Abstract
Capsule Bare ground increases artificial nest predation in olive groves.
Aims To assess the effect of different soil management regimes on nest predation rates in olive groves.
Methods We performed nest predation experiments with artificial nests during the breeding season in 2013, in two areas of southern Spain. Each artificial nest (n = 300) contained three quail Coturnix eggs, two of which were unmanipulated and the third one was emptied and injected with plaster. Predators were identified by marks on eggs filled with plaster.
Results Ground nests were significantly more depredated, irrespective of the presence of ground cover; tree nests were less depredated in fields with ground cover. There was a clear difference in nest predators of ground and tree nests. Rodents were the most frequent predators of tree nests.
Conclusion Lower predation rates of tree nests in orchards with ground cover are probably linked to a change in the foraging behaviour of rodents, which in these more complex habitats might be restricted by rodents' own risk of predation. This study underscores the important role of agricultural practices in preserving farmland bird communities, particularly tree-nesting species, suggesting that for this group, implementation of ground cover in olive groves might enhance breeding success by reducing nest predation rates.
In recent decades, many farmland songbirds have experienced population declines in Europe (Donald et al. Citation2006). Degradation of habitats, particulary the simplification and homogenization of agricultural landscapes, has been suggested as the main factor affecting the decline of these bird populations (Benton et al. Citation2003, Tscharntke et al. Citation2005). The alteration of farmland ecosystems has created an environment in which prey populations might be more sensitive to predation, i.e. habitat change may interact with predation rates (Evans Citation2004). Nest predation is the primary cause of nest losses for a wide range of bird species, in different habitats and geographic locations (Ricklefs Citation1969, Martin Citation1993, Schmidt & Whelan Citation1999), and it has probably contributed to the decline of passerine populations in landscapes heavily modified by agriculture and other human developments (Robinson et al. Citation1995, Bayne & Hobson Citation1997, Donovan et al. Citation1997, Willson et al. Citation2001). For example, in Spain most farmland birds favour fallow fields for nesting; however, due to the intense use of herbicides, fallow fields are nowadays a rare habitat type and the few favourable patches have high nest densities, attracting predators, and thus exposing nests to very high predation rates (Pescador & Peris Citation2001). A similar situation occurs with Sky Larks Alauda arvensis, which preferentially nest in set-aside fields, but suffer high nest predation rates owing to high nest densities in this habitat type (Donald Citation1999). Such decoupling of habitat attractiveness from suitability may lead to the development of an ‘ecological trap’ (Robertson & Hutto Citation2006).
In the Mediterranean Basin, olive orchards are one of the primary agro-ecosystems and they are important winter quarters and breeding areas for numerous European bird species (Rey Citation2011). In Europe, the largest area of olive farming is in Spain, where 2.5 million ha are dedicated to this crop. In recent decades, agricultural intensification and changes in land use have replaced the traditional mosaic structure of olive groves interspersed with other crops, with extensive monocultures, resulting in a more homogeneous landscape (Sokos et al. Citation2013). Conventional farming, involving the intensive use of agrochemicals, is the traditional and most common production system (85% of the crop area), which has lead to significant negative environmental consequences including water pollution and, especially, soil erosion (Gómez et al. Citation2009). However, to prevent erosion, many farmers are now maintaining (or implementing) herbaceous ground cover within crops, which likely increases and provides structural complexity and resources for foraging birds (Wilson et al. Citation1999, Vickery et al. Citation2009). For instance, Castro-Caro et al. (Citation2014) have shown that herbaceous ground cover consistently favoured the abundance and richness of songbirds in the olive groves of southern Spain. In addition, breeding birds select their territories in olive orchards according to the availability of food resources such as seeds and arthropods (Muñoz-Cobo Citation2009). As a result, birds prefer to settle on fields with herbaceous ground cover, and songbird density in these fields can be at least twice as high compared to bare ground in conventional olive groves (Muñoz-Cobo Citation2009, Castro-Caro et al. Citation2014).
According to theoretical models (see patch use theory; Stephens & Krebs Citation1986) the foraging effort of predators may be directed towards patches with the highest cumulative prey availability. In Mediterranean farmland, isolated good-quality olive orchards with ground cover embedded in a bare-ground-dominated olive matrix are expected to attract breeding birds, but also their predators. Empirical data have shown that land use intensification in Mediterranean farmland is associated with an increase in the abundance of generalist predators, such as foxes, feral dogs and cats, which exert a significant predatory pressure on some farmland species, particulary ground-nesting birds (Yanes & Suárez Citation1996, Pita et al. Citation2009). Therefore, olive groves with ground cover may act as an ecological trap for birds, because they may not experience an increase in fitness in terms of reproductive success from settling in these preferred habitats (Robertson & Hutto Citation2006).
On the other hand, there is a debate as to whether nest predation rate is related to spatial structural complexity. Some studies have shown higher nest predation rates in agricultural landscapes compared to those in contiguous forests (Bayne & Hobson Citation1997, Hannon & Cotterill Citation1998), while others have found a higher risk predation in the more structurally complex habitats (Zuria et al. Citation2007). However, these studies focused mostly on field-forest areas, and little work has addressed nest predation in non-forested habitats (but see Ludwig et al. Citation2012). In the present study, we compared nest predation in olive orchards of southern Spain under two different soil management regimes (herbaceous ground cover vs. bare ground) using artificial ground and tree nests. If orchards with herbaceous ground cover are attracting predators, and thus acting as an ‘ecological trap’ we should predict that (1) predation rates could be higher in olive orchards with ground cover and (2) ground nests will suffer from higher predation pressure than tree nests, because ground nests are exposed to a greater diversity of predators.
METHODS
Study area and study design
The study was conducted in 2013 in three study sites of Southern Spain from mid-April to early June, coinciding with the breeding period of most common nesting species birds in the area (Muñoz-Cobo et al. Citation2001). Two sites were located in Villa del Río (37° 58′ N, 4° 17′ W), and the other in Montilla (37° 34′ N, 4° 37′ W), Córdoba province. All sites were embedded in an olive-dominated landscape, where agricultural intensification has eliminated most of the natural vegetation (Rey Citation2011). A more detailed description of the study area is provided by Castro-Caro et al. (Citation2014). In each study site, we selected two independent plots of 4 ha each, one with ground cover and the other with bare ground. The distance between plots was at least 500 m. The herbaceous ground cover comprised annual species that are adapted to Mediterranean climate and set their seeds before the summer drought. Cover was present throughout the groves except in the area below tree crowns, which was kept plant-free by the application of contact and systemic herbicides. The amount of area covered by ground cover varied among plots (50–75%). Ground cover was not mown during the experiment. All experimental plots were olive orchards that were managed under conventional olive grove farming practices and were selected at random, although an effort was made to choose olive groves of the same age and tree density. All of the olive orchards had trees >100 years old at a density of ∼100 trees/ha, and were subjected to the same pruning schemes.
The community of tree-nesters in the studied olive groves was dominated by Cardueline finches, mainly European Serin Serinus serinus, European Greenfinch Carduelis chloris and Common Linnet Carduelis cannabina, while Crested Larks Galerida cristata and Woodlarks Lullula arborea represent the ground-nesting community (Muñoz-Cobo Citation2009, Castro-Caro et al. Citation2014; Castro-Caro et al. unpubl. data).
The assemblage of potential nest predators includes corvids like Ravens Corvus corax. The most common mammalian carnivores are Red Fox Vulpes vulpes, feral dogs and cats (Duarte & Vargas Citation2001). Small mammals have been reported to be one of the main predator guilds of nests (e.g. Rats Rattus sp. and Garden Dormouse Eliomys quercinus; Gil-Delgado et al. Citation2010): see Supplemental Material.
Nest predation experiment
Artificial nests resembled those of Crested Larks, which build ground nests, and of Serins, which build open-cup nests on the outer parts of olive tree branches. Both bird species breed in olive agro-ecosystems in Spain; their breeding season extends from March to early June, and two or three broods per year are common. Clutch size for Crested Larks ranges between three and seven, and for Serins between two and five eggs; incubation time is around 13 days for both species (Cramp & Perrins Citation1994).
We exposed 100 artificial nests in every study site, 50 of them in the plot with ground cover and 50 in the plot with bare ground. In each plot, half of the nests were placed on the ground and the other half were placed on trees, following a 30 × 30 m grid in alternating positions (following Ludwig et al. Citation2012). Therefore, the distance between two nests of the same type was 60 m. Ground nests were placed in a small hollow dug on the ground at the border of the tree canopies and were oriented north, while tree nests were fixed to branches at a height of about 2 m and oriented randomly (see Supplemental Material). Altogether, 300 nests were exposed to predators for a two-week period and were checked every three or four days. The first period of exposure took place in the study site of Villa del Río from 18 April to 2 May, the second period was in Montilla from 6 to 20 May and the third period was in Villa del Río again, from 23 May to 6 June. A nest was considered as depredated, if any of the eggs was damaged or lost. Nest predation rate was estimated as the percentage of nests depredated in every plot.
We used artificial nests to overcome the extreme difficulty of finding real nests in the study area (Castro-Caro unpubl. data), thereby obtaining sufficiently large sample sizes to test ecological hypothesis. The use of artificial nests is an indirect method to estimate the impact of predation and has been widely used in bird studies (Zanette Citation2002, Beja et al. Citation2013). We used commercially available open-cup nests made of hempen braid 8 cm in diameter and 5 cm deep. Nests were exposed to the weather for at least 14 days before use, to dispel any artificial scent (Zuria et al. Citation2007). In each nest we placed three quail Coturnix coturnix eggs, two were unmanipulated and the third one was emptied and injected with plaster. In this way the three eggs had the same external appearance (Yanes & Suárez Citation1997), and plaster eggs could be used to identify teeth marks left by the predator (Major Citation1991, Willson et al. Citation2001, Carpio et al. Citation2013). Quail eggs have been useful to estimate spatial variation in nest failure risk for ground-nesting passerines (Cortés-Avizanda et al. Citation2009, Vögeli et al. Citation2011). Latex gloves and clean footwear were used during the placing of the nests to prevent scents that might be attractive to predators (Beja et al. Citation2013). Predators were identified by marks on eggs filled with plaster (Yanes & Suárez Citation1997, Duarte & Vargas Citation2001). In addition, four automatic cameras (Bushnell Trophy Cam) were placed in each plot to identify predators and were moved to another nest if the nest was depredated. Automatic camera systems have been used extensively to identify potential predator species (Laurance & Grant Citation1994). Photographic evidence was used to confirm the identification based on marks on the plaster moulds (Herranz, Suárez et al. Citation2002, Herranz, Yanes et al. Citation2002); identification was correct in 100% of cases (see Supplemental Material).
Statistical analysis
Predation level of a plot or nests inside each plot may be influenced by the presence of landscape features that promote landscape heterogeneity, such as hedges, ditches or roads (Chalfoun et al. Citation2002, Whittingham & Evans Citation2004, Zuria et al. Citation2007). To account for these effects we calculated the distance from each nest to the nearest hedge, ditch and road using ArcGIS 9.3.
Chi-squared tests were used to compare nest predation rate between groups of predators depending on the vegetation cover (ground cover vs. bare ground), and on the type of nest (ground vs. tree). To evaluate the relationships between the level of nest predation and several experimental variables we used a generalized linear mixed model, in which ‘site’ was considered a random variable and ‘plot’ was nested within ‘site’ for the random effect. In this model, nest site (categorical as ground vs. tree), vegetation cover (categorical as ground cover vs. bare ground), and the distance to road and the distance to hedge–ditch were included as explanatory variables. The dependent variable used in the model was whether the nest was depredated or not. We used a binomial distribution, with a logit-link function.
Akaike's Information Criterion corrected for small sample sizes (AICc) (Burnham & Anderson Citation2002) was used to perform a backward model selection; the model with the lowest AICc was considered the best one (Zuur et al. Citation2009). The statistical software INFOSTAT proposed by Balzarini et al. (Citation2001) was used.
RESULTS
A total of 300 nests were exposed during the breeding season, of which 157 nests were depredated (52%). In orchards with ground cover, 65 nests were depredated (41%) vs. 92 nests (59%) in plots with bare ground. Ground nests were significantly more depredated, either in bare ground or with ground cover (Mann–Whitney U test, Z = −0.1, P > 0.05), whereas tree nests were less depredated with ground cover (Mann–Whitney U test, Z = −4.8, P < 0.01; ).
Figure 1. Nest predation rate as a function of the interaction between cover type (ground cover vs. bare ground) and nest site (ground vs. tree) based on the 25 nests in each of the treatments ± sd.
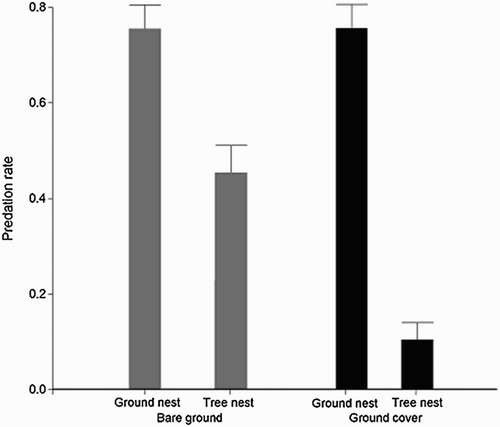
The full model was the best model, which included nest site, ground cover, distance to road and distance to hedge–ditch and the interaction between ground cover × nest site (). Finally, we found that the most frequent predators of tree nests were rodents (65% of nests predated, χ2 = 23.2, df = 6, P < 0.001). However, no single type of predator of ground nests was predominant (χ2 = 1.8, df = 6, P = 0.11) – see . When considering all plots (ground cover and bare ground) rodents turned out to be the main predator group (χ2 = 3.8, df = 6, P < 0.001 and χ2 = 3.8, df = 6, P < 0.001, respectively; ).
Figure 2. Percentage of nests depredated for each predator group in each cover type (ground cover vs. bare ground), and at each nest site (ground vs. tree).
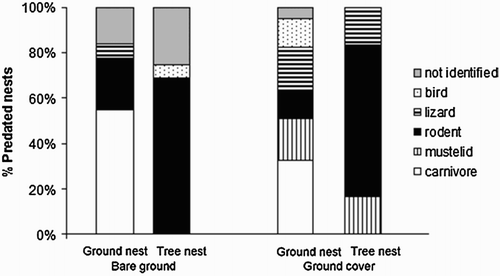
Table 1. The best model to predict nest predation. The intercept for the variable vegetation cover is ‘Bare ground’ and for the nest site is ‘Ground nests’.
DISCUSSION
Our results show that nest predation was lower in plots with ground cover than in those placed in the bare ground plots. However, these results may be attributed to the lower predation rate on tree nests in fields with herbaceous cover, while similar predation rate was found on ground nests when soil management regimes were compared. Rodents were the most frequent predator on tree nests and we found that predation rate on tree nests was relatively low in orchards with ground cover. It has been shown that ground cover increases the structural complexity in perennial woody systems (Arlettaz et al. Citation2012, Castro-Caro et al. Citation2014), which benefits invertebrates, particularly if the sward is species-rich and structurally complex (Wilson et al. Citation1999, Vickery et al. Citation2009). Potentially, such patches constitute a suitable habitat for small farmland mammals such as rodents. For instance, the total autumn small mammal biomass found in a UK farmland was three times higher on 6-m wide field margins than on arable field without such margins (Shore et al. Citation2005). Rodents select microhabitats where they can optimize their anti-predation and foraging requirements (Mandelik et al. Citation2003, Macdonald et al. Citation2007). In southern Spain, Boulay et al. (Citation2009) showed that rodents preferred to forage in covered microhabitats, where they consumed a larger proportion of seeds, probably because they were less visible to potential predators.
Some studies have shown that the observed variations in patterns of nest predation are determined by the distribution, abundance or species composition of nest predators and their specific foraging behaviours in different landscapes and habitats (Martin Citation1987, Ricklefs Citation1989, Andrén Citation1995). For instance, seasonal patterns in habitat use by rodents appeared to be largely a response to seasonal disturbance and the availability of cover in the fields (Todd et al. Citation2000). In fields with ground cover, rodents could extend their foraging areas beyond the olive trees to the ground which provides food resources and shelter from their predators. Therefore, predation pressure on tree nests is likely to be lower in the more structurally complex olive orchards, whereas this pressure might be higher in bare ground orchards where the foraging effort of rodents could be more intensive in the olive trees.
An alternative explanation for the lower nest predation rate on tree nests when ground cover occurs is through what is known as the ‘mesopredator release hypothesis’ which states that larger predators reduce density of smaller predators with concomitant decreases in predation pressure on their prey (Terborgh et al. Citation1999, Citation2001). This hypothesis has been used to explain the decline in nest success of many tropical migrants (Soulé et al. Citation1988, Ritchie & Johnson Citation2009). In our study, ground cover is likely to be more suitable habitat for some predators such as mustelids and reptiles which consume mainly rodents (McDonald et al. Citation2000). In fact, in our study, these predators only depredated nests in orchards with ground cover. As a result, higher predation pressure on rodents in ground cover orchards may decrease their predation rates on nests.
Artificial nests placed on the ground were more depredated than those placed in trees regardless of the type of soil management regime. This result agrees with established patterns of nest predation noted in the literature (Ricklefs Citation1969, Wilcove Citation1985, Melampy et al. Citation1999) which postulates that ground nests have higher rates of predation because of the presumed greater diversity of terrestrial predators. Furthermore, in this study, carnivores were the main predators of ground nests (), particulary red foxes Vulpes vulpes and feral dogs. Nevertheless, experimental studies carried out with captive foxes have shown that aural cues (e.g. chick alarm calls) are particulary important in stimulating and directing search behaviour (Österholm Citation1964). For instance, captive foxes and trained hunting dogs were unable to find nests without chicks unless in close proximity (<1 m) to the nest (Österholm Citation1964, Storaas et al. Citation1999). When chicks are present, mammalian predators can detect them from a much greater distance (Storaas et al. Citation1999), suggesting that in real nests the predation pressure on ground nesters may be even greater because chicks are more susceptible to mammalian predator than their clutches (Storaas et al. Citation1999). On the other hand, if foxes are relatively inefficient predators of nests when only eggs are present, we suggest that in our experimental study, ground nest losses to foxes are expected to be ‘incidental’ (sensu Vickery et al. Citation1992). Incidental predation occurs when secondary prey items are encountered and subsequently consumed, not through directed search for such prey, but through their casual encounter by a predator engaged in search for primary prey (Schmidt et al. Citation2001). In Mediterranean farmland, rabbits are primary prey for most of the carnivores (Delibes-Mateos et al. Citation2008); however, carnivores may depredate ground nests as secondary prey when found during their foraging bouts. Incidential predation because of rabbit abundance has been used to explain, for example, the declining population of larks in an Iberian semiarid shrubsteppe (Yanes and Suárez Citation1996). Interestingly, Carpio et al. (unpub. data) found high density and abundance of rabbits in the olive groves under study here.
The idea that complex habitats have lower predation rates was supported by this study, which may be the result of a greater biodiversity of either predators or microhabitats. Indeed, the variety of predators may promote intraguild competition and mesopredator release, with larger predators controlling smaller ones, which may be an important factor in structuring predator communities (Ritchie & Johnson Citation2009). Herbaceous ground cover may effectively increase microhabitat diversity and niche availability in the olive orchards, making them more suitable for foraging and shelter for both rodents and their potential predators (Kisel et al. Citation2011). Olive orchards with ground cover are known to be preferred by birds when compared to those having bare ground (Muñoz-Cobo Citation2009, Castro-Caro et al. Citation2014). Therefore, the lower nest predation rates of tree nests in groves with ground cover provides some evidence that, at least for tree-nesting songbirds, these orchards are not acting as ecological traps. This might increase the intrinsic value of this practice in enhancing biodiversity in olive groves, in addition to their agronomic benefits and soil erosion protection. Nevertheless, more research is needed to disentangle the magnitude of predator–prey interactions, which should be taken into account as a tool to promote biodiversity in farmland systems.
Supplementary material
Download MS Word (1.3 MB)Acknowledgements
We thank all the farmers who gave us permission to work in their fields. Special thanks go to Juanma and Stelle for their help in collecting data. We are also thankful to Isabel C. Barrio for her thorough review of this manuscript. The University of Córdoba and the Institute for Sustainable Agriculture of the Spanish National Research Council (CSIC) funded this project.
Supplemental Material
A supplementary online appendix giving predation rates by predator type, nest position and with/without herbaceous cover (Table S1) and showing examples of artificial nests, photo-trapped predation attempts and the marks left on artificial and real eggs by different predator species (Figs S1–S8) can be accessed at http://dx.doi.org/10.1080/00063657.2014.961894.
References
- Andrén, H. 1995. Effects of landscape composition on predation rates at habitat edges. In Hansson, L., Fahrig, L., & Merriam, G. (eds) Mosaic Landscapes and Ecological Processes, 225–255. Chapman & Hall, New York.
- Arlettaz, R., Maurer, M.L., Mosimann-Kampe, P., Nusslé, S., Abadi, F. & Schaub, M. 2012. New vineyard cultivation practices create patchy ground vegetation, favouring Woodlarks. J. Ornithol. 153: 229–238.
- Balzarini, M., Casanoves, F., Di Rienzo, J.A., González, I.A., Robledo, C.W. & Tablada, M.E. 2001. Software estadístico INFOSTAT. Manual de usuario. Versión 1. Universidad Nacional de Córdoba, Argentina.
- Bayne, E.M. & Hobson, K.A. 1997. Comparing the effects of landscape fragmentation by forestry and agriculture on predation of artificial nests. Conserv. Biol. 11: 1418–1429.
- Beja, P., Schindler, S., Santana, J., Porto, M., Morgado, R., Moreira, F. & Reino, L. 2013. Predators and livestock reduce bird nest survival in intensive Mediterranean farmland. Eur. J. Wildl. Res. 60: 249–258.
- Benton, T.G., Vickery, J.A. & Wilson, J.D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18: 182–188.
- Boulay, R., Carro, F., Soriguer, R.C. & Cerdá, X. 2009. Small-scale indirect effects determine the outcome of a tripartite plant–disperser–granivore interaction. Oecologia 161: 529–537.
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. Springer-Verlag, New York.
- Carpio, A.J., Guerrero-Casado, J., Tortosa, F.S. & Vicente, J. 2013. Predation of simulated red-legged partridge nests in big game estates from South Central Spain. Eur. J. Wildl. Res. 60: 391–394. doi:10.1007/s10344-013-0786-8
- Castro-Caro, J.C., Barrio, I.C. & Tortosa, F.S. 2014. Is the effect of farming practices on songbird communities landscape-dependent? A case study in olive groves in southern Spain. J. Ornithol. 155: 357–365.
- Chalfoun, A.D., Thompson III, F.R. & Ratnaswamy, M.J. 2002. Nest predators and fragmentation: a review and meta-analysis. Conserv. Biol. 16: 306–318.
- Cortés-Avizanda, A., Carrete, M., Serrano, D. & Donázar, J.A. 2009. Carcasses increase the probability of predation of ground–nesting birds: a caveat regarding the conservation value of vulture restaurants. Anim. Conserv. 12: 85–88.
- Cramp, S. & Perrins, C.M. (eds) 1994. Handbook of the Birds of Europe, the Middle East & North Africa, Vol. 8. Oxford University Press, Oxford.
- Delibes-Mateos, M., Ferreras, P. & Villafuerte, R. 2008. Rabbit populations and game management: the situation after 15 years of rabbit haemorrhagic disease in central southern Spain. Biodivers. Conserv. 17: 559–574.
- Donald, P.F. 1999. The ecology and conservation of Skylarks Alauda arvensis on lowland farmland. DPhil Thesis, Oxford University.
- Donald, P.F., Sanderson, F.J., Burfield, I.J. & Van Bommel, F.P.J. 2006. Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agric. Ecosyst. Environ. 116: 189–196.
- Donovan, T.M., Jones, P.W., Annand, E.M. & Thompson, F.R. 1997. Variation in local-scale edge effects: mechanisms and landscape context. Ecology 78: 2064–2075.
- Duarte, J. & Vargas, J.M. 2001. Mamíferos predadores de nidos de Perdiz Roja (Alectoris rufa Linnaerus, 1758) en olivares del sur de España. Galemys 13: 47–58.
- Evans, K.L. 2004. The potential for interactions between predation and hábitat change to cause population declines of farmland birds. Ibis 147: 1–13.
- Gil-Delgado, J.A., Mira, Ó., Viñals, A., Gómez, J., Banyuls, N. & Vives-Ferrándiz, C. 2010. Diet of the garden dormouse (Eliomys quercinus Linnaeus 1766) in orange groves: seasonal variation and use of available resources. Mammalia 74: 147–151.
- Gómez, J.A., Sobrinho, T.A., Giráldez, J.V. & Fereres, E. 2009. Soil management effects on runoff erosion and soil properties in an olive grove of Southern Spain. Soil Till. Res. 102: 5–13.
- Hannon, S.J. & Cotterill, S.E. 1998. Nest predation in aspen woodlots in an agricultural area in Alberta: the enemy from within. Auk 115: 16–25.
- Herranz, J., Suárez, F. & Yanes, M. 2002. A low-cost photographic system to identify bird nest predators and its effects on artificial nest predation rates. Game Wildl. Sci. 19: 179–195.
- Herranz, J., Yanes, M. & Suárez, F. 2002. Does photo-monitoring affect nest predation. J. Field Ornithol. 73: 97–101.
- Kisel, Y., McInnes, L., Toomey, N.H. & Orme, C.D.L. 2011. How diversification rates and diversity limits combine to create large-scale species–area relationships. Phil. Trans. R. Soc. B 366: 2514–2525.
- Laurance, W.F. & Grant, J.D. 1994. Photographic identification of ground-nest predators in Australian tropical rainforest. Wildl. Res. 21: 241–248.
- Ludwig, M., Schlinkert, H., Holzschuh, A., Fischer, C., Scherber, C., Trnka, A., Tscharntke, T. & Batáry, P. 2012. Landscape-moderated bird nest predation in hedges and forest edges. Acta Oecol. 45: 50–56.
- Macdonald, D.W., Tattersall, F.H., Service, K.M., Firbank, L.G. & Feber, R.E. 2007. Mammals, agri-environment schemes and set-aside: what are the putative benefits? Mammal Rev. 37: 259–277.
- Major, R.E. 1991. Identification of nest predators by photography, dummy eggs, and adhesive tape. Auk 108: 190–195.
- Mandelik, Y., Jones, M. & Dayan, T. 2003. Structurally complex habitat and sensory adaptations mediate the behavioural responses of a desert rodent to an indirect cue for increased predation risk. Evol. Ecol. Res. 5: 501–515.
- Martin, T.E. 1987. Artifcial nest experiments: effects of nest appearance and type of predators. Condor 89: 925–928.
- Martin, T.E. 1993. Nest predation and nest sites: new perspectives on old patterns. Bioscience 43: 523–532.
- McDonald, R.A., Webbon, C. & Harris, S. 2000. The diet of stoats (Mustela erminea) and weasels (Mustela nivalis) in Great Britain. J. Zool. (Lond) 252: 363–371.
- Melampy, M.N., Kershner, E.L. & Jones, M.A. 1999. Nest predation in suburban and rural woodlots of northern Ohio. Am. Midland Natur. 141: 284–292.
- Muñoz-Cobo, J. 2009. Olivar y biodiversidad. In Gómez Calero, J.A. (ed) Sostenibilidad de la producción de Olivar en Andalucia, 162–220. Consejería de Agricultura y Pesca – Junta de Andalucía, Sevilla. http://hdl.handle.net/10261/24985
- Muñoz-Cobo, J., Moreno-Montesino, J., Romero, C. & Ruíz, M. 2001. Análisis cuantitativo y cualitativo de las comunidades de aves en cuatro tipos de olivares de Jaén. (I) comunidades primaverales. Bol. San. Veg. de Plagas 27: 259–275.
- Österholm, H. 1964. The significance of distance receptors in the feeding behaviour of the fox Vulpes vulpes L. Acta Zool. Fenn. 106: 1–31.
- Pescador, M. & Peris, S. 2001. Effects of land-use on nest predation: an experimental study in Spanish croplands. Folia Zool. 50: 127–136.
- Pita, R., Mira, A., Moreira, F., Morgado, R. & Beja, P. 2009. Influence of landscape characteristics on carnivore diversity and abundance in Mediterranean farmland. Agric. Ecosyst. Environ. 132: 57–65.
- Rey, P.J. 2011. Preserving frugivorous birds in agro-ecosystems: lessons from Spanish olive orchards. J. Appl. Ecol. 48: 228–237.
- Ricklefs, R.E. 1969. An analysis of nesting mortality in birds. Smithson. Contrib. Zool. 9: 1–48.
- Ricklefs, R.E. 1989. Nest predation and the species diversity of birds. Trends Ecol. Evol. 4: 184–186.
- Ritchie, E.G. & Johnson, C.N. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12: 982–998.
- Robertson, B.A. & Hutto, R.L. 2006. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87: 1075–1085.
- Robinson, S.K., Thompson, F.R., Donovan, T.M., Whitehead, D.R. & Faaborg, J. 1995. Regional forest fragmentation and the nesting success of migratory birds. Science 267: 1987–1990.
- Schmidt, K.A. & Whelan, C.J. 1999. The relative impacts of nest predation and brood parasitism on seasonal fecundity in songbirds. Conserv. Biol. 13: 46–57.
- Schmidt, K.A., Goheen, J.R., Naumann, R., Ostfeld, R.S., Schauber, E.M. & Berkowitz, A. 2001. Experimental removal of strong and weak predators: mice and chipmunks preying on songbird nests. Ecology 82: 2927–2936.
- Shore, R.F., Meek, W.R., Sparks, T.H., Pywell, R.F. & Nowakowski, M. 2005. Will Environmental stewardship enhance small mammal abundance on intensively managed farmland? Mammal Rev. 35: 277–284.
- Sokos, C.K., Mamolos, A.P., Kalburtji, K.L. & Birtsas, P.K. 2013. Farming and wildlife in Mediterranean agroecosystems. J. Natur. Conserv. 21: 81–92.
- Soulé, M.E., Bolger, D.T., Alberts, A.C., Wrights, J., Sorice, M. & Hill, S. 1988. Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Conserv. Biol. 2: 75–92.
- Stephens, D.W. & Krebs, J.R. 1986. Foraging Theory. Princeton University Press, Princeton, NJ.
- Storaas, T., Kastdalen, L. & Wegge, P. 1999. Detection of forest grouse by mammalian predators: a possible explanation for high brood losses in fragmented landscapes. Wildl. Biol. 5: 187–192.
- Terborgh, J., Estes, J.A., Paquet, P., Ralls, K., Boyd-Heger, D., Miller, B.J. & Noss, R.F. 1999. The role of top carnivores in regulating terrestrial ecosystems. In Soule, M.E. & Terborgh, J. (eds) Continental Conservation, 39–64. Island Press, Washington, DC.
- Terborgh, J., Lopez, I., Nunez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G.H., Lambert, T.D. & Balbas, L. 2001. Ecological meltdown in predator-free forest fragments. Science 294: 1923–1926.
- Todd, I.A., Tew, T.E. & Macdonald, D.W. 2000. Arable habitat use by wood mice (Apodemus sylvaticus). 1. Macrohabitat. J. Zool. 250: 299–303.
- Tscharntke, T., Kleijn, A.M., Kruess, A., Steffan-Dewenter, I. & Thies, C. 2005. Landscape perspectives on agricultural intensification and biodiversity ecosystem service management. Ecol. Lett. 8: 857–874.
- Vickery, P.D., Hunter, M.L. & Wells, J.V. 1992. Evidence of incidental nest predation and its effects on nests of threatened grassland birds. Oikos 63: 281–288.
- Vickery, J.A., Feber, R.E. & Fuller, R.J. 2009. Arable field margins managed for biodiversity conservation: a review of food resource provision for farmland birds. Agric. Ecosyst. Environ. 133: 1–13.
- Vögeli, M., Laiolo, P., Serrano, D. & Tella, J.L. 2011. Predation of experimental nests is linked to local population dynamics in a fragmented bird population. Biol. Lett. 7: 954–957.
- Whittingham, M.J. & Evans, K.L. 2004. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 146: 210–220.
- Wilcove, D.S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66: 1211–1214.
- Willson, M.F., Morrison, J.L., Sieving, K.E., De Santo, T.L., Santiesteban, L. & Diaz, I. 2001. Patterns of predation risk and survival of bird nests in a Chilean agricultural landscape. Conserv. Biol. 15: 447–456.
- Wilson, J.D., Morris, A.J., Arroyo, B.E., Clark, S.C. & Bradbury, R.B. 1999. A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric. Ecosyst. Environ. 75: 13–30.
- Yanes, M. & Suárez, F. 1996. Incidental nest predation and lark conservation in an Iberian Semiarid Shrubsteppe. Conserv. Biol. 10: 881–887.
- Yanes, M. & Suárez, F. 1997. Nest predation and reproductive traits in small passerines: a comparative approach. Acta Oecol. 18: 413–426.
- Zanette, L. 2002. What do artificial nests tells us about nest predation? Biol. Conserv. 103: 323–329.
- Zuria, I., Gates, J.E. & Castellanos, I. 2007. Artificial nest predation in hedgerows and scrub forest in a human-dominated landscape of central Mexico. Acta Oecol. 31: 158–167.
- Zuur, A.F., Leno, E.N., Walker, N.J., Saveliev, A.A. & Smith, G.M. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
