Abstract
Capsule Song Thrushes introduced to New Zealand differ in a number of life history traits compared to their source population in Britain.
Aim To survey life history traits in an introduced population of Song Thrushes, for comparison with the source population.
Methods Nests were monitored near Kaikoura, New Zealand. We recorded clutch size, egg size, length of incubation and nestling stages, hatching success, levels of brood reduction, and nest success. Data on life history traits of Song Thrushes in Britain were collated from the literature.
Results Mean clutch size declined from 4.1 eggs in Britain to 3.6 eggs in New Zealand. Egg size, nestling period length, and breeding success of Song Thrushes in New Zealand also decreased, while incubation period lengthened. There were changes in six out of seven life history traits studied.
Conclusion The general direction of changes in life history traits suggests that Song Thrushes in New Zealand invest less in each reproductive bout than in their native range. These changes may be the result of the warmer climate and longer breeding season in New Zealand. Understanding the fitness effects of these life history changes may provide insight into how a species adapts to a warmer environment.
Many life history traits in birds exhibit broad geographic patterns that are poorly understood. For example, clutch size is generally smaller at lower latitudes, while birds in the southern hemisphere have lower clutch sizes than northern hemisphere species (Jetz et al. Citation2008). Similarly, tropical species of Parulid warblers have longer incubation and nestling periods, and lower levels of nest attentiveness than their temperate counterparts (Cox & Martin Citation2009). Why these traits differ is not clear, but northern hemisphere species that have been introduced in the southern hemisphere provide a natural ‘experiment’ for understanding the evolution of life history traits at the global scale (Diamond Citation1986). Many introduced species often occur in places distant from the source population and in habitats that differ from that in which the species evolved (Long Citation1981). Thus, introduced species are likely to experience strong and novel selection pressures on their life history traits in the new environment. By studying the life history traits of species that have become established outside their natural range, one might be able to link changes in life history with differences in the environment between native and introduced ranges.
The Song Thrush (Turdus philomelos) is one of many European birds that was introduced to New Zealand in the late 19th century (Thomson Citation1922, Long Citation1981). Acclimatisation Societies introduced birds at each main city in New Zealand, releasing an estimated 400 thrushes overall, and the species is now widely established throughout the country. Song Thrushes are particularly common in farmland, open native forest, and in urban areas in New Zealand (Robertson et al. Citation2007). As in the native range, Song Thrushes in New Zealand feed on Lumbricidae earthworms and Brown Garden Snails (Cantareus asperses), which were also introduced from Britain (Cramp Citation1988, Higgins et al. Citation2006). Similarly, introduced European predators such as rats (Rattus spp.) and Stoats (Mustela ermina) are a major cause of nest failure in New Zealand (Moors Citation1983). However, thrushes in New Zealand also feed on a variety of native fruits (e.g. Coprosma spp.) and native invertebrates (e.g. Powelliphanta snails), and use native tree species for nesting (e.g. Kanuka Kunzea ericoides). The biotic environment inhabited by Song Thrushes in New Zealand is, therefore, broadly similar to that in Britain, but at the same time includes novel elements from the native flora and fauna.
The physical environment in New Zealand is likewise comparable to Britain, although there are some climatic differences that could affect life history evolution. The two land masses are similar in size, span similar temperate latitudes and experience similar seasonal patterns; however, New Zealand summers are warmer and drier and the winters milder (MacLeod et al. Citation2007), and New Zealand is closer to the equator than Britain. If transposed onto the northern hemisphere, New Zealand would lie at a position similar to that of France and the Iberian Peninsula. Perhaps because of this difference in climate, the breeding season of Song Thrushes is significantly longer in New Zealand compared to Britain (164.5 days vs. 116.0 days), which enables birds in New Zealand to raise more broods per season than their British counterparts (Evans et al. Citation2005). Milder winters may also increase adult survival, which could also allow individuals to raise more broods over their lifetimes (Evans et al. Citation2005).
Life history traits are the product of evolutionary adaptation to local conditions, and changes in such traits might not be expected in populations only established within the historical period. Nevertheless, changes in life history traits have been found in other studies of introduced species (Lahti Citation2008), suggesting either rapid evolutionary change or plastic responses to local conditions. For example, clutch size and egg volume have both significantly decreased in some introduced birds in New Zealand, including Song Thrushes (Cassey et al. Citation2005, Evans et al. Citation2005, Congdon & Briskie Citation2010, Samaš et al. Citation2013). Whether other life history traits have changed is unknown, and the objective of our study was to survey a range of life history traits in New Zealand Song Thrushes to determine how they differ from their ancestral European populations. In addition to clutch size and egg size, we also compared hatching success, incubation and nestling period duration, nest success and outcome (including rates of predation and nest desertion), and breeding success (reproductive output per attempt (ROA)).
How life history traits might be expected to change in a new environment is not always clear. For example, as in the native range, there is likely to be selection on clutch size in the introduced range to optimize the number of offspring produced per reproductive attempt (Nur Citation1986). Clutch size might be expected to decrease in the introduced range if seasonal productivity is lower (e.g. Ashmole's hypothesis; Evans et al. Citation2005), as a function of changes in the trade-off between current and future reproduction (Martin Citation2002, de Heij et al. Citation2006), or even other factors such as poorer female condition (Monaghan & Nager Citation1997). On the other hand, larger clutches might be favoured if predation risk is lower in the introduced range (Skutch Citation1949, Slagsvold Citation1984). Similarly, egg size could increase in the introduced range if there is a trade-off with a reduced clutch size, or both clutch and egg size could decrease due to the inability of females in the introduced environment to accumulate the required resources for egg production (Wilkin et al. Citation2009).
Differences between the native and introduced range in food availability and predation risk are also likely to drive changes in nesting success and investment in parental efforts, potentially leading to changes in lengths of incubation and nestling periods, levels of brood reduction, and nesting productivity (Martin Citation1995; Martin & Briskie Citation2009). In this study, we report how Song Thrushes in an introduced population differ from those in the native range as a first step in understanding how environmental changes may cause changes in life history traits.
METHODS
Study species
The Song Thrush is a medium-sized passerine that builds a large, open-cup nest in trees and shrubs. The female builds the nest and is the sole incubator. Intraspecific parasitism is rare (Moskát et al. Citation2003). Both parents provision the nestlings, with the male providing the bulk of the food (Hill et al. Citation1999). New Zealand thrushes are the descendants of birds caught in Britain (Thomson Citation1922). Song Thrushes in Britain are primarily non-migratory, and there is no evidence of migratory populations in the New Zealand range. However, birds from central and eastern Europe over-winter in southern Britain (Cramp Citation1988), so it is possible that some European birds may have been among those released in New Zealand.
Life history data
Data on a range of life history traits associated with breeding in both New Zealand and the British range were collected from the literature, analyses of nest record cards, and field work by the authors. From 2002–2008, we studied a Song Thrush population at Kowhai Bush, Kaikoura (42°23′S, 173°37′E), a 240 ha forest on the floodplain of the Kowhai River. In our core study area of ∼30 ha, the forest is dominated by Kanuka and an understorey of native and introduced shrubs. The forest is bordered by farmland and has been fenced off from grazing animals since the late 1970s, but introduced Hares (Lepus europaeus), Brush-tailed Possums (Trichosurus vulpecula), European Hedgehogs (Erinaceus europaeus), Stoats, Feral Cats (Felis catus), Ship Rats (Rattus rattus), and House Mice (Mus musculus) are present. Song Thrushes are the most abundant species of bird in the forest, with an estimated density of 6–11 pairs/ha in our study plot (unpubl. data).
A variety of life history traits associated with breeding were included in this study: (1) clutch size; (2) egg size and shape; (3) duration of incubation and (4) nestling periods; (5) hatching success; (6) fledgling success and levels of brood reduction; and (7) nest success. Clutch size was defined as the number of eggs laid per nesting attempt and confirmed when the same number of eggs was present for two consecutive visits during incubation, or if the visits were < 9 days apart and the eggs had hatched by the second visit (which confirmed that incubation was underway on the first visit). Eggs from a subset of nests were measured (length and maximum width) using digital callipers (accurate to the nearest 0.01 mm) to estimate volume, using the formula 0.51 × length × width2 (Hoyt Citation1979). Shape was defined as the ratio between egg length and egg width. The duration of the incubation and nestling periods was measured from the day the last egg was laid until the day the last egg hatched, and from the day the first egg hatched until the day the last nestling fledged, respectively. Both were estimated to the nearest day. Hatching success was defined as the proportion of eggs that hatched out of the total number of eggs present at hatching, and could only be measured for nests where clutch and brood size were both known (i.e. not including eggs that were abandoned or preyed upon during the incubation period). Fledgling success was measured as the number of fledglings leaving the nest in nests where at least one nestling fledged. Rate of brood reduction (mortality of individual nestlings within a nest) was measured for successful nests where both brood size and the number of fledglings were known. Nesting attempts were classed as successful if at least one nestling fledged. Daily nest survival was calculated using the Mayfield method (Mayfield Citation1961, Citation1975), whereby the probability of a nest surviving for one day was determined by calculating daily nest failure rates, counted as the number of nests which failed out of the total number of ‘exposure days’ (the time span of observations). The cause of nest failure (i.e. predation and abandonment) was noted to compare the causes of failure between this population and British populations. To estimate productivity, we used the equation given by Paradis et al. (Citation2000) to calculate ROA:
where BS is the brood size (at fledging), DSE and DSN the daily nest survival rates for the egg and nestling stages, and IT/NT the length of the incubation/nestling periods. This measure takes into account both nest survival and number of young fledged and thus provides a measure of the number of young produced per year.
Nest searching at our study site in New Zealand primarily consisted of searching vegetation for nests. Nests were also located using Song Thrush alarm calls or the sound of a female flushing from the nest. The majority of nests were monitored from discovery until the nest either succeeded (fledging at least one chick) or failed (nest abandonment or loss due to predation, poor nest construction, or severe weather). The final outcome for 57 of 416 nests was unknown (e.g. insufficient visits or we left study site before nest attempt completed) and these were omitted from analyses of nest success and breeding success. Nests were revisited every two to three days, to follow nest progress. However, visits were typically made daily as laying, hatching, and fledging approached, to determine the onset of laying and the length of the incubation and fledging periods.
Data on life history traits of British populations were taken from the literature, except egg size, for which we measured 30 clutches from the collection at the British Museum (Congdon & Briskie Citation2010). These were clutches from Song Thrushes that were clearly labelled as being collected in Britain. We only used data from the literature when it was clear the authors used the same criteria we used for defining each life history trait (e.g. length of incubation period from day last egg laid to last egg hatched).
Analyses
Statistical tests were carried out using R 3.0.3 (R Development Core Team Citation2011). We used generalized linear models (GLMs; Crawley Citation2005) to analyse trends within our study population at Kowhai Bush. All variables and their interactions were initially included in the model, then non-significant variables were successively removed until only significant variables remained, to develop a minimal adequate model. Excluded variables were then reinserted into the final model to confirm they had no effect. To compare the Kowhai Bush data with data from British populations, we used Welch's t-tests. This test was used because we could not always determine if variances were equal from published literature sources (Ruxton Citation2006). Egg volume, egg shape, and incubation period were log-transformed to normalize the data. Number of hatchlings, fledglings, and hatching success were tested using GLMs assuming a negative binomial distribution. As the effects of clutch size were not necessarily expected to conform to a linear relationship with the variables tested, we treated it as a factor in all tests.
In the results reported here, data from all years were combined because annual variation was not significant for any variable in the final models (the closest was variation in hatching success across years: χ2 = 11.3, df = 6, P = 0.08). It should be noted that as we were unable to band most birds in our study, it is possible we sampled some individuals more than once and this could lead to pseudo-replication. However, given the high density of birds in our study plot, we estimate that we discovered less than a third of nesting attempts and this may have reduced the risk of resampling the same individuals repeatedly. All means are given ± se.
RESULTS
Clutch size
Mean clutch size of Song Thrushes at our New Zealand study site in Kowhai Bush was 3.56 eggs ± 0.031 (n = 365 nests, range 2–5), which was significantly lower than in Britain (; t = 13.7, df = 739, P < 0.001). This value was also significantly lower than the mean of 3.74 calculated by Evans et al. (Citation2005) using nest records from the whole of New Zealand (t = −4.9, df = 675, P < 0.001) and the mean of 3.73 found by Samaš et al. (Citation2013) for Song Thrushes nesting in two urban centres on the North Island (t = −3.9, df = 675, P < 0.001). However, our estimate and those of Evans et al. (Citation2005) and Samaš et al. (Citation2013) indicate that mean clutch size is about half an egg smaller in New Zealand than in Britain. There was a significant difference between months (F = 14.1, df = 3, 361, P < 0.001), with clutch size decreasing over the breeding season at Kowhai Bush, as it does in Britain (). The smaller clutch size in New Zealand thrushes appears to occur across the entire breeding season ().
Figure 1. Mean monthly clutch size of Song Thrushes over the breeding season for Kowhai Bush (unshaded bars ± se) and a British population (shaded bars; means from Cramp Citation1988).
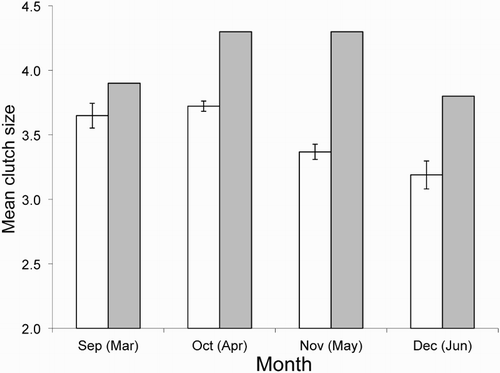
Table 1. Life history values (mean ± se) for Song Thrush populations in an introduced population at Kowhai Bush, Kaikoura (New Zealand), and native (British) populations, as reported in the literature. Percent differences between New Zealand and the native range are given in parentheses (**Significant at P < 0.01). The sample sizes stated are the number of nests used to estimate each trait; for those used to estimate ROA, see .
Egg size
Mean egg volume of Song Thrushes at Kowhai Bush, calculated using the mean volume within each of 154 nests, was 5.60 cm3 ± 0.039 and the mean ratio of egg shape (L/W) was 1.325 ± 0.0050. There was no relationship between egg volume and clutch size (F = 0.6, df = 3, 136, P = 0.64), but egg volume increased significantly as the season progressed (month of first egg vs. volume, F = 2.9, df = 3, 149, P < 0.05) and there was a significant difference in volume between eggs from Kowhai Bush and Britain (; t = −4.2, df = 40, P < 0.001). Mean egg shape was not significantly different between clutch sizes (F = 0.7, df = 3, 136, P = 0.55), but eggs from Kowhai Bush were relatively shorter and fatter than British eggs, and this difference was significant (; t = 2.3, df = 41, P < 0.026). Both Cassey et al. (Citation2009) and Samaš et al. (Citation2013) also found that Song Thrushes laid smaller eggs in New Zealand than in Europe.
Length of nesting stages
Mean incubation period of Song Thrushes at Kowhai Bush was 14.7 days ± 0.20 (n = 54), which is significantly longer than the incubation period in Britain (13.4 ± 0.14 days, ; t = 5.6, df = 103, P < 0.001). There was no effect of clutch size on length of incubation period in New Zealand Song Thrushes (F = 0.7, df = 3, 50, P = 0.57). Mean nestling period of thrushes at Kowhai Bush was significantly shorter than the nestling period in Britain (12.7 days ± 0.13 vs. 13.2 ± 0.095 days; t = 3.2, df = 166, P < 0.002). There was no effect of clutch size on length of nestling period in New Zealand Song Thrushes (F = 2.0, df = 2, 76, P = 0.14). The opposing direction of changes in the lengths of the incubation and nestling period did not appear to cancel out, because the total duration of the nesting cycle in New Zealand Song Thrushes (27.4 days) was still almost a day longer than in British Song Thrushes (26.6 days). This difference could not be compared statistically because the definitions of incubation period (last egg laid to last nestling hatched) and nestling period (first nestling hatched to last nestling fledged) meant that these measures overlapped during the hatching period.
Hatching success
The mean number of hatchlings per nest for Song Thrushes in Kowhai Bush was 3.23 ± 0.057 (n = 209 nests). After controlling for the significant relationship with clutch size (χ2 = 17.5, df = 3, 205, P < 0.01), there was no significant difference in the number of hatchlings per nest between months (χ2 = 0.285, df = 3, 202, P = 0.96). Hatching success (total number hatchlings/total number eggs in nests at hatching) of thrushes in Kowhai Bush was 89.8% ± 0.013 (675 hatchlings from 752 eggs in 209 nests). Lack (Citation1949) reported 86–91% hatching success for Song Thrush clutches of three to five eggs in Britain (n = 175 nests), and although we could not compare these estimates statistically, the range includes the mean at Kowhai Bush. There was also no significant difference between hatching success of Song Thrushes at Kowhai Bush and in Ireland (; Wilcoxon rank sum: W = 11175, P = 0.71).
Fledging success and brood reduction
Mean number of fledglings per successful nest at Kowhai Bush was 2.63 ± 0.085 (n = 124). There was no significant difference between clutch sizes (χ2 = 2.8, df= 3, 105, P = 0.25) or months (χ2 = 2.0, df = 3, 120, P = 0.57). Of 101 successful nests where clutch size, hatching success and number of fledglings were known, 30 (29.7%) experienced brood reduction and 46 nestlings (17.0%) died before fledging. Although some nestling mortality was the result of falls from tilted nests, 93% of deaths in successful nests appeared to be the result of starvation. In comparison, Lack (Citation1949) reported nestling mortality of only 4% for Song Thrushes from British nest record data (n = 280 nests).
Nest success
Daily nest survival rates of Song Thrushes were similar during the egg (laying + incubation) and nestling stages at Kowhai Bush (). The overall probability of a thrush nest surviving at Kowhai Bush was 23.5% (based on average egg-stage duration period of 18.3 days and nestling stage of 12.7 days). This is higher than the daily survival rates in two Irish populations (19.4%, ), where nest survival was lower than at Kowhai Bush during the egg stage, but slightly higher for the nestling stage. Daily survival rates of thrush nests in England appeared higher than Kowhai Bush for both nest stages (), although this difference could not be tested statistically. These results contrast with estimates of nest success reported in Song Thrushes on the North Island: Cassey et al. (Citation2009) found that only 5.2% of nests survived in horticultural areas (primarily berry farms), while Samaš et al. (Citation2013) calculated a daily predation rate of 0.0248 for Song Thrushes breeding in two urban centres, which we estimate represents a survival rate of just over 45%.
Table 2. Daily nest survival rates (± se) and ROA (number of fledglings per nest) for Song Thrushes in New Zealand and British populations. For daily nest survival, the nesting period has been separated into the egg stage (laying + incubation) and nestling stage.
Early nesting attempts were more successful at Kowhai Bush, with significantly more nests being predated (χ2 = 5.3, df = 357, P < 0.05) and abandoned (χ2 = 4.8, df = 317, P < 0.05) later in the season. Nest failure was caused by predation (58.8% of failures), abandonment (37.3%), and nests tipping over or falling (3.9%). These values are similar to those found in Ireland (; predation: χ2 = 2.2, df = 1, P = 0.14; desertion: χ2 = 0.0007, df = 1, P = 0.98). Overall, breeding success was significantly lower at Kowhai Bush than in Britain (; t = −6.1, df = 337, P < 0.01). Using the formula for ROA (Paradis et al. Citation2000), we calculated a value of 0.78 for Song Thrushes in New Zealand. This is lower than that reported for Britain ().
Table 3. Causes of nest failure for the egg and nestling stages for Song Thrushes in New Zealand (Kowhai Bush, this study) and Ireland (Kelleher & O'Halloran Citation2006).
DISCUSSION
We found that several life history traits appear to have changed in a New Zealand population of Song Thrushes since their introduction from Britain, with significant changes observed in clutch size, egg size and shape, incubation and nestling period lengths, and levels of brood reduction. Song Thrushes in New Zealand laid smaller clutches and smaller eggs, had shorter nestling periods, and produced fewer fledglings, which suggests a decrease in the overall investment per nesting attempt. One trait which appears to have increased compared with Britain is the incubation period. However, this may be the result of females reducing incubation attentiveness (percent time spent incubating), and is, therefore, another possible example of decreasing investment. Together, these findings suggest that Song Thrushes in New Zealand have reduced investment as a result of environmental constraints (e.g. reduced food availability during breeding) or a trade-off with other life history traits (e.g. increased adult survival or increased number of broods per breeding season). If reduced investment is adaptive in New Zealand, the changes in life history traits may be an example of rapid evolutionary change.
One reason for a bird to adaptively reduce its investment in any single reproductive bout is if the value of that bout has decreased. For example, higher levels of predation or brood reduction may select for reduced clutch sizes (Skutch Citation1949, Slagsvold Citation1984). Although there are conflicting lines of evidence that rates of predation differ between New Zealand and Britain (see below), the ROA of Song Thrushes was lower at Kowhai Bush than in Britain (Cramp Citation1988, Paradis et al. Citation2000, Kelleher & O'Halloran Citation2006), suggesting that the benefits of each clutch have decreased relative to the source population. Kelleher & O'Halloran (Citation2006) also found lower reproductive success in Ireland compared to Britain, and they proposed that Ireland's milder climate allowed for higher adult survival and increased competition during the breeding season. A similar situation could be the cause of lower reproductive success of thrushes in New Zealand, because high rates of brood reduction suggest many pairs cannot provide enough food for their young. For successful nests, 29.7% of Song Thrushes at Kowhai Bush experienced brood reduction. In contrast, Lack (Citation1949) reported that only 4% of nestlings starved in successful nests in Britain (see also Kelleher & O'Halloran Citation2006). Both MacLeod et al. (Citation2009) and Samaš et al. (Citation2013) reported that the breeding densities of Song Thrushes were much higher in New Zealand than in the native range. Higher density could increase intraspecific competition and lead to greater brood reduction in a density-dependent fashion. If starvation risk limits the number of nestlings Song Thrushes can raise in New Zealand, selection may have favoured a decrease in clutch size as an adaptation to lower levels of food resources and increased competition. However, this explanation appears unlikely, because Samaš et al. (Citation2013) found no evidence that the higher densities of introduced birds in New Zealand was related to a reduction in clutch size. Instead, it is possible that increased adult survivorship ‘devalues’ individual bouts by enabling birds to initiate more bouts over their lifespan or by selecting for lower reproductive effort via trade-offs between survival and reproduction (Stearns Citation1992). We were unable to estimate survival of adults at Kowhai Bush to assess this possibility, but there is anecdotal evidence to suggest that Song Thrushes and some other introduced birds live longer in New Zealand than in their native ranges (Evans et al. Citation2005).
An alternative hypothesis for adaptive changes in life history traits is that Song Thrushes in the introduced range face conditions that diverge sufficiently from their native range to cause stress that leads to poorer body condition and diminished productivity. We were unable to compare the condition of birds to determine if they differ from that in the native range, although Debruyne (Citation2008) found that Song Thrushes in New Zealand were significantly smaller in a number of skeletal features compared to Britain and this could be an indication of reduced condition. As in Britain, Song Thrushes in New Zealand feed on earthworms, snails, and other invertebrates (Higgins et al. Citation2006). It is possible the warmer and drier summers in much of the Song Thrush's New Zealand range reduce the availability of these prey items, particularly at a time when females are producing eggs and feeding young. The reduced clutch size, smaller egg size, increased length of the incubation period, and high rate of brood reduction in New Zealand thrushes are consistent with this hypothesis. However, skeletal changes could also reflect changes in body size as a result of selection, and further work (e.g. food supplementation experiments) would be needed to determine if lower food availability in New Zealand is the cause of changes in either body size or life history.
Increased predation risk plays a key role in shaping the life history traits of birds (Skutch Citation1949, Slagsvold Citation1984, Jetz et al. Citation2008), and might be expected to drive selection for shorter nesting periods to reduce the time nests are exposed to predators. Song Thrushes at Kowhai Bush had incubation periods that were more than a day longer than in the source population but nestling periods that were half a day shorter. Whether this is related to predation risk is not clear, because estimates of nest success in both the native and New Zealand range are not consistent, with some studies showing higher nest success in New Zealand (Samaš et al. Citation2013; this study) and others showing much lower success (Cassey et al. Citation2009). As a result, other factors may have driven this change. For example, females may be investing less energy into incubation, or the decrease in clutch size may increase cooling of the clutch (Reid et al. Citation2000), thus requiring more energy to achieve the same degree of incubation. As a result, additional effort to maintain a short incubation period may not be adaptive, despite an increase in predation risk of longer incubation periods. On the other hand, lower investment in incubation may reduce nest conspicuousness to predators, if parental activity affects predation risk and is correlated with incubation effort (Conway & Martin Citation2000). A comparison of incubation attentiveness and number of visits to the nest between British and New Zealand birds would clarify whether female incubation behaviour has changed, while temperature probes could determine if females are providing the same degree of incubation during on-bouts. It may be that food limitation requires females to forage for longer to attain the same nutritional gains, which in turn could reduce time spent incubating (Chalfoun & Martin Citation2007). As the decrease in clutch size reduces the length of the laying period (one day fewer per egg not laid), it is possible that selection for smaller clutches is also related to trade-offs between the length of each nesting stage and the duration of the most favourable breeding conditions.
Although Song Thrushes in New Zealand had lower reproductive output, it is unclear why it is lower than in Britain. Predation and desertion rates were similar between the two countries (at least using data from our study site), and as a result, numbers of successful nests were similar. Instead, the relative output of successful nests differed due to smaller clutches and a greater occurrence of brood reduction in New Zealand. Experimental clutch size manipulations and food supplementation are needed to assess the ability of parents to raise more offspring in any one reproductive bout and to determine whether the New Zealand environment restricts the number of nestlings song thrushes can fledge (i.e. if the decrease in clutch size is adaptive). Alternatively, data on adult survivorship and the effects of enlarged broods on subsequent parental fitness would provide information regarding the likelihood of higher survival probabilities in New Zealand selecting for an overall reduction in investment per nesting attempt, because more attempts are possible in New Zealand.
Further studies on the life history traits of other Song Thrush populations in New Zealand and in Britain would enable more detailed comparisons and confirm the widespread nature of changes in life history traits. Although Song Thrush numbers have been declining in Britain (Peach et al. Citation2004, Robinson et al. Citation2004), they remain one of the most common birds in New Zealand (Robertson et al. Citation2007). The reason for this difference is not clear, but their abundance in New Zealand, despite lower breeding success, suggests that post-fledging survival may be a factor affecting British populations, as proposed by Robinson et al. (Citation2004). Indeed, a decrease in brood size of Song Thrushes in central Europe has been linked to warming climatic conditions on the breeding grounds (Najmanová & Adamík Citation2009). If climate change results in the British climate becoming warmer and drier (i.e. more similar to that of New Zealand), this may further affect Song Thrush populations through decreases in reproductive success, which could select for reduced investment in reproduction. The life history changes observed among thrushes in New Zealand may thus provide a glimpse into how this species may be affected by environmental warming in their native range.
ACKNOWLEDGEMENTS
This research was carried out as part of a PhD at the University of Canterbury and approved by the University of Canterbury Animal Ethics Committee. We are grateful to the students and research assistants who contributed with fieldwork and to Raphael Didham, who provided advice on data analysis. Jack van Berkel provided accommodation and facilities at the Edward Percival Field Station during fieldwork in Kaikoura.
FUNDING
This work was supported by a Top Achiever Doctoral Scholarship from the New Zealand Tertiary Education Commission, the School of Biological Sciences and the Erskine Fund of the University of Canterbury.
REFERENCES
- Cassey, P., Blackburn, T.M. & Evans, K.L. 2005. Changes in egg size of exotic passerines introduced to New Zealand. Notornis 52: 243–246.
- Cassey, P., Boulton, R.L., Ewen, J.G. & Hauber, M.E. 2009. Reduced clutch-size is correlated with increased nest predation in exotic Turdus thrushes. Emu 109: 294–299. doi: 10.1071/MU09017
- Chalfoun, A.D. & Martin, T.E. 2007. Latitudinal variation in avian incubation attentiveness and a test of the food limitation hypothesis. Anim. Behav. 73: 579–585. doi: 10.1016/j.anbehav.2006.09.010
- Congdon, N.M. & Briskie, J.V. 2010. Effect of population bottlenecks on the egg morphology of introduced birds in New Zealand. Ibis 152: 136–144. doi: 10.1111/j.1474-919X.2009.00975.x
- Conway, C.J. & Martin, T.E. 2000. Evolution of passerine incubation behaviour: influence of food, temperature, and nest predation. Evolution 54: 670–685. doi: 10.1111/j.0014-3820.2000.tb00068.x
- Cox, W.A. & Martin, T.E. 2009. Breeding biology of the Three-striped Warbler in Venezuela: a contrast between tropical and temperate Parulids. Wilson J. Orn. 121: 667–678. doi: 10.1676/08-133.1
- Cramp, S. 1988. Handbook of the Birds of Europe, the Middle East and North Africa: The Birds of the Western Palaearctic, Volume V: Tyrant Flycatchers to Thrushes. Oxford University Press, Oxford.
- Crawley, M.J. 2005. Statistics: An Introduction Using R. John Wiley & Sons, Chichester.
- Debruyne, C.A. 2008. Fluctuating asymmetry and body morphology in relation to population bottlenecks of introduced birds in New Zealand. PhD Thesis, University of Canterbury.
- Diamond, J. 1986. Overview: laboratory experiments, field experiments, and natural experiments. In Diamond, J. & Case, T.J. (eds) Community Ecology, 3–22. Harper & Row, New York.
- Evans, K.L., Duncan, R.P., Blackburn, T.M. & Crick, H.Q.P. 2005. Investigating geographic variation in clutch size using a natural experiment. Funct. Ecol. 19: 616–624. doi: 10.1111/j.1365-2435.2005.01016.x
- de Heij, M.E., van den Hout, P.J. & Tinbergen, J.M. 2006. Fitness cost of incubation in Great Tits (Parus major) is related to clutch size. Proc. R. Soc. London, Ser. B 273: 2353–2361. doi: 10.1098/rspb.2006.3584
- Higgins, P.J., Peter, J.M. & Cowling, S.J. 2006. Handbook of Australian, New Zealand and Antarctic Birds, Volume 7: Boatbill to Starlings. Oxford University Press, Oxford.
- Hill, I.F., Cresswell, B. & Kenward, R.E. 1999. Comparison of brooding patterns between Blackbird Turdus merula and Song Thrush T. philomelos. Bird Study 46: 122–126. doi: 10.1080/00063659909461124
- Hoyt, D.F. 1979. Practical methods of estimating volume and fresh weight of birds’ eggs. Auk 96: 73–77.
- Jetz, W., Sekercioglu, C.H. & Böhning-Gaese, K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6: 2650–2657. doi: 10.1371/journal.pbio.0060303
- Kelleher, K.M. & O'Halloran, J. 2006. Breeding biology of the Song Thrush Turdus philomelos in an island population. Bird Study 53: 142–155. doi: 10.1080/00063650609461427
- Lack, D. 1949. Family size in certain thrushes (Turdidae). Evolution 3: 57–66. doi: 10.2307/2405452
- Lahti, D.C. 2008. Population differentiation and rapid evolution of egg color in accordance with solar radiation. Auk 125: 796–802. doi: 10.1525/auk.2008.07033
- Long, J.L. 1981. Introduced Birds of the World. David & Charles, London.
- MacLeod, C.J., Parish, D.B.M. & Robinson, R.A. 2007. Niche opportunities and introduced birds: temporal variation in resource abundance. In Bissonette, J.A. & Storch, I. (eds) Temporal Dimensions of Landscape Ecology: Wildlife Responses to Variable Resources, 252–268. Springer, New York.
- MacLeod, C.J., Newson, S.E., Blackwell, G. & Duncan, R.P. 2009. Enhanced niche opportunities: can they explain the success of New Zealand's introduced bird species? Diversity Distrib. 15: 41–49. doi: 10.1111/j.1472-4642.2008.00498.x
- Martin, T.E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65: 101–127. doi: 10.2307/2937160
- Martin, T.E. 2002. A new view of avian life-history evolution tested on an incubation paradox. Proc. Roy. Soc. Lond. B 269: 309–316. doi: 10.1098/rspb.2001.1879
- Martin, T.E. & Briskie, J.V. 2009. Predation on dependent offspring: a review of the consequences for mean expression and phenotypic plasticity in avian life history traits. Ann. N.Y. Acad. Sci. 1168: 201–217. doi: 10.1111/j.1749-6632.2009.04577.x
- Mayfield, H.F. 1961. Nesting success calculated from exposure. Wilson Bull. 73: 255–261.
- Mayfield, H.F. 1975. Suggestions for calculating nest success. Wilson Bull. 87: 456–466.
- Monaghan, P. & Nager, R.G. 1997. Why don't birds lay more eggs? Trends Ecol. Evol. 12: 270–274. doi: 10.1016/S0169-5347(97)01094-X
- Moors, P.J. 1983. Predation by mustelids and rodents on the eggs and chicks of native and introduced birds in Kowhai Bush, New Zealand. Ibis 125: 137–154. doi: 10.1111/j.1474-919X.1983.tb03095.x
- Moskát, C., Karcza, Z. & Csörgo, T. 2003. Egg rejection in European Blackbird (Turdus merula): effect of mimicry. Ornis Fenn. 80: 86–91.
- Najmanová, L. & Adamík, P. 2009. Effect of climatic change on the duration of the breeding season in three European thrushes. Bird Study 56: 349–356. doi: 10.1080/00063650902937305
- Nur, N. 1986. Is clutch size variation in the Blue Tit (Parus caeruleus) adaptive? An experimental study. J. Anim. Ecol. 55: 983–999. doi: 10.2307/4428
- Paradis, E., Baillie, S.R., Sutherland, W.J., Dudley, C., Crick, H.Q.P. & Gregory, R.D. 2000. Large-scale spatial variation in the breeding performance of Song Thrushes Turdus philomelos and blackbirds T. merula in Britain. J. Appl. Ecol. 37 (Suppl. 1): 73–87. doi: 10.1046/j.1365-2664.2000.00547.x
- Peach, W.J., Robinson, R.A. & Murray, K.A. 2004. Demographic and environmental causes of the decline of rural Song Thrushes Turdus philomelos in lowland Britain. Ibis 146: 50–59. doi: 10.1111/j.1474-919X.2004.00362.x
- R Development Core Team. 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. URL: http://www.R-project.org.
- Reid, J.M., Monaghan, P. & Ruxton, G.D. 2000. The consequences of clutch size for incubation conditions and hatching success in starlings. Funct. Ecol. 14: 560–565. doi: 10.1046/j.1365-2435.2000.t01-1-00446.x
- Robertson, C.J.R., Hyvönen, P., Fraser, M.J. & Pickard, C.R. 2007. Atlas of bird distribution in New Zealand 1999–2004. The Ornithological Society of New Zealand, Inc., Wellington.
- Robinson, R.A., Green, R.E., Baillie, S.R., Peach, W.J. & Thomson, D.L. 2004. Demographic mechanisms of the population decline of the Song Thrush Turdus philomelos in Britain. J. Anim. Ecol. 73: 670–682. doi: 10.1111/j.0021-8790.2004.00841.x
- Ruxton, G.D. 2006. The unequal variance t-test is an underused alternative to Student's t-test and the Mann-Whitney U test. Behav. Ecol. 17: 688–690. doi: 10.1093/beheco/ark016
- Samaš, P., Grim, T., Hauber, M.E., Cassey, P., Weidinger, K. & Evans, K.L. 2013. Ecological predictors of reduced avian reproductive investment in the southern hemisphere. Ecography 36: 809–818. doi: 10.1111/j.1600-0587.2012.07927.x
- Silva, E.T. 1949. Nest records of the Song-Thrush. British Birds 42: 97–111.
- Skutch, A.E. 1949. Do tropical birds rear as many young as they can nourish? Ibis 91: 430–455. doi: 10.1111/j.1474-919X.1949.tb02293.x
- Slagsvold, T. 1984. Clutch size variation of birds in relation to nest predation: on the cost of reproduction. J. Anim. Ecol. 53: 945–953. doi: 10.2307/4669
- Stearns, S.C. 1992. The Evolution of Life Histories. Oxford University Press, Oxford.
- Thomson, G.M. 1922. The Naturalisation of Animals and Plants in New Zealand. Cambridge University Press, Cambridge. doi: 10.5962/bhl.title.28093
- Wilkin, T.A., Gosler, A.G., Garant, D., Reynolds, S.J. & Sheldon, B.C. 2009. Calcium effects on life-history traits in a wild population of the great tit (Parus major): analysis of long-term data at several spatial scales. Oecologia 159: 463–472. doi: 10.1007/s00442-008-1222-8
