Abstract
Capsule The occurrence of oilseed rape increased main prey abundance and breeding success of Common Buzzards.
Aims We tested whether the occurrence of oilseed rape influences the abundance of Common Voles, i.e. the main prey of Common Buzzards and so also nesting activity and breeding success of Common Buzzards.
Methods The study was carried out in 2005–2012 in a 38 km2 area in western Poland, where oilseed rape plantations (12–106 ha) covered 18% of the agricultural land. The number of active burrow entrances was used as an index of vole abundance in various crops, and Buzzard breeding performance, i.e. the occurrence of annual nesting attempts in individual long-term nesting sites as well as the presence and number of fledglings, was estimated by observations of their nests.
Results The index of vole abundance was highest in oilseed rape, and judging by the proportion of active burrow entrances (33–77%), the plantations of rape typically supported a larger portion of the local vole population than other crops. The acreage of oilseed rape fields around individual nesting sites of Buzzards did not affect the probability of nesting attempts in these sites. However, the probability of successful nesting and the number of fledglings per successful nest increased with the area of oilseed rape around the Buzzard nesting sites.
Conclusion The occurrence of oilseed rape may positively affect prey availability and in turn the breeding success of Buzzards. The spread of oilseed rape may therefore also be beneficial for other vole-eating raptors hunting in the agricultural landscapes.
In the recent decades a considerable decline in the abundance of many birds living in rural areas has been observed in Europe, and this phenomenon is linked to the intensification of agriculture and land use changes, including enlarging the size of arable fields and modifying the composition of crop species (Stoate et al. Citation2001, Citation2009, Green et al. Citation2005, Kleijn et al. Citation2009). One of the observed changes in the crop structure is a clear increase in the popularity of oilseed rape Brassica napus (FAO statistics, http://faostat.fao.org). For example in Poland, the area of this plant nearly doubled during the period 2000–2011, and its share recently reached 8% of the sown area (GUS Citation2012).
The spread of oilseed rape could have various effects on birds inhabiting agricultural landscapes. The increase in acreage of this plant was estimated as unfavourable for birds that prefer spring crops during the nesting period and that avoid winter crops which quickly become too high in the spring, e.g. for the Skylark Alauda arvensis (Newton Citation2004). In addition, oilseed rape fields frequently replaced set-aside plots which constituted the favourable habitat of Skylarks (Gillings et al. Citation2010). On the other hand, for certain birds, such as the Reed Bunting Emberiza schoeniclus, oilseed rape fields may create additional breeding habitat (Newton Citation2004). Moreover, oilseed rape is an important food source for some birds. For example, its leaves are included in the autumn – winter diet of Grey Partridges Perdix perdix (Orłowski et al. Citation2011) and constitute the most important winter food resource of Great Bustards Otis tarda in Central Europe, where this species is on the edge of extinction (Streich et al. Citation2006). The increase in acreage of oilseed rape improved the availability of foliar food and the status of Woodpigeons Columba palumbus in Britain (Newton Citation2004). Oilseed rape may also provide additional food for several species of birds feeding on seeds, especially the Common Linnet Carduelis cannabina (Newton Citation2004).
In this paper we test the hypothesis that the increase in acreage of oilseed rape may also indirectly affect food resources of raptors. The Common Vole Microtus arvalis is the primary or important prey of many raptor species hunting in the agricultural landscape (Newton Citation1979). This small rodent is particularly numerous in perennial vegetation, for example, alfalfa and grasses (Ryszkowski Citation1982). Hence, the change in land use involving the elimination of grassland can lead to significant reduction of food resources for some raptors and contribute to a decline in their numbers, as indicated, for example, in the UK (Newton Citation2004). However, high densities of Common Voles have also been reported in plantations of oilseed rape (Ryszkowski Citation1982).
The most common and widespread raptor species in Europe is the Common Buzzard Buteo buteo (Newton Citation1979, Cramp & Simmons Citation1980). Its food varies regionally, for example, in some areas of Britain the Common Rabbit Oryctolagus cuniculus is the main prey (Graham et al. Citation1995, Sim et al. Citation2001). However, on the European continent, including Poland, a major component of Common Buzzard diet in areas dominated by agriculture is usually the Common Vole (Mebs Citation1964, Goszczyński & Piłatowski Citation1986, Spidsø & Selås Citation1988, Jędrzejewska & Jędrzejewski Citation1998, Skierczyński Citation2006, Jankowiak & Tryjanowski Citation2013).
The aim of this study was to estimate the impact of oilseed rape occurrence on the abundance of the Common Vole and in turn on the breeding performance of the Common Buzzard. We predicted that nesting attempts, breeding success and high number of fledglings in Buzzard nests will be more frequently observed in nesting sites surrounded by larger acreage of oilseed rape plantations.
METHODS
Study area
The study was carried out in 2005–2012 in a 38 km2 area located near Czempiń, south of Poznań, in western Poland. The study area consisted mainly of arable land with relatively large areas of individual fields, varying in size from 10 to 100 ha. The main crops were cereals, but oilseed rape, maize, sugar beet, potatoes and alfalfa were also cultivated. There were scattered clumps of forest formed by both deciduous and coniferous trees among crop fields. They had a surface area of <1 to 100 ha and covered a total of only 8% of the study area. The Common Buzzard was the most abundant raptor species in western Poland, and its regional density increased from an average of 20 pairs per 100 km² in the early 1980s (Pielowski Citation1991), to about 37 pairs per 100 km² in 2005–2012 (M. Panek unpubl. data). During the study a few pairs of regularly nesting Goshawks Accipiter gentilis, Sparrowhawks Accipiter nisus, Marsh Harriers Circus aeruginosus and isolated cases of breeding by other raptor species were found (M. Panek unpubl. data).
Field methods
Every year all oilseed rape plantations situated in the study area were recorded on maps (scale 1:10 000; Head Office of Geodesy and Cartography, Warsaw), on which roads, ditches and hedgerows, i.e. field borders, were marked. The size of individual crop fields was then estimated from these maps (to the nearest 1 ha).
The abundance of Common Voles was estimated by counting their burrow entrances. It has been found in agricultural areas in Poland that the number of burrows used by Common Voles was positively correlated with the numbers of these animals (Mackin-Rogalska et al. Citation1986). Similar methods for assessing relative vole abundance have been successfully applied in some previous Buzzard studies (Krüger Citation2002, Schindler et al. Citation2012). Five transect routes were established, with a total length of 35 km, covering the study area evenly. Burrow counts were carried out every year in March. Only active burrow entrances with clear signs of use (fresh digging, droppings, pieces of food), located up to 3 m on both sides of the transect routes, were recorded. Moreover, the type of vegetation in which the burrows were placed was categorized as winter cereals, oilseed rape, alfalfa and other (stubbles, mustard and other green cover crops, wild plants; no active entrances were found on ploughed fields). During the vole counts, the type of crop (or the presence of ploughed land) on each crossed arable field was noted on the above mentioned maps. Next, the total length of sections running through the selected vegetation types on each transect route was measured from these maps (to the nearest 10 m). Finally, the annual and long-term average numbers of burrow entrances per transect length unit for each vegetation type were calculated (burrow index hereafter). The index obtained primarily reflected relative vole abundance at the beginning of Buzzard breeding season, i.e. during the pre-laying and laying period. However, the index seems to be less representative for the period of young rearing in May and July, because the rate of spring changes in vole numbers may vary between years and crop types (Ryszkowski Citation1982).
The occurrence of occupied nests of Common Buzzards and their reproductive success in the study area was estimated every year using the methodology commonly utilized in research on birds of prey (Newton Citation1979, Jędrzejewska & Jędrzejewski Citation1998). Namely, the area was visited from the second half of March to mid-July, once every two weeks. In March and April, clumps of forest were searched to find Buzzard nests. The low cover of forest in the study area allowed the unambiguous designation of individual long-term nesting sites of Buzzards. A nesting site was defined as an isolated clump of forest or a group of neighbouring clumps, surrounding by treeless agricultural land, in which in at least one study year a nesting attempt was observed. So, if in a given forest clump Buzzard nesting attempts were found only in some years, this part of the study area was also regarded as the same nesting site in the remaining years. It was assumed that within a given nesting site a nesting attempt occurred in a given year, if during the incubation period, i.e. in April or May, a Buzzard sitting on the nest was observed at least once. The locations of active nests were marked on the above mentioned maps and nest coordinates were calculated based on the kilometre grid of the map (accuracy 50 m). Further visits to occupied nests were aimed at evaluating reproductive success. A breeding attempt was considered as successful when at least one fully feathered offspring (i.e. without white down feathers on the head) was observed on the nest before the anticipated fledging time. At this stage of their development, the number of offspring was also counted. Observations were carried out from the ground, from a distance of several dozen metres from the nests using binoculars.
Based on the above mentioned maps showing the distribution of oilseed rape fields in the study area, we measured the acreage of this crop around all Buzzard nesting sites, both in the years with nesting attempts and without an occupied nest. The measurements (to the nearest 1 ha) took place within a radius of 1 km from the nest location, i.e. in a circle of about 3 km², which approximately corresponded to the area obtained by dividing the area of the study by the average number of Buzzard pairs found there (see Results). In the case of some nesting sites, breeding attempts found in successive years occurred in the same nest, and in other nesting sites breeding attempts took place in different nests from year to year (typically spaced apart by several dozen metres within a given clump of forest, and only rarely up to 300 m). Therefore, the average nest locations were estimated from the nest coordinates and used to plot the circles in which the annual area of oilseed rape was measured.
In the study area 19 Buzzard nesting sites were found; 15 of these were located in small forest patches from <1 to 15 ha and 4 in larger forests of 45 and 100 ha. In all these cases the mean nest locations were up to 150 m from the edges of the forest.
Statistical analysis
Differences in the burrow index between the four crop categories and between the years were tested using two-factor analysis of variance implemented in Statistica Software. Next, we fitted a mixed effect model to the measures of the Buzzard breeding performance where oilseed rape area was considered as a fixed explanatory variable and year nested within the nesting site were considered as random effects. First, we fitted a mixed model on the nest occupancy (nesting attempt vs. no active nest) as a response variable where we assumed binomial error structure. Only year was considered as a random effect in this particular model to ensure model convergence. Next, we fitted a model on the nesting success (at least one fledgling produced vs. nest failure) with binomial error structure and a model on the number of fledglings where we assumed a Poisson error structure. These last two models were fitted to the data from only active nesting sites and from nests with successful nesting, respectively. The mixed models were fitted using library lme4 in R (Bates et al. Citation2014).
RESULTS
The size of individual oilseed rape fields ranged from 12 to 106 ha, with a mean of 37 ha (n = 104, se = 2). In individual years the oilseed rape plantations covered from 5.6% to 23.9% (mean = 17.8%, n = 8, se = 2.4) of the agricultural land. The occurrence of all selected crop types in the study area (from the transect routes), was mainly winter cereals (24.5–55.5% of total transect length in individual years, mean = 36.8%, n = 8, se = 3.6), followed by oilseed rape (6.5–22.4%, mean = 17.6%, n = 8, se = 2.6), alfalfa (3.7–12.6%, mean = 8.2%, n = 8, se = 1.3) and other vegetation (mainly stubbles and cover crops; 2.0–20.9%, mean = 11.5%, n = 8, se = 2.4), while the remaining parts constituted ploughed land.
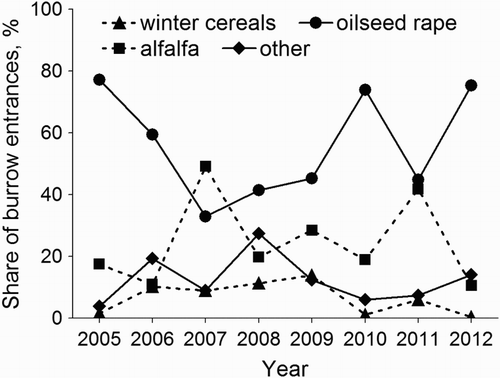
The burrow index differed between years (F 7,93 = 14.4, P < 0.001) and crop types (F 3,93 = 35.3, P < 0.001), with a significant interaction between these variables (F 21,93 = 6.3, P < 0.001). During five of the eight study years, the highest values of this index were found in oilseed rape (long-term mean = 64.2 burrow entrances per km, n = 33, se = 13.1); another crop with abundant voles was alfalfa (mean = 46.4, n = 18, se = 16.5), while in other crops (mean = 15.6, n = 37, se = 4.0) and especially in winter cereals (mean = 3.0, n = 37, se = 0.6) the burrow index was low (). Based on the numbers of burrow entrances per km of transects in various crop types and on the share of crop types in the study area, the distribution of voles between these crops was evaluated. Despite the dominance of winter cereals in the study area, they apparently contained only a small portion of the local Common Vole population because the contribution of burrow entrances found in these crops varied in different years only from 0% to 13.9% of the total number found on the transects. In some years, a high percentage of these entrances was reported in alfalfa fields (up to 49.1%). During seven out of the eight study years, however, most vole burrow entrances occurred in oilseed rape, where their contribution ranged from 32.9% to 77.2% ().
Figure 1. The index of common vole abundance (average number of burrow entrances per km of transects ±se) in individual crop types near Czempiń, western Poland, in the years 2005–2012.
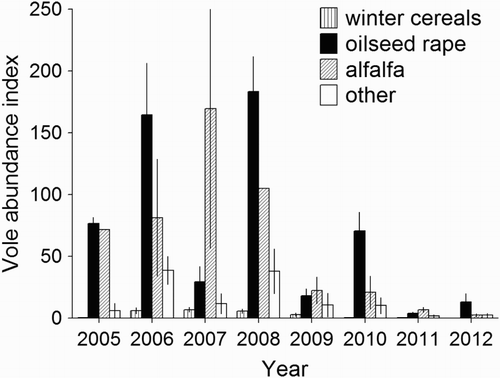
Figure 2. The proportion (share) of common vole burrow entrances in individual crops (the ratio of entrance number in a given crop type to the total number of entrances found in a given year) near Czempiń, western Poland, in the years 2005–2012.
In the 19 Buzzard nesting sites found in the study area, 11–16 nesting attempts were recorded in individual years (mean = 14.2, n = 8, se = 0.6). This gave a density of 28.9 to 42.1 pairs per 100 km² (mean = 37.4 pairs per 100 km², n = 8, se = 1.7). In total, 114 nesting attempts were observed on 152 occasions (nesting sites × years), 84 of the nesting attempts were successful (74%), and the mean number of fledglings per successful nest amounted to 1.5 (range 1–3, se = 0.1).
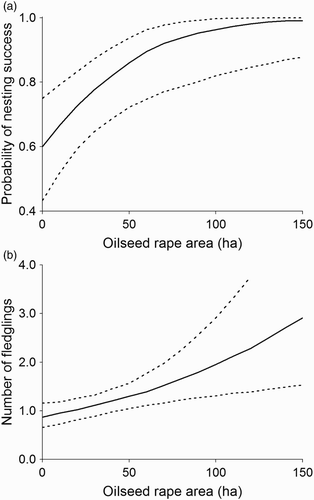
The probability of a nesting attempt in a given nesting site was not related to the area of oilseed rape around the nest (b ± se = 0.006 ± 0.006, z = 0.99, P = 0.32). The probability of nesting success was positively related to the area of oilseed rape around the nest (b ± se = 0.029 ± 0.010, z = 2.8, P = 0.005, a), as was the number of fledglings in successful nests (b ± se = 0.008 ± 0.002, z = 2.94, P = 0.003, b).
Figure 3. The relationship between area of oilseed rape in a diameter of 1 km around the nest and (a) the probability of nesting success and (b) the number of fledglings in the Common Buzzard, western Poland. Shown is the fit on the original scale from the mixed effect model with 95% confidence intervals.
DISCUSSION
During most spring seasons, the highest index of Common Vole density occurred in the oilseed rape fields. Moreover, judging by the number of active burrow entrances (Mackin-Rogalska et al. Citation1986), plantations of rape contained the majority of the population of voles living in the study area compared to other crops. The high densities of Common Voles in oilseed rape fields were probably connected with relatively large green biomass in this crop, because plant production is known as a factor influencing small rodent populations (Laine & Henttonen Citation1983, Tkadlec et al. Citation2006). Therefore, the occurrence of oilseed rape positively affected the local abundance of Buzzard food resources.
According to Newton (Citation1979), most raptor populations are limited by the availability of their prey. For example, in England, it was found that the local density of breeding Common Buzzards was dependent on the abundance of their main prey (Graham et al. Citation1995, Sim et al. Citation2001). In our study, however, the occurrence of oilseed rape plantations with numerous voles apparently had little effect on the probability of nesting attempts by Buzzards. On the other hand, territory occupation and nesting attempts by Buzzards could be locally affected also by vole resources in other vegetation. Alfalfa was less common than oilseed rape in the study area, but in some years a considerable part of agricultural land was covered in winter and early spring with stubbles and cover crops (mainly mustard) where the burrow index was sometimes high. Moreover, Newton (Citation1979) emphasized that the abundance of prey may be the most important factor affecting the breeding populations of raptors mainly in areas where there are no limits in the availability of nesting sites. Wooded patches covered only a small part of the study area. It is therefore possible that the choice of nesting sites for these birds may have been limited there. Thus, they probably bred in the only places which provided suitable nesting conditions (e.g. trees with good nest bases and safety), and so this was to a large extent independent of the distribution of crops rich in food in any given year.
Reproductive success of Buzzards, i.e. both the probability of successful nesting and the number of fledglings, was higher in nests surrounded by a larger area of oilseed rape fields. In our study area Buzzards nested mainly in small forest patches and only a few nests were located near the edges of larger forests. It is therefore unlikely that the higher proportion of forested area (and so potentially a smaller area of oilseed rape) in the diameter of 1 km around some nesting sites introduced any significant bias in the above conclusion. Thus, the relationship between the occurrence of oilseed rape and the breeding parameters of Buzzards is likely to be linked with the abundance of Common Voles. The influence of food resources, changing in time or space, on the breeding success of Common Buzzards has been observed in a number of studies (Newton Citation1979, Swann & Etheridge Citation1995, Austin & Houston Citation1997, Selås Citation2001, Sim et al. Citation2001; although see Krüger Citation2004). According to Goszczyński & Piłatowski (Citation1986): Common Buzzards are generalist predators switching to other prey, mostly birds, when the availability of small rodents is low. On the other hand, they are not then able to achieve an equivalent hunting efficiency which would enable them to maintain high breeding success.
Oilseed rape fields were characterized by a higher vole density index in early spring than other crops. However, during the feeding of young by Buzzards in May and June, oilseed rape becomes high and dense, which may limit the location and capture of voles by these predators. On the other hand, high densities of voles in oilseed rape plantations may result in their subsequent dispersal to adjacent crops, especially to some spring crops (Ryszkowski Citation1982), and therefore higher availability of this prey in the territories of Buzzards containing oilseed rape fields. In addition, the abundance of food may affect the course and results of breeding in raptors to a large extent by the effects on the condition of females at the beginning of the breeding season and on the clutch size (Newton Citation1979). It has been found that territorial pairs of the Common Buzzard often stay in their territories throughout the winter and during this time they prefer vegetation patches rich in voles (Weir & Picozzi Citation1975, Schindler et al. Citation2012). Furthermore, it seems unlikely that the capture of voles in oilseed rape by Buzzards is limited (in comparison to other vegetation) during the winter and at the beginning of spring when these plants are not fully grown.
Although the occurrence of oilseed rape did not affect the breeding activity of Buzzards in their individual territories, it was positively related to reproductive success, and thus potentially positively influenced the abundance of this species. Thus, changes in agriculture, consisting of increased planting of oilseed rape should be beneficial for the species. It might therefore be one of the reasons for the increase in Common Buzzard numbers observed in Europe in the last decades (BirdLife International Citation2014). The population of Common Buzzards also increased in Poland in the second half of the 20th century (Tomiałojć & Stawarczyk Citation2003); however, in the years 2000–2013 a slight decline was observed (Chodkiewicz el al. Citation2013). Therefore, even if the relatively high reproductive success of Buzzards nesting near oilseed rape plantations in our study area is not important for the viability of the local population, the higher production of young may still support emigration to other locations, including regions with decreasing populations.
The importance of oilseed rape occurrence may also apply to other birds of prey hunting small rodents in the agricultural landscape. For example, the European Kestrel Falco tinnunculus is a raptor species feeding mainly on voles, and the density of this prey correlates positively with its reproductive output (Village Citation1982, Korpimäki Citation1986). Moreover, the availability of voles in crop fields determined the size of clutches and breeding success in Montagu's Harriers Circus pygargus in France (Salamolard et al. Citation2000). For Marsh Harriers C. aeruginosus in Spain, the beneficial factor was the presence of areas with intensive agriculture, especially where crops such as alfalfa probably contained high densities of small rodents (Cardador et al. Citation2011). Similarly, the observed increase in oilseed rape acreage may be beneficial for the conservation of raptor species hunting in the agricultural landscape. In other words, the presence of this crop can potentially compensate for adverse effects that some changes of agricultural land use have on the birds of prey, as described by Newton (Citation2004).
On the other hand, some birds of prey feeding primarily on small rodents at least occasionally hunt other animals, such as game birds, and thus could potentially limit these populations (Valkama et al. Citation2005). For example in France, the decrease in Grey Partridge numbers was caused mainly by an increase in female losses (Bro et al. Citation2001). The female mortality rate was correlated with the abundance of harriers, mainly the Hen Harrier Circus cyaneus, suggesting that these predators could have some influence on partridge populations (Bro et al. Citation2001, Citation2006). Moreover, the occurrence of harriers increased with the larger crop fields (Bro et al. Citation2001), so it was dependent on habitat, probably through its impact on food availability. Therefore, in a similar way, if the increase in oilseed rape acreage favours some birds of prey, it may also cause higher losses in their secondary prey. Similar relationships may also apply to certain predatory mammals. For example, in Polish farmland, Common Voles constitute a major part of the diet of Red Foxes Vulpes vulpes, which hunt also hares and partridges (Goszczyński Citation1995). High vole numbers typically limit predation of vole-eating predators on alternative prey species, because the predators switch between food sources with changes in their relative abundance, but such response may be too weak to affect prey species which constitute only a small part of predator diet, i.e. secondary prey (Norrdahl & Korpimäki Citation2000). Increased abundance of main prey may cause higher predator pressure on accidentally predated secondary prey especially when these two types of prey coexist in the same area (Vickery et al. Citation1992). This condition is fulfilled in the case of Common Voles and some wild birds or small game animals. However, it has also been suggested that the increase in predation pressure on farmland birds may not be related to increased predator abundance but rather to unfavourable habitat changes resulting in higher vulnerability of prey species to predation (Evans Citation2004).
This study showed that the development of oilseed rape plantations may affect animals living in agricultural landscapes through increasing the availability of resources crucial for breeding success, and not only by modifying habitat features and plant food resource availability. The increase in acreage of this crop may improve prey availability for vole-eating predators and, in this way, positively influence their populations. It may, however, also potentially increase the pressure of such predators on some of their secondary prey, although this remains to be tested.
ACKNOWLEDGEMENTS
We are grateful to colleagues from the Research Station PHA in Czempiń for their help in data collection, and to Maciej Budny, Will Cresswell, Robert Kamieniarz and two anonymous referees for their valuable comments to the manuscript. We also thank Ian Hatcher for language improvements.
FUNDING
This study was financed by the Research Station PHA in Czempiń.
REFERENCES
- Austin, G.E. & Houston, D.C. 1997. The breeding performance of the Buzzard Buteo buteo in Argyll, Scotland and a comparison with other areas in Britain. Bird Study 44: 146–154. doi: 10.1080/00063659709461050
- Bates, D., Maechler, M., Bolker, B. & Walker, S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. Available from: http://CRAN.R-roject.org/package=lme4 (Accessed 21 August 2014).
- BirdLife International. 2014. Species factsheet: Buteo buteo. Available from: http://www.birdlife.org (Accessed 28 August 2014).
- Bro, E., Reitz, F., Clobert, J., Migot, P. & Massot, M. 2001. Diagnosing the environmental cause of the decline in Grey Partridge Perdix perdix survival in France. Ibis 143: 120–132. doi: 10.1111/j.1474-919X.2001.tb04176.x
- Bro, E., Arroyo, B. & Migot, P. 2006. Conflict between grey partridge Perdix perdix hunting and hen harrier Circus cyaneus protection in France: a review. Wildl. Biol. 12: 233–247. doi: 10.2981/0909-6396(2006)12[233:CBGPPP]2.0.CO;2
- Cardador, L., Carrete, M. & Maňosa, S. 2011. Can intensive agricultural landscapes favour some raptor species? The Marsh harrier in north-eastern Spain. Anim. Conserv. 14: 382–390. doi: 10.1111/j.1469-1795.2011.00449.x
- Chodkiewicz, T., Neubauer, G., Chylarecki, P., Sikora, A., Cenian, Z., Ostasiewicz, M., Wylegała, P., Ławicki, Ł., Smyk, B., Betleja, J., Gaszewski, K., Górski, A., Grygoruk, G., Kajtoch, Ł., Kata, K., Krogulec, J., Lenkiewicz, W., Marczakiewicz, P., Nowak, D., Pietrasz, K., Rohde, Z., Rubacha, S., Stachyra, P., Świętochowski, P., Tumiel, T., Urban, M., Wieloch, M., Woz´niak, B., Zielińska, M. & Zieliński, P. 2013. Monitoring ptaków Polski w latach 2012–2013. Biuletyn Monitoringu Przyrody 11: 1–72.
- Cramp, S. & Simmons, K.E.L. 1980. The Birds of the Western Palearctic, vol. II. Oxford University Press, London.
- Evans, K.L. 2004. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146: 1–13. doi: 10.1111/j.1474-919X.2004.00231.x
- Gillings, S., Henderson, I.G., Morris, A.J. & Vickery, J.A. 2010. Assessing the implications of the loss of set-aside for farmland birds. Ibis 152: 713–723. doi: 10.1111/j.1474-919X.2010.01058.x
- Goszczyński, J. 1995. Lis. OIKOS, Warsaw.
- Goszczyński, J. & Piłatowski, T. 1986. Diet of common buzzards (Buteo buteo L.) and goshawks (Accipiter gentilis L.) in the nesting period. Ekologia Polska 34: 655–667.
- Graham, I.M., Redpath, S.M. & Thirgood, S.J. 1995. The diet and breeding density of Common Buzzards Buteo buteo in relation to indices of prey abundance. Bird Study 42: 165–173. doi: 10.1080/00063659509477162
- Green, R.E., Cornell, S.J., Scharlemann, J.P.W. & Balmford, A. 2005. Farming and the fate of wild nature. Science 307: 550–555. doi: 10.1126/science.1106049
- GUS. 2012. Statistical Yearbook of Agriculture 2012. Central Statistical Office, Warsaw.
- Jankowiak, Ł. & Tryjanowski, P. 2013. Cooccurrence and food niche overlap of two common predators (red fox Vulpes vulpes and common buzzard Buteo buteo) in an agricultural landscape. Turk. J. Zool. 37: 157–162.
- Jędrzejewska, B. & Jędrzejewski, W. 1998. Predation in Vertebrate Communities. The Białowieża Forest as a Case Study. Springer, Berlin.
- Kleijn, D., Kohler, F., Báldi, A., Batáry, P., Concepción, E.D., Clough, Y., Díaz, M., Gabriel, D., Holzschuh, A., Knop, E., Kovács, A., Marshall, E.J.P., Tscharntke, T. & Verhulst, J. 2009. On the relationship between farmland biodiversity and land-use intensity in Europe. Proc. R. Soc. B 276: 903–909. doi: 10.1098/rspb.2008.1509
- Korpimäki, E. 1986. Diet variation, hunting habitat and reproductive output of the Kestrel Falco tinnunculus in the light of the optimal diet theory. Ornis Fenn. 63: 84–90.
- Krüger, O. 2002. Dissecting common buzzard lifespan and reproductive success: the relative importance of food, competition, weather, habitat and individual attributes. Oecologia 133: 474–482. doi: 10.1007/s00442-002-1053-y
- Krüger, O. 2004. The importance of competition, food, habitat, weather and phenotype for the reproduction of Buzzard Buteo buteo. Bird Study 51: 125–132. doi: 10.1080/00063650409461344
- Laine, K. & Henttonen, H. 1983. The role of plant production in microtine cycles in northern Fennoscandia. Oikos 40: 407–418. doi: 10.2307/3544313
- Mackin-Rogalska, R., Adamczewska-Andrzejewska, K. & Nabagło, L. 1986. Common vole numbers in relation to the utilization of burrow systems. Acta Theriol. 31: 17–44. doi: 10.4098/AT.arch.86-2
- Mebs, T. 1964. Zur Biologie und Populationsdynamik des Mäusebussards (Buteo buteo) (Unter besonderer Berücksichtigung der Abhängigkeit vom Massenwechsel der Feldmaus Microtus arvalis). J. Ornithol. 105: 247–306. doi: 10.1007/BF01672243
- Newton, I. 1979. Population Ecology of Raptors. Poyser, Berkhamsted.
- Newton, I. 2004. The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146: 579–600. doi: 10.1111/j.1474-919X.2004.00375.x
- Norrdahl, K. & Korpimäki, E. 2000. Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos 91: 528–540. doi: 10.1034/j.1600-0706.2000.910315.x
- Orłowski, G., Czarnecka, J. & Panek, M. 2011. Autumn-winter diet of Grey Partridge Perdix perdix in winter crops, stubble fields and fallows. Bird Study 58: 473–486. doi: 10.1080/00063657.2011.606498
- Pielowski, Z. 1991. The population and breeding success of predatory birds on farmland near Czempiń (Western Poland). Acta Ornithol. 26: 107–118.
- Ryszkowski, L. 1982. Structure and function of the mammal community in an agricultural landscape. Acta Zool. Fenn. 169: 45–59.
- Salamolard, M., Butet, A., Leroux, A. & Bretagnolle, V. 2000. Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 81: 2428–2441. doi: 10.1890/0012-9658(2000)081[2428:ROAAPT]2.0.CO;2
- Schindler, S., Hohmann, U., Probst, R., Nemeschkal, H.-L. & Spitzer, G. 2012. Territoriality and habitat use of Common Buzzards (Buteo buteo) during late autumn in northern Germany. J. Raptor Res. 46: 149–157. doi: 10.3356/JRR-11-22.1
- Selås, V. 2001. Breeding density and brood size of common buzzard Buteo buteo in relation to snow cover in spring. Ardea 89: 471–479.
- Sim, I.M.W., Cross, A.V., Lamacraft, D.L. & Pain, D.J. 2001. Correlates of Common Buzzard Buteo buteo density and breeding success in the West Midlands. Bird Study 48: 317–329. doi: 10.1080/00063650109461231
- Skierczyński, M. 2006. Food niche overlap of three sympatric raptors breeding in agricultural landscape of Western Pomerania region of Poland. Buteo 15: 17–22.
- Spidsø, T.K. & Selås, V. 1988. Prey selection and breeding success in the common buzzard Buteo buteo in relation to small rodents cycles in southern Norway. Fauna Norv. Ser. C 11: 61–66.
- Stoate, C., Boatman, N.D., Borralho, R.J., Rio Carvalho, C., de Snoo, G.R. & Eden, P. 2001. Ecological impacts of arable intensification in Europe. J. Environ. Manage. 63: 337–365. doi: 10.1006/jema.2001.0473
- Stoate, C., Báldi, A., Beja, P., Boatman, N.D., Herzon, I., van Doorn, A., de Snoo, G.R., Rakosy, L. & Ramwell, C. 2009. Ecological impacts of early 21st century agricultural change in Europe – a review. J. Environ. Manage. 91: 22–46. doi: 10.1016/j.jenvman.2009.07.005
- Streich, W.J., Litzbarski, H., Ludwig, B. & Ludwig, S. 2006. What triggers facultative winter migration of Great Bustard (Otis tarda) in Central Europe? Eur. J. Wildl. Res. 52: 48–53. doi: 10.1007/s10344-005-0007-1
- Swann, R.L. & Etheridge, B. 1995. A comparison of breeding success and prey of the Common Buzzard Buteo buteo in two areas of northern Scotland. Bird Study 42: 37–43. doi: 10.1080/00063659509477146
- Tkadlec, E., Zbořil, J., Losík, J., Gregor, P. & Lisická, L. 2006. Winter climate and plant productivity predict abundance of small herbivores in central Europe. Clim. Res. 32: 99–108. doi: 10.3354/cr032099
- Tomiałojc´, L. & Stawarczyk, T. 2003. Awifauna Polski. Rozmieszczenie, liczebność i zmiany. PTPP “pro Natura”, Wrocław.
- Valkama, J., Korpimäki, E., Arroyo, B., Beja, P., Bretagnolle, V., Bro, E., Kenward, R., Mañosa, S., Redpath, S.M., Thirgood, S. & Viñuela, J. 2005. Birds of prey as limiting factors of gamebird populations in Europe: a review. Biol. Rev. 80: 171–203. doi: 10.1017/S146479310400658X
- Vickery, P.D., Hunter, M.L. & Wells, J.V. 1992. Evidence of incidental nest predation and its effects on nests of threatened grassland birds. Oikos 63: 281–288. doi: 10.2307/3545389
- Village, A. 1982. The diet of Kestrels in relation to vole abundance. Bird Study 29: 129–138. doi: 10.1080/00063658209476747
- Weir, D. & Picozzi, N. 1975. Aspects of social behaviour in the Buzzard. Br. Birds 68: 125–141.
