and the gradient between ambient and incubation temperature is reduced. It has also been shown that the incubation period increases with egg size (Parsons Citation1972, Martin & Arnold Citation1991), clutch size (Moreno & Carlson Citation1989, Hepp et al. Citation2005, Dobbs et al. Citation2006), and clutch mass (Hepp et al. Citation1990), because a larger number of and/or larger eggs will be more costly to incubate. However, recent studies regarding various species have found that shorter incubation periods occur in larger clutches (Wesołowski Citation2000, Ardia et al. Citation2006, García-Navas & Sanz Citation2011). Explanations put forward include that good-quality females could both produce larger clutches and provide higher incubation attendance, and that larger clutches cool more slowly during female absences and therefore are incubated at higher average temperatures (see also Reid et al. Citation2002). On the other hand, the brood patch is not completely developed and incubation behaviour not fully established until sometime after clutch completion (Bailey Citation1952, Jones Citation1971, Sockman et al. Citation2006), so birds starting incubation before clutch completion might then be expected to have longer incubation periods than those starting later (but see Ardia et al. Citation2006, García-Navas & Sanz Citation2011).
The most important consequence of starting incubation before clutch completion is that eggs will hatch asynchronously (i.e. hatching occurs over a period of 24 hours or more; Haftorn Citation1981a, Ardia et al. Citation2006). Thus, the same factors potentially affecting the onset of incubation relative to clutch completion (temperatures during the laying period, date, clutch size, and egg size) have been found to be related with the degree of hatching asynchrony (Slagsvold Citation1986, Potti Citation1998, Ardia et al. Citation2006, Stenning Citation2008, Theofanellis et al. Citation2008, Podlas & Richner Citation2013), the most frequent trends being an increase of hatching asynchrony with clutch size and as the season progresses.
In birds, embryonic development typically starts when eggs are actively incubated, and this usually means that eggs are constantly warm (Drent Citation1975, Haftorn Citation1983, Wiebe et al. Citation1998). In Great Tits Parus major, only females incubate, and they usually cover the eggs with nest material during the day until incubation starts (Haftorn & Slagsvold Citation1995). Therefore, the onset of incubation could be assumed to occur when eggs are seen uncovered and warm during the day, or when females are seen incubating during the day (Gibb Citation1950, Stenning Citation2008, García-Navas & Sanz Citation2011). Great Tit females roost in the nest during the night during the laying period (Pendlebury & Bryant Citation2005) until the onset of incubation, suggesting that early eggs may gain more incubation than later eggs (Lord et al. Citation2011). Furthermore, we found that, on many occasions, eggs were completely uncovered yet cold at the end of the laying period (Barba Citation1991), suggesting also that eggs may receive some incubation before the start of full incubation. Both of these processes may allow Great Tits to shorten incubation but at the expense of increasing hatch asynchrony (Cresswell & McCleery Citation2003).
The Great Tit is a widespread species in Europe. Its use of nest boxes has encouraged many research groups to use it as a model species for a wide range of ecological and evolutionary studies, some of them carried out over several decades (Lambrechts et al. Citation2010). One of these studies has been carried out in an extensive orange plantation in Sagunto, Eastern Spain, where the breeding performance of Great Tits has been followed since 1986 (Encabo et al. Citation2001, Tomás et al. Citation2012). For most of these years, data on the onset of incubation and on the duration of the incubation and hatching periods of a sample of nests are available.
Our main objective was to describe within-population variation in the onset of incubation relative to clutch completion, the factors affecting this, and its effects on the duration of incubation and hatching periods in the Great Tit population of Sagunto. To our knowledge, this is the first study dealing with the incubation and hatching periods, and factors affecting them, in a southern European Great Tit population. Based on the studies cited above, we predicted that: (1) the onset of incubation will be earlier relative to clutch completion, (2) the incubation period will be shorter, and (3) the hatching period longer if ambient temperatures are higher, as the date of laying of the first egg (or onset of incubation for incubation period) is later, and as clutch size (or clutch volume for incubation period) increases. We also tested for the effects of uncovering the eggs before the onset of full incubation, because this may indicate partial incubation (sensu Wang & Beissinger Citation2011). If this is the case we predict that the ‘full’ incubation period will be shorter, and hatching period longer.
METHODS
Study area and data selection
The study was performed on a Great Tit population breeding in nest boxes in an extensive orange plantation in Sagunto, Eastern Spain (39°42′N, 0°15′W, 30 m a.s.l.). Basic breeding parameters have been recorded each year since 1986 (Encabo et al. Citation2001, Tomás et al. Citation2012) during periodic visits to the nests. These were the laying date of the first egg, clutch size, and egg volume. Nest boxes were visited at least once per week, and laying of the first egg was estimated assuming that one egg was laid per day. Laying gaps (days during the laying period where no egg was laid) occurred only very occasionally under unfavourable environmental conditions (Monrós et al. Citation1998), and we considered that the bias they could introduce was negligible. Once a nest was found with eggs, it was visited daily from the day when the fifth egg was expected to be laid until clutch completion. Laying was assumed to have finished, and the clutch to be complete, when no more eggs where laid during two consecutive days and the female started full incubation. The length (L) and width (W) of the eggs of a sample of nests each year were measured with a calliper (±0.01 mm) when the clutch was complete. The volume (V) of each egg was calculated as V = (0.4673 × L × W2) + 0.042 (Ojanen et al. Citation1978, Encabo et al. Citation2001). Clutch volume (sum of the volumes of all the eggs in a clutch) was computed for each clutch.
For the present study, we included only first clutches laid between 1988 and 2010. As some of the parents were not individually identified (none in early years), we considered first clutches as those where the first egg was laid within 30 days from the laying of the first egg of that year (Nager & van Noordwijk Citation1995, Møller et al. Citation2014). Most pairs (70%, n = 1674 pairs) only attempted one clutch per year, but some (19%) produced a second clutch if the first clutch failed, and others (11%) attempted a second clutch after a successful first clutch (own unpubl. data, period 1988–2010). From this initial sample size we excluded nests used in experiments not part of this study which could affect the parameters under study here, and nests where the laying date and clutch size could not be determined. From these, the exact determination of the parameters of interest (onset of incubation relative to clutch completion and duration of the incubation and hatching periods) could be achieved for a variable number of nests each year (see criteria below). After application of these restrictions, we completely excluded years with less than ten valid nests for all of the studied parameters (1989, 1997, 1998, 2002–5, and 2008). From the remaining 15 years, with a total of 1098 first clutches initiated in the study area, subsamples for analyses concerning the onset of incubation included 609 nests from 15 years, those concerning the incubation period included 356 nests from 12 years, and those concerning the hatching period, 429 nests from 15 years. In all cases, selected nests are thought to be a random sample of the population.
Ambient temperature data (daily maximum and minimum) of a nearby (ca. 4 km) meteorological station were obtained from the Spanish Meteorological Agency. Daily mean temperatures were computed as the average of maximum and minimum of each day. For each nest, we computed the mean of the mean daily temperatures for the laying period (from the day of laying of the first egg to the day prior to incubation start), and for the incubation period (see below).
The incubation period
We defined the incubation period as the number of days elapsing from the day when incubation was first observed and the day prior to the hatching of the first egg. We only included nests where the incubation period was determined precisely by daily visits to the nests from at least one day before incubation started and from at least one day before hatching started. For each nest the onset of incubation relative to clutch completion was calculated by subtracting the date of start of incubation from the date of clutch completion, being ‘0’ if incubation started on the day of laying of the last egg, ‘−1’ if it started with the penultimate egg, ‘+1’ if it started the day after clutch completion, and so on (Loos & Rohwer Citation2004, Ardia et al. Citation2006).
In our daily visits, we noted whether the eggs were covered or uncovered by nest material prior to the onset of incubation. We classified nests into two groups, those in which eggs were uncovered the day of onset of full incubation (i.e. eggs were warm the first day they were uncovered), and those where eggs were completely uncovered, but cold, before starting full incubation.
The hatching period
In a sample of nests each year, nests were visited daily from incubation day 12 until all the eggs had hatched, or the remaining eggs did not hatch in two consecutive days. We used these nests to estimate the hatching period, defined as the time in days from hatching of the first egg to hatching of the last successful egg (i.e. excluding those that did not hatch) inclusive (Potti Citation1998). Therefore, the hatching period was one day if all the eggs hatched on the same day. Great Tits are unable to remove unhatched eggs from the nest, but they do remove nestlings which die in their first days of life. Because we made daily visits, we assumed that all disappeared eggs had hatched, and that missing nestlings had died and were taken out of the nest by the parents.
Statistical analyses
We used general linear models (GLMs) to explore the relationships between the target variables (onset of incubation relative to clutch completion, and duration of the incubation and hatching periods) and the factors predicted to affect them. The year and whether the eggs were uncovered or not before the onset of incubation were included as factors in all the models. The rest of the independent variables were included as covariates in each model as appropriate. In the model of the hatching period, the onset of incubation relative to clutch completion and its square value were included to account for a potential nonlinear relationship – for example, starting incubation before clutch completion may affect hatching asynchrony but starting incubation after clutch completion may have little or no effect. As this variable had negative and positive values, the lower value (−3) was considered to be ‘1’, and the rest of the values were arranged consequently (i.e. adding four units to each one) for this analysis, although we preserved the original nomenclature in the text and figures.
Means ± sd are presented when appropriate. The sample sizes used to estimate mean onset of incubation or the duration of the incubation and hatching periods are smaller when performing GLMs because not all nests had complete sets of measurements. Statistical analyses were carried out using SPSS v. 19.0.
RESULTS
Onset of incubation
The onset of incubation relative to clutch completion varied between years (). Yearly means were positive (i.e. incubation starting after clutch completion) for 8 out of the 15 years, negative for 6 years, and 0.00 in 1988. Incubation started from 3 days before to 6 days after clutch completion (). Overall, females started incubation on the day of laying of the last egg (mean = 0.06 ± 0.30, n = 15 years, including 609 nests).
Figure 1. Frequency distribution of the onset of incubation relative to clutch completion. ‘0’ is the date of laying of the last egg, negative values indicate that incubation started before clutch completion, and positive values indicate that incubation started after clutch completion. Sample sizes (number of nests) are indicated above the bars.
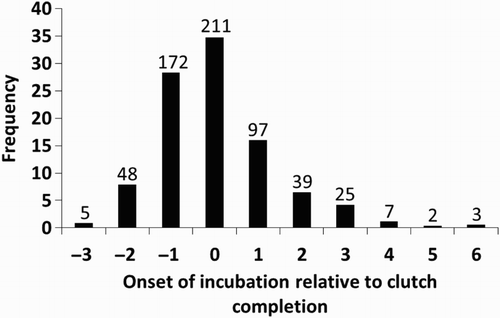
Table 1. Results of a GLM of the influence of year, uncovering of the eggs before incubation (UNC), mean temperature during the laying period (TLAY), laying date, and clutch size on the onset of incubation relative to clutch completion. The biological significance is shown in and given in the text for uncovering of the eggs before incubation.
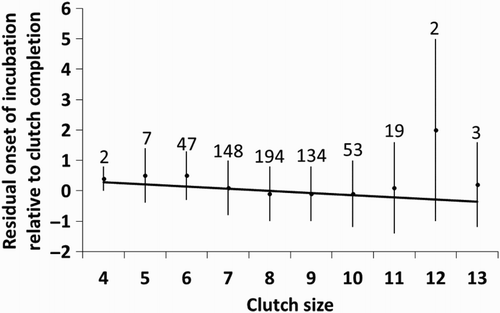
The onset of incubation was later relative to clutch completion if the eggs were uncovered before the onset of incubation (0.7 ± 1.4, n = 134 vs. −0.2 ± 1.4, n = 475; ). Of the 134 nests where eggs appeared uncovered and cold before the onset of incubation, eggs appeared uncovered on the day of clutch completion in 28 nests, eggs appeared uncovered before clutch completion in 87 nests (mean = −1.7 ± 1.7 days), and eggs appeared uncovered after clutch completion in 19 nests (mean = 2.1 ± 1.8 days). Nests where eggs appeared uncovered on the date of clutch completion started incubation 1.4 ± 0.8 days after clutch completion, those where eggs were uncovered before clutch completion started incubation, on average, on the day of clutch completion (mean = −0.1 ± 1.1 days), while those where eggs appeared uncovered after clutch completion started incubation 3.1 ± 1.6 days after clutch completion. Also, incubation started earlier relative to clutch completion as temperatures during the laying period were higher, and as clutch size was larger (; ).
Figure 2. Relationship between the onset of incubation relative to clutch completion (expressed as residuals after controlling for year, uncovering of the eggs before incubation and clutch size; see ) and mean temperature during the laying period of each clutch.
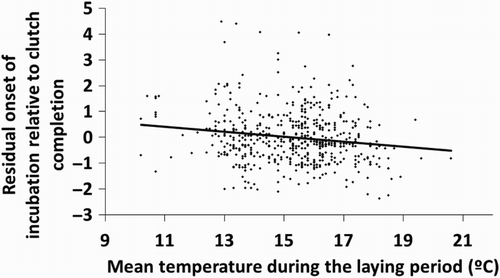
Figure 3. Relationship between the onset of incubation relative to clutch completion (expressed as residuals after controlling for year, uncovering of the eggs before incubation and temperature during laying; see ) and clutch size. The mean ± sd residual of onset of incubation is shown for each clutch size (sample size above bars), but the regression line was fitted using the individual data points.
Incubation period
The mean incubation period was 13.2 ± 0.4 days (n = 12 years, including 356 nests), though it varied between years (), ranging from 12.6 (years 1992 and 2006) to 13.8 days (1996). Among the factors considered to potentially affect the incubation period, we only found that incubation period was shorter as incubation started later relative to clutch completion (; ).
Figure 4. Relationship between the incubation period and the onset of incubation relative to clutch completion. The mean ± sd residual of the incubation period is shown for each day of onset of incubation relative to clutch completion (sample size above bars), but the regression line was fitted using the individual data points.
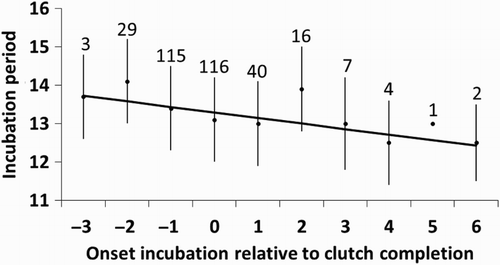
Table 2. The results of a GLM of the influence of year, uncovering of the eggs before incubation (UNC), mean temperature during the incubation period (TINC), date of the start of incubation (ID), clutch volume, and the onset of incubation relative to clutch completion (OINC) on the duration of the incubation period as illustrated by a GLM. The biological significance is shown in .
Hatching period
The hatching period also varied between years (), and ranged between 1.0 and 2.9 days (mean 1.7 ± 0.6 days, n = 15 years, including 429 nests). The hatching period was longer as incubation started earlier relative to clutch completion and as the incubation period was shorter (; & ). Starting incubation 5–6 days after clutch completion was rare (0.82% of the clutches; see ), and we only have one nest with a complete data set that was included in this analysis. We repeated the analysis removing this single point, and in this case both the onset of incubation relative to clutch completion and its squared term (F 1,148 = 8.9, P = 0.003) were significant (). Thus, starting incubation before clutch completion had an effect on hatching asynchrony while starting on, or after clutch completion, had virtually none.
Figure 5. Duration of the hatching period (expressed as residuals after controlling for year and incubation period; see ) and onset of incubation relative to clutch completion. The mean ± sd residual of hatching period is shown for each day of onset of incubation relative to clutch completion (sample size above bars), but the regression line was fitted using the individual data points. The regression line has been adjusted removing the point corresponding to a nest where incubation started six days after clutch completion (see text).
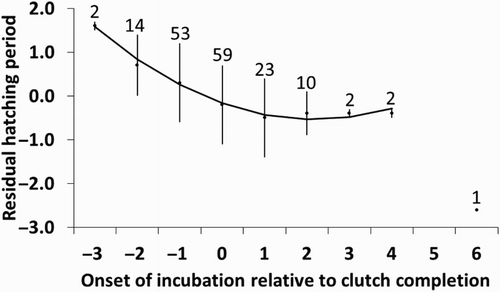
Figure 6. Relationship between the duration of the hatching period (expressed as residuals after controlling for year and onset of incubation relative to clutch completion; see ) and the duration of the incubation period. The mean ± sd residual of hatching period is shown for each incubation period (sample size above bars), but the regression line was fitted using the individual data points.
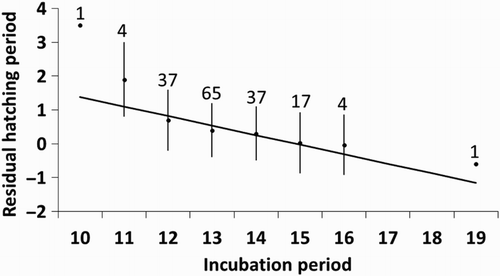
Table 3. The results of a GLM of the influence of year, uncovering of the eggs before incubation (UNC), mean temperature during the laying period (TLAY), clutch size, date of the start of incubation (ID), incubation period, and the onset of incubation relative to clutch completion (OINC) and its square (OINCSQ) on the duration of the hatching period. The biological significance is shown in & .
DISCUSSION
This study emphasizes the crucial role of the time of starting incubation relative to clutch completion on the duration of the incubation period and the degree of hatching asynchrony. The fact that passerine birds can start incubation some days before or after clutch completion has been known for decades (Gibb Citation1950, Zink Citation1959, Stenning Citation2008), and we confirmed it here in our Great Tit population. However, most studies have concentrated on the incubation before clutch completion, because this usually leads to hatching asynchrony, a phenomenon which has attracted much attention (Clark & Wilson Citation1981, Magrath Citation1990, Stenning Citation1996). The fact that many species could start incubation after clutch completion has been so overlooked that it was claimed to have been ‘discovered’ quite recently (Rowe & Weatherhead Citation2009: ‘we are unaware of any studies of either altricial or precocial birds that have reported a delay of incubation onset of >24 h beyond clutch completion’). But several preceding and recent studies have shown that starting incubation after clutch completion is not only common, but it also contributes to the adjustment of the hatching dates to prevalent environmental conditions, thus enhancing breeding success (Monrós et al. Citation1998, Cresswell & McCleery Citation2003, Stenning Citation2008, García-Navas & Sanz Citation2011).
Concerning proximate causes, we found that Great Tits started incubation earlier in the laying sequence when temperatures during the laying period were higher. Slagsvold (Citation1986) suggested that higher temperatures around laying allow females to be in a better condition, so that they can start incubation before clutch completion. Potti (Citation1998) also suggested that female condition has a causal role in the onset of incubation. Nilsson (Citation1993) showed that female Marsh Tits Poecile palustris receiving extra food during laying started incubation earlier relative to clutch completion than control pairs, showing an energetic limitation to the start of incubation. Alternatively, starting incubation early when temperatures are higher could be viewed as a strategic decision to synchronize hatching dates with food availability (García-Navas & Sanz Citation2011). The negative relationship between temperature and onset of incubation also gives support for the egg viability hypothesis (Arnold et al. Citation1987, Amundsen & Stokland Citation1988), which states that the viability of unincubated eggs could decline with time, especially if temperatures are high, so incubation should start during the laying period to avoid egg losses.
Another proximate factor affecting the onset of incubation relative to clutch completion was clutch size: females laying larger clutches tended to start incubation earlier relative to clutch completion. This trend has also been found in several other species, and has been interpreted as either the willingness of females to accelerate hatching if the laying period is prolonged, since recruitment probabilities of fledglings decline with time, or as a correlation between female condition and clutch size, with females which are in better condition laying larger clutches and being able to start laying earlier relative to clutch completion (Magrath Citation1992, Potti Citation1998, Wang & Beissinger Citation2009, García-Navas & Sanz Citation2011).
For Great Tits, calculation of the incubation period in other European populations has frequently included the hatching period, and its values range between 13.5 and 16.3 days (Gibb Citation1950, Kluijver Citation1950, Winkel Citation1970, Citation1975, Haftorn Citation1981b). The incubation and hatching periods defined here do not overlap, so their sum (13.2 + 1.7 = 14.9 days) is comparable to the incubation periods in these previous studies. Many studies have shown that incubation periods are generally longer in species breeding at lower latitudes (Ricklefs Citation1969, Martin et al. Citation2007, Robinson et al. Citation2008). With the caution that available estimates were calculated slightly differently, and using different criteria, the Sagunto population has a longer incubation period than all northern populations, excluding that from Norway (16.3 days, Haftorn Citation1981b).
Given the predictions derived from the previous studies, it was surprising that the incubation period was only related to the day of the start of incubation relative to clutch completion. The negative relationship supports our hypothesis that incubation is less effective during the laying period, though there is also the possibility that nests which start incubation later relative to clutch completion receive more partial incubation. This contrasts with previous studies, where clutches where incubation started before clutch completion had shorter incubation periods compared to clutches where incubation started on or after laying of the last egg (Viñuela Citation1997, Ardia et al. Citation2006, García-Navas & Sanz Citation2011). Prolactin is one of the hormones triggering the formation of the brood patch and maintaining incubation behaviour (Lea & Klandorf Citation2002, Angelier & Chastel Citation2009). However, this hormone also causes a regression of the gonads (Dawson et al. Citation2001), probably creating conflicts between producing new eggs and incubation behaviour (Haftorn Citation1981b, Mead & Morton Citation1985, Sockman et al. Citation2000, Citation2006). It is, therefore, possible that, if incubation starts during the laying period, it might be less effective than starting once the physical structures and ethological repertory is fully developed, which probably occurs after clutch completion (see Runde & Barrett Citation1981, St. Clair Citation1998). Most previous studies on the duration of the incubation period have not included the day of onset of incubation relative to clutch completion among the predictor variables. Here, we show that this factor might be crucially important in determining the incubation period.
The duration of the hatching period in the studied population (1.7 days) was in the lower extreme of the range found for other Great Tit populations (1.8–4 days) (Gibb Citation1950, Neub Citation1979, Haftorn Citation1981b, Orell Citation1983, Amundsen & Slagsvold Citation1998, Tilgar & Mänd Citation2006, Theofanellis et al. Citation2008, Matysioková & Remeš Citation2011, Podlas & Richner Citation2013). This was not unexpected, taking into account that an average female in Sagunto started incubation the day the final egg was laid.
Our data also show the expected negative relationship between the onset of incubation relative to clutch completion and the duration of the hatching period if incubation started before clutch completion, while it virtually had no effect if it started on or after laying the last egg. Therefore, starting incubation before clutch completion could control both hatching asynchrony and date of hatching, while delaying the onset of incubation beyond clutch completion would delay the hatching date, without apparent effects on hatching asynchrony. The (relatively small) variability in the degree of hatching asynchrony observed when incubation started on or after clutch completion, could have partly been caused by some eggs receiving partial incubation before clutch completion. We have shown above that temperatures during laying and clutch size affect the onset of incubation relative to clutch completion, so these factors also have an indirect effect on hatching asynchrony.
Contrary to expectations, we found that hatching asynchrony increased as the incubation period decreased. A potential explanation for this relationship is that partial incubation, occurring over several days (both prior and after clutch completion, before the onset of full incubation) initiated embryo development, resulting in both a shortening of the (full) incubation period and an increase in hatching asynchrony. Lord et al. (Citation2011) showed that differences in the amount of incubation received by individual Great Tit eggs prior to clutch completion significantly affected the time they needed to hatch after full incubation started. For example, Potti (Citation1998) noticed that many Pied Flycatcher Ficedula hypoleuca clutches where incubation started after the last egg was laid exhibited hatching asynchrony; he suggested that the amount of incubation received by individual eggs prior to clutch completion was the main reason of this unexpected asynchrony.
In conclusion, temperatures during laying and clutch size were related to the onset of incubation relative to clutch completion, which in turn has important consequences for the duration of the incubation and hatching periods. Therefore, starting incubation before or after clutch completion could, respectively, advance or delay the hatching date, though any advancement would be at the cost of increasing the degree of hatching asynchrony.
ACKNOWLEDGEMENTS
We would like to thank all of the people who helped in the field during the study years, and to Will Cresswell, Alex Lord, and an anonymous reviewer for useful comments on previous drafts. We also thank the Spanish Meteorological Agency for providing temperature data, and the Spanish Ministry of Agriculture, Food and Environment for providing nest boxes.
Funding
This long-term study of Great Tits was partially supported by: GV-2517/94 (Generalidad Valenciana), CGL2004-00787, CGL2007-61395 (Spanish Ministry of Education and Science), and CGL2010-21933-C02-02 (Spanish Ministry of Science and Innovation).
REFERENCES
- Amundsen, T. & Slagsvold, T. 1998. Hatching asynchrony in great tits: a bet-hedging strategy? Ecology 79: 295–304. doi: 10.1890/0012-9658(1998)079[0295:HAIGTA]2.0.CO;2
- Amundsen, T. & Stokland, J.N. 1988. Adaptive significance of asynchronous hatching in the shag: a test of the brood reduction hypothesis. J. Anim. Ecol. 57: 329–344. doi: 10.2307/4909
- Angelier, F. & Chastel, O. 2009. Stress, prolactin and parental investment in birds: a review. Gen. Comp. Endocrinol. 163: 142–148. doi: 10.1016/j.ygcen.2009.03.028
- Ardia, D.R., Cooper, C.B. & Dhondt, A.A. 2006. Warm temperatures lead to early onset of incubation, shorter incubation periods and greater hatching asynchrony in tree swallows Tachycineta bicolor at the extremes of their range. J. Avian Biol. 37: 137–142. doi: 10.1111/j.0908-8857.2006.03747.x
- Ardia, D.R., Pérez, J.H. & Clotfelter, E.D. 2010. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc. R. Soc. B 277: 1881–1888. doi: 10.1098/rspb.2009.2138
- Arnold, T.W., Rohwer, F.C. & Armstrong, T. 1987. Egg viability, nest predation, and the adaptive significance of clutch size in prairie ducks. Am. Nat. 130: 643–653. doi: 10.1086/284736
- Bailey, R.E. 1952. The incubation patch of passerine birds. Condor 54: 121–136. doi: 10.2307/1365062
- Barba, E. 1991. Ecología de reproducción del carbonero común Parus major en el naranjal valenciano. PhD Thesis, University of Valencia.
- Clark, A.B. & Wilson, D.S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction and nest failure. Quart. Rev. Biol. 56: 253–277. doi: 10.1086/412316
- Cresswell, W. & McCleery, R.H. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72: 356–366. doi: 10.1046/j.1365-2656.2003.00701.x
- Dawson, A., King, G.E., Bentley, G.E. & Ball, G.F. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 16: 365–380. doi: 10.1177/074873001129002079
- Dobbs, R.C., Styrsky, J.D. & Thompson, C.F. 2006. Clutch size and the costs of incubation in the house wren. Behav. Ecol. 17: 849–856. doi: 10.1093/beheco/arl019
- Drent, R. 1975. Incubation. In Farner, D.S. & King, J.R. (eds) Avian Biology, Vol. V: 333–420. Academic Press, New York.
- Encabo, S.I., Monrós, J.S. & Barba, E. 2001. Egg size variation in a Mediterranean Great Tit Parus major population. Ardeola 48: 63–70.
- García-Navas, V. & Sanz, J.J. 2011. Short-term alterations in songbird breeding schedule lead to better synchronization with food availability. Auk 128: 146–155. doi: 10.1525/auk.2010.10094
- Gibb, J.A. 1950. The breeding biology of the Great and Blue Titmice. Ibis 92: 507–539. doi: 10.1111/j.1474-919X.1950.tb01759.x
- Haftorn, S. 1981a. Incubation during the egg-laying period in relation to clutch-size and other aspects of reproduction in the Great Tit Parus major. Ornis Scand. 12: 169–185. doi: 10.2307/3676074
- Haftorn, S. 1981b. Incubation rhythm in the Great Tit Parus major. Fauna norv. Ser. C Cinclus 4: 9–26.
- Haftorn, S. 1983. Egg temperature during incubation in the Great Tit Parus major, in relation to ambient temperature, time of day, and other factors. Fauna norv Ser C Cinclus 6: 22–38.
- Haftorn, S. & Slagsvold, T. 1995. Egg covering in birds: description of the behaviour in tits (Parus spp.) and a test of hypotheses of its function. Fauna norv. Ser. C Cinclus 18: 85–106.
- Hepp, G.R., Kennamer, R.A. & Harvey VI, W.F. 1990. Incubation as reproductive cost in female Wood Ducks. Auk 107: 756–764. doi: 10.2307/4088008
- Hepp, G.R., Folk, T.H. & Manlove, C.A. 2005. Nest temperature, incubation period, and investment decisions of incubating wood duck Aix sponsa. J. Avian Biol. 36: 523–530. doi: 10.1111/j.0908-8857.2005.03462.x
- Hepp, G.R., Kennamer, R.A. & Johnson, M.H. 2006. Maternal effects in Wood Ducks: incubation temperature influences incubation period and neonate phenotype. Funct. Ecol. 20: 307–314. doi: 10.1111/j.1365-2435.2006.01108.x
- Husby, A., Gustafsson, L. & Qvarnström, A. 2012. Low genetic variance in the duration of the incubation period in a collared flycatcher (Ficedula albicollis) population. Am. Nat. 179: 132–136. doi: 10.1086/663193
- Jones, R.E. 1971. The incubation patch of birds. Biol. Rev. 46: 315–339. doi: 10.1111/j.1469-185X.1971.tb01048.x
- Kluijver, H.N. 1950. Daily routines of the Great Tit, Parus m. major L. Ardea 38: 99–135.
- Lambrechts, M.M., Adriaensen, F., Ardia, D.R., Artemyev, A.V., Atiénzar, F., Bańbura, J., Barba, E., Bouvier, J.-C., Camprodon, J., Cooper, C.B., Dawson, R.D., Eens, M., Eeva, T., Faivre, B., Garamszegi, L.Z., Goodenough, A.E., Gosler, A.G., Grégoire, A., Griffith, S.C., Gustafsson, L., Johnson, L.S., Kania, W., Keišs, O., Llambias, P.E., Mainwaring, M.C., Mänd, R., Massa, B., Mazgajski, T.D., Møller, A.P., Moreno, J., Naef-Daenzer, B., Nilsson, J.-Å., Norte, A.C., Orell, M., Otter, K.A., Park, C.R., Perrins, C.M., Pinowski, J., Porkert, J., Potti, J., Remes, V., Richner, H., Rytkönen, S., Shiao, M.-T., Silverin, B., Slagsvold, T., Smith, H.G., Sorace, A., Stenning, M.J., Stewart, I., Thompson, C.F., Tryjanowski, P., Török, J., Van Noordwijk, A.J., Winkler, D.W. & Ziane, N. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 45: 1–26. doi: 10.3161/000164510X516047
- Lea, R.W. & Klandorf, H. 2002. The brood patch. In Deeming, D.C. (ed.) Avian Incubation: Behaviour, Environment and Evolution, 100–118. Oxford University Press, New York.
- Loos, E.R. & Rohwer, F.C. 2004. Laying-stage nest attendance and onset of incubation in prairie nesting ducks. Auk 121: 587–599. doi: 10.1642/0004-8038(2004)121[0587:LNAAOO]2.0.CO;2
- Lord, A.M., McCleery, R.H. & Cresswell, W. 2011. Incubation prior to clutch completion accelerates embryonic development and so hatch date for eggs laid earlier in a clutch in the great tit Parus major. J. Avian Biol. 42: 187–191. doi: 10.1111/j.1600-048X.2010.05256.x
- Magrath, R.D. 1990. Hatching asynchrony in altricial birds. Biol. Rev. 65: 587–622. doi: 10.1111/j.1469-185X.1990.tb01239.x
- Magrath, R.D. 1992. Roles of egg mass and incubation pattern in establishment of hatching hierarchies in the blackbird (Turdus merula). Auk 109: 474–487.
- Martin, P.A. & Arnold, T.W. 1991. Relationships among fresh mass, incubation time, and water loss in Japanese Quail eggs. Condor 93: 28–37. doi: 10.2307/1368602
- Martin, T.E., Auer, S.K., Bassar, R.D., Niklison, A.M. & Lloyd, P. 2007. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61: 2558–2569. doi: 10.1111/j.1558-5646.2007.00204.x
- Matysioková, B. & Remeš, V. 2011. Responses to increased costs of activity during incubation in a songbird with female-only incubation: does feather colour signal coping ability? J. Ornithol. 152: 337–346. doi: 10.1007/s10336-010-0594-9
- Mead, P.S. & Morton, P.L. 1985. Hatching asynchrony in the mountain white-crowned sparrow (Zonotrichia leucophrys oriantha): a selected or incidental trait? Auk 102: 781–792.
- Møller, A.P., Adriaensen, F., Artemyev, A. Bańbura, J., Barba, E., Biard, C., Blondel, J., Bouslama, Z., Bouvier, J.-C., Camprodon, J., Cecere, F., Chaine, A., Charmantier, A., Charter, M., Cichoń, M., Cusimano, C., Czeszczewik, D., Doligez, B., Doutrelant, C., Dubiec, A., Eens, M., Eeva, T., Faivre, B., Ferns, P.N., Forsman, J.T., García-del-Rey, E., Goldshtein, A., Goodenough, A.E., Gosler, A.G., Goz´dz´, I., Gregoire, A., Gustafsson, L., Hartley, I.R., Heeb, P., Hinsley, S.A., Isenmann, P., Jacob, S., Järvinen, A., Juškaitis, R., Kania, W., Korpimaki, E., Krams, I., Laaksonen, T., Leclercq, B., Lehikoinen, E., Loukola, O., Lundberg, A., Mainwaring, M.C., Mänd, R., Massa, B., Mazgajski, T.D., Merino, S., Mitrus, C., Mönkkönen, M., Morales-Fernaz, J., Moreno, J., Morin, X., Nager, R.G., Nilsson, J.-Å., Nilsson, S.G., Norte, A.C., Orell, M., Perret, P., Perrins, C.M., Pimentel, C.S., Pinxten, R., Priedniece, I., Quidoz, M.-C., Remeš, V., Richner, H., Robles, H., Russell, A., Rytkonen, S., Senar, J.C., Seppanen, J.T., da Silva, L.P., Slagsvold, T., Solonen, T., Sorace, A., Stenning, M.J., Török, J., Tryjanowski, P., van Noordwijk, A.J., von Numers, M., Walankiewicz, W. & Lambrechts, M.M. 2014. Clutch size variation in Western Palearctic secondary hole-nesting passerine birds in relation to nest box design. Methods Ecol. Evol. 5: 353–362.
- Monrós, J.S., Belda, E.J. & Barba, E. 1998. Delays of the hatching dates in Great Tits Parus major: effects on breeding performance. Ardea 86: 213–220.
- Moreno, J. & Carlson, A. 1989. Clutch size and the costs of incubation in the Pied Flycatcher Ficedula hypoleuca. Ornis Scand. 20: 123–128. doi: 10.2307/3676879
- Nager, R.G. & van Noordwijk, A.J. 1995. Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. Am. Nat. 146: 454–474. doi: 10.1086/285809
- Neub, M. 1979. Brutbiologische Konsequenzen des asynchronen Schlüpfens bei Kohlmeise (Parus major) und Blaumeise (Parus caeruleus). J. Orn. 120: 196–214. doi: 10.1007/BF01642998
- Nilsson, J.Å. 1993. Energetic constraints on hatching asynchrony. Am. Nat. 141: 158–166. doi: 10.1086/285466
- Ojanen, M., Orell, M. & Väisänen, R.A. 1978. Egg and clutch sizes in four passerine species in northern Finland. Ornis Fennica 55: 60–68.
- Orell, M. 1983. Nestling growth in the Great Tit Parus major and the Willow Tit P. montanus. Ornis Fennica 60: 65–82.
- Parsons, J. 1972. Egg size, laying date and incubation period in the Herring Gull. Ibis 114: 536–541. doi: 10.1111/j.1474-919X.1972.tb00855.x
- Pendlebury, C.J. & Bryant, D.M. 2005. Night-time behaviour of egg-laying tits. Ibis 147: 342–345. doi: 10.1111/j.1474-919x.2005.00409.x
- Podlas, K. & Richner, H. 2013. The adaptive function of hatching asynchrony: an experimental study in great tits. Anim Behav. 86: 567–576. doi: 10.1016/j.anbehav.2013.06.012
- Potti, J. 1998. Variation in the onset of incubation in the pied flycatcher (Ficedula hypoleuca): fitness consequences and constraints. J. Zool. Lond. 245: 335–344. doi: 10.1111/j.1469-7998.1998.tb00108.x
- Reid, J.M., Cresswell, W., Holt, S., Mellanby, R.J., Whitfield, D.P. & Ruxton, G.D. 2002. Nest scrape design and clutch heat loss in Pectoral Sandpipers (Calidris melanotos). Funct. Ecol. 16: 305–312. doi: 10.1046/j.1365-2435.2002.00632.x
- Ricklefs, R.E. 1969. The nesting cycle of songbirds in tropical and temperate regions. Living Bird 8: 165–175.
- Robinson, W.D., Styrsky, J.D., Payne, B.J., Harper, R.G. & Thompson, C.F. 2008. Why are incubation periods longer in the tropics? A common-garden experiment with house wrens reveals it is all in the egg. Am. Nat. 171: 532–535. doi: 10.1086/528964
- Rowe, K.M.C. & Weatherhead, P.J. 2009. A third incubation tactic: delayed incubation by American Robins (Turdus migratorius). Auk 126: 141–146. doi: 10.1525/auk.2009.07210
- Runde, O.J. & Barrett, R.T. 1981. Variations in egg size and incubation period of the kittiwake Rissa tridactyla in Norway. Ornis Scand. 12: 80–86. doi: 10.2307/3675908
- Slagsvold, T. 1986. Asynchronous versus synchronous hatching in birds: experiments with the pied flycatcher. J. Anim. Ecol. 55: 1115–1134. doi: 10.2307/4437
- Sockman, K.W., Schwabl, H. & Sharp, P.J. 2000. The role of prolactin in the regulation of clutch size and onset of incubation behaviour in the American kestrel. Horm. Behav. 38: 168–176. doi: 10.1006/hbeh.2000.1616
- Sockman, K.W., Sharp, P.J. & Schwabl, H. 2006. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biol. Rev. 81: 629–666. doi: 10.1017/S1464793106007147
- St. Clair, C.C. 1998. What is the function of first eggs in crested penguins? Auk 115: 478–482. doi: 10.2307/4089208
- Stenning, M. 1996. Hatching asynchrony, brood reduction and other rapidly reproducing hypotheses. Trends Ecol. Evol. 11: 243–246. doi: 10.1016/0169-5347(96)10030-6
- Stenning, M. 2008. Hatching asynchrony and brood reduction in Blue Tits Cyanistes caeruleus may by a plastic response to local oak Quercus robur bud burst and caterpillar emergence. Acta Ornithol. 43: 97–106. doi: 10.3161/000164508X345383
- Theofanellis, T., Galinou, E. & Akriotis, T. 2008. The role of hatching asynchrony in brood size reduction of the great tit Parus major in a Mediterranean pine forest. J. Natl. Hist. 42: 375–380. doi: 10.1080/00222930701835324
- Tilgar, V. & Mänd, R. 2006. Sibling growth patterns in great tits: does increased selection on last-hatched chicks favour an asynchronous hatching strategy? Evol. Ecol. 20: 217–234. doi: 10.1007/s10682-005-5877-x
- Tomás, G., Barba, E., Merino, S. & Martínez, J. 2012. Clutch size and egg volume in great tits (Parus major) increase under low intensity electromagnetic fields: a long-term field study. Environ. Res. 118: 40–46. doi: 10.1016/j.envres.2012.07.007
- Vedder, O. 2012. Individual birds advance offspring hatching in response to increased temperature after the start of laying. Oecologia 170: 619–628. doi: 10.1007/s00442-012-2335-7
- Viñuela, J. 1997. Laying order affects incubation duration in the black kite (Milvus migrans): counteracting hatching asynchrony. Auk 114: 192–199. doi: 10.2307/4089160
- Wang, J.M. & Beissinger, S.R. 2009. Variation in the onset of incubation and its influence on avian hatching success and asynchrony. Anim. Behav. 78: 601–613. doi: 10.1016/j.anbehav.2009.05.022
- Wang, J.M. & Beissinger, S.R. 2011. Partial incubation in birds: its occurrence, function, and quantification. Auk 128: 454–466. doi: 10.1525/auk.2011.10208
- Wesołowski, T. 2000. Time-saving mechanisms in the reproduction of Marsh Tits (Parus palustris). J. Ornithol. 141: 309–318. doi: 10.1007/BF02462240
- Wiebe, K.L., Wiehn, J. & Korpimäki, E. 1998. The onset of incubation in birds: can females control hatching patterns? Anim. Behav. 55: 1043–1052. doi: 10.1006/anbe.1997.0660
- Winkel, W. 1970. Experimentelle Untersuchungen zur Brutbiologie von Kohl – und Blaumeise (Parus major und P. caeruleus). Uber die Legeperiode, Eigrösse, Brutdauer, Nestlingsentwicklung und Reaktion bei Veränderung der Eizahl. J. Orn. 111: 154–174. doi: 10.1007/BF01675593
- Winkel, W. 1975. Vergleichend–brutbiologische Untersuchungen an fünf Meisen–Arten (Parus spp.) in einem niedersächsischen Aufforstungsgebiet mit Japanischer Lärche Larix leptolepis. Vogelwelt 96: 41–63, 104–114.
- Zink, G. 1959. Zeitliche Faktoren im Brutablauf der Kohlmeise (Parus major). Untersuchungen an einer gekennzeichneten Population von Kohlmeisen in Möggingen–Radolfzell (II). Vogelwarte 20: 128–134.
