Abstract
Capsule Wetland occupation by breeding Marsh Harriers is influenced by wetland and vegetation area, distance to other wetlands where conspecifics are present and also by the characteristics and conditions of the vegetation such as predominant species and its height at the start of the breeding period.
Aims To determine factors influencing the probability of wetland occupation by Marsh Harrier in relation to wetland dimensions, vegetation and hydric conditions, and to determine the effect of the predominant helophyte species in the wetlands and its physical characteristics.
Methods Three hundred and thirty-two wetlands were monitored during a breeding season in NW Spain, a Mediterranean area that hosts 10% of the total number of breeding pairs of Marsh Harriers in Spain. The probability of wetland occupation for breeding in terms of wetland size and proximity, vegetation characteristics, human disturbance and also hydrological variables, was analysed using logistic regression.
Results One hundred and sixty-five pairs were located in 131 wetlands. Variables affecting occupation include vegetation composition and characteristics, wetland dimensions and distance to other occupied wetlands. Wetlands with the highest probability of being occupied were larger, with greater areas of marsh vegetation, taller vegetation, rush and reed communities as the predominant species and which were closer to another wetland occupied by Marsh Harriers.
Conclusions The dimensions and predominant species of helophyte vegetation, and size and location determine the occupation of wetlands as breeding grounds by Marsh Harrier. Changes in the natural supply of water and nutrients due to the implementation of intensive irrigation farming are likely modifying flora in wetlands and affecting the distribution and population size of Marsh Harriers in this region of Spain.
Determining the spatial distribution of threatened species as well as their requirements and selection of habitat are key aspects in the development of adequate management and conservation measures. This is particularly relevant in species occupying agricultural areas where original habitats have undergone profound changes and human impact can greatly affect how species use them (Chamberlain et al. Citation2000, Donald et al. Citation2001). These changes affect the entire territory, including wetlands distributed within a matrix of agricultural habitats.
Wetlands are habitats of the highest conservation interest due to their high biological diversity (Weller Citation1999, Keddy Citation2010) and because they are the main habitat of a large number of threatened avian species. They also play a vital role in the life cycle of many of these species, for instance during breeding and migration (Tucker & Evans Citation1997). Wetlands are amongst the most altered habitats and, as is the case in other ecosystems, a consequent decrease in the number of habitat specialists has been detected (Olden et al. Citation2004). Anthropic modifications in the hydrological conditions of wetlands, burning and water pollution caused by harmful substances or fertilizers can affect the flora inhabiting them (Burke & Grime Citation1996). These alterations often result in native species being gradually replaced by others with a high capacity to colonize altered habitats (Galatowitsch et al. Citation1999). For example, different species of Typha have a greater adaptive and invasive capacity and are replacing original helophyte communities. These changes in favour of cattail communities are linked to changes in water level and particularly to an increase in nutrients (Runhaar et al. Citation1996, Newman et al. Citation1998, Woo & Zedler Citation2002). Species of flora of the highest conservation interest in Mediterranean wetlands have adapted to unstable water conditions and therefore, the change in water regime (extra input from irrigation crops) often means they lose their adaptive advantages and inevitably disappear.
Very few studies examine the relationship between changes in floristic composition in wetlands and their occupation by different avian species (Benoit & Askins Citation1999, Arbeiter & Tegetmeyer Citation2011) despite the fact that changes in emerging invasive helophytes is currently a phenomenon of great significance, and will probably become much more so in the future. In the case of the Marsh Harrier Circus aeruginosus, although there are a number of studies on breeding habitat selection, little is known about aspects such as selection depending on the helophyte community in wetlands (Stanevicius Citation2004, Cardador et al. Citation2011) and water regime.
This study analyses wetland occupation by Marsh Harrier as a nesting habitat in a Mediterranean agricultural environment. Aspects that usually determine wetland occupation by the species, such as their size, size of marsh vegetation, water regime, proximity of other occupied sites and human disturbance are considered. Vegetation characteristics including the predominant species and its height are also considered, as well as the possible effects of water input and nutrients from irrigation crops on marsh vegetation and on the use of these wetlands as a breeding habitat. We predicted larger wetlands to be occupied because they have higher water levels, are less affected by disturbances and are located close to other occupied wetlands. Moreover, changes occurring to, and agricultural intensification in the matrix where the wetlands are located, could result in water and nutrient inputs altering the composition of their floristic community, which, in turn, could affect their occupation by Marsh Harriers.
METHODS
Study system
The study area is located in the North West of the North Iberian Plateau (León region, 42°21′N, 5°26′W, ) and covers 4200 km2. The climate and topography are relatively homogenous, with gentle relief situated between 730 and 920 m altitude. The climate is Mediterranean, 400–550 mm mean annual rainfall, low rainfall in summer and 10–11°C mean annual temperature. Traditionally, this area was dedicated to rain-fed agriculture, but since the 1960s a considerable area has been transformed into irrigation crops. Water for irrigation comes from headwater reservoirs in nearby rivers in the Cantabrian mountain range, an area of high rainfall (up to 2000 mm annually). Barley, wheat and alfalfa are grown in the rain-fed areas, and there are also some vineyards, whereas corn, wheat, beans and sugar beet are the main crops in the irrigated areas.
Figure 1. Study area and census results. (a) Distribution of the Marsh Harrier Circus aeruginosus in Spain in a Universal Transverse Mercator grid of 10 km, in 2006 (Molina & Martínez Citation2008). (b) Wetlands visited (triangles) and occupied (circles) by one or more pairs of Marsh Harrier in this study.
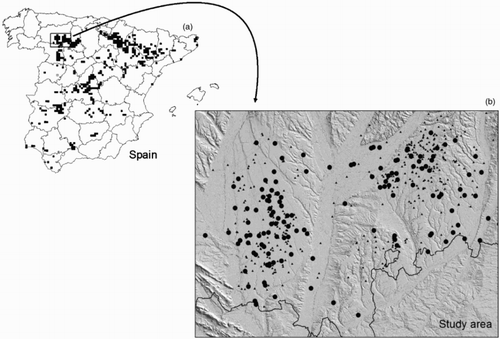
The wetlands occupied by the Marsh Harrier are mostly natural ponds and to a lesser extent streams or oxbow lakes whereas scarcely 3% of pairs nest in water bodies of anthropogenic origin. The ponds are generally endorheic and in the case of those with a natural water regime, their water level depends on rainfall. Ponds situated in irrigated areas receive extra water input from surplus water used for crop irrigation, mostly during the summer months. Rainfall in 2004 and 2005 was below normal values (compared to a mean of the last 30 years), but normal in 2002 and 2003.
The Marsh Harrier is an average-sized bird of prey (405–800 g), widely distributed throughout the European continent, though it occurs less frequently in the Mediterranean region (Cramp & Simmons Citation1980). It mostly nests in wetlands and has a generalist diet of small and medium-sized vertebrates (Cramp & Simmons Citation1980). Marsh Harriers are sensitive to alterations and changes in habitat, though they also shows a high degree of plasticity in habitat choice (Stanevicius Citation2004, Cardador et al. Citation2011, Sternalski et al. Citation2013).
During the middle of the 20th century, Marsh Harrier populations decreased noticeably in Europe, but since then they have recovered, particularly between 1970 and 1990, and now appear to be stable, or fluctuating slightly (Bird Life International Citation2004). The decrease in population appears to have been the result of direct persecution, poisoning and hunting, and the drying up of wetlands (Jubete Citation2003, Molina & Martínez Citation2008). In Spain, populations have changed positively from minimum numbers of fewer than 300 pairs at the end of the 1980s to almost 1500 pairs in 2006. In 1990, there were 16 pairs of Marsh Harriers in the study area and serious conservation problems due to nest plundering and burning in wetlands were reported (Martínez et al. Citation1990). Subsequent surveys show a positive trend because in 1994 there were 26 pairs and 56 in 1999 (García et al. Citation2008). The study area has one of the largest populations in the Iberian Peninsula, approximately 10% of all breeding pairs in Spain (Molina & Martínez Citation2008).
The wetlands in the study area were selected by reviewing published maps (Military Mapping of Spain, Series L Scale 1:50 000 and the National Topographic Map of Spain. IGN Scale 1:25 000), and by aerial orthophotography (Ministry of Agriculture SIGPAC (System for Geographic Information on Agricultural Parcels) equivalent Scale 1:10 000). The review of wetlands included all those occupied by the Marsh Harrier in previous censuses and studies (Martínez et al. Citation1990, Martínez et al. Citation1996). Five hundred and twelve wetlands were located in the study area and explored. Those lacking vegetation due to drought, burning, dumping rubble, drainage and so on were considered unsuitable. Three hundred thirty-two wetlands with marsh vegetation were selected to be studied and monitored.
The wetlands were visited on two occasions during the 2005 breeding season, first during the pre-laying period, between 20 March and 20 April, when the adults remain in the area close to the nest for a longer period of time and signs of display and nest-building are more frequent (Fernández & Azkona Citation1993, Citation1994), and then in May to confirm occupancy and the number of pairs. A wetland was considered to be occupied when one or more pairs of Marsh Harriers showed signs of breeding behaviour, mating, carrying nest material, delivering prey or defending a territory. The number of pairs or breeding units was established according to the maximum number of located females showing breeding behaviour given that polygyny is common in the species (Cramp & Simmons Citation1980), and despite the fact that some males display female plumage (Sternalski et al. Citation2012) Intensive sampling was not carried out to detect nesting pairs amongst cereal crops but visits were made to similar areas where nesting had previously occurred. Overall, in the 131 occupied wetlands, 165 pairs bred (mean ± sd: 1.26 ± 0.93, range: 1–10). One further pair was detected in cereal crops and was not considered in subsequent analyses.
Data analysis
To evaluate the occupation of wetlands by the Marsh Harrier, we applied a logistic regression model (generalized linear model) with a binomial error structure and a logit-link function. The dependent variable was coded as either 1 (wetland occupied) and 0 (wetland not occupied). As many as eight variables considered a priori as factors determining the presence of the Marsh Harrier were measured. These variables were related to characteristics of wetlands and marsh vegetation, hydrological factors, human disturbance and conspecific distribution (). The characteristics of the wetlands with regard to water level and regime (artificial regime is water is supplied from nearby irrigated plots and natural if water input is rainwater only) and marsh vegetation (predominant helophyte species, vegetation height and dimensions) were measured in the wetlands themselves during field trips. As values for vegetation height and water level can vary noticeably throughout the census period, these data were analysed based on the values obtained on the first visit, at the time when Marsh Harriers occupied the wetland. Three types of community were identified according to the physiognomy of the vegetation and predominant helophyte species: a cattail community dominated by Typha domingensis and Typha latifolia, a reed community dominated by Phragmites australis, and a rush community mainly dominated by Schoenoplectus lacustris and Bolboschoenus maritimus to a lesser extent. Although communities usually have one predominant species, they are normally accompanied by others belonging to the genera Carex, Sparganium, Scirpoides and Juncus. Geographical and topographical data, wetland dimensions and distance to human infrastructures were calculated by analysing aerial orthophotographs (Ministry of Agriculture. SIGPAC (Sistema de Información Geográfica de Parcelas Agrícolas) equivalent Scale 1:10 000). Floristic composition was analysed between the different water regimes using a Chi-square test.
Table 1. Explanatory variables used to model the probability of wetland occupation by Marsh Harriers Circus aeruginosus in the study area.
Two logistic regression models were made: a full model that included all the considered variables () and the minimum adequate model, calculated by removing non-significant variables (α = 0.05) through backwards stepwise selection. Variables were eliminated based on Akaike's information criterion (AIC) (Burnham & Anderson Citation2002) and the procedure for simplification of the model continued until the AIC criterion could not be improved. Data analyses were carried out with the R, version 3.1.2 (R Core Team Citation2014).
RESULTS
The minimum adequate model for wetland occupation shows that the variables that contribute most are surface area of wetland, minimum width of helophytic vegetation, predominant species of helophytic communities, height of helophytic vegetation and proximity to other occupied sites. However, distance to human disturbance, and hydrological factors such as water regime do not appear in the most parsimonious models ().
Table 2. Generalized linear model results obtained for the probability of wetland occupancy by Marsh Harriers Circus aeruginosus in the study area. Surfacewetland: Surface of wetland, Minwidhtveg: Minimum width of helophytic vegetation, Distancedisturbance: Distance to human disturbance, Surroundingareas: Surrounding areas (irrigation or rain-fed), Watereg: Water regime, Heightvegetation: Height of helophytic vegetation, Helophytic: Helophytic communities (Reed: Reed communities, Cattail: Cattail communities), Waterlevel: Water level and Distanceoccupied: Proximity to other sites occupied by Marsh Harrier.
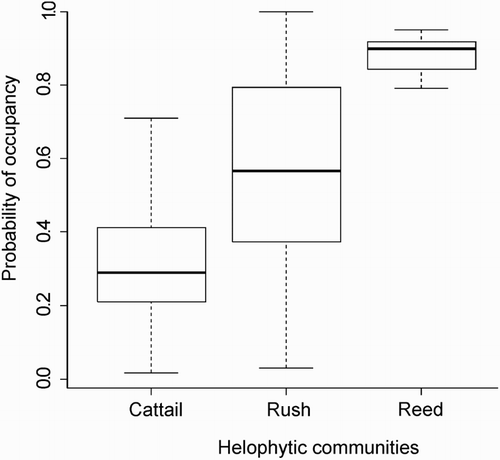
Larger wetlands, with greater dimensions of helophytic vegetation showed a greater probability of being occupied. The mean probability of occupation when vegetation height at the start of breeding was low was scarcely 8.3% whereas it reached 44.7% when vegetation height exceeded 0.5 m. With regard to distance to the nearest occupied wetland, an increase of 250 m decreased the probability of occupation by 1%. Significant differences were detected in the occupation rate between wetlands dominated by Typha spp. and those dominated by S. lacustris and P. australis. Whereas all the studied P. australis wetlands were occupied, the probability of occupation (mean ± sd) of wetlands with rush communities was 0.55 ± 0.28, and 0.32 ± 0.20 for those with cattail communities ().
Figure 2. Probability of occupancy of wetlands by Marsh Harrier depending on the predominant helophyte species.
Significant differences were also observed in floristic composition in the different water regimes (Chi-square value = 104.9 ; P < 0.001) because reed communities only appeared in wetlands with a natural regime, and cattail communities were predominant in 90.0% of wetlands with an artificial regime, in comparison with only 40.7% of wetlands with a natural water regime. Wetlands with a natural regime had a much lower water level than those with an artificial one. Thus, more than half of wetlands with a natural regime were dry at the beginning of the breeding period compared to only 7% of wetlands with an artificial regime.
DISCUSSION
The main factors determining occupation of a wetland in the study area by Marsh Harriers were wetland and marsh vegetation dimensions, and hydrological factors. Marsh Harriers occupied larger wetlands with greater marsh vegetation dimensions for breeding, preferably close to other wetlands where the species nests. Apart from this, vegetation characteristics, including predominant species and vegetation height at the start of breeding, also influenced wetland occupancy. Wetland and vegetation characteristics are vital for a species vulnerable to egg and chick predation and changes in water level (Witkowski Citation1989, Dijkstra & Zijlstra Citation1997, Iñigo & Atienza Citation2003, Stanevicius Citation2004). Also, as in other species, the presence of conspecifics in the wetland or nearby areas can also influence the selection of a wetland for nesting (Cardador et al. Citation2011).
Wetlands dominated by different species of Typha were occupied noticeably less than those occupied by rush and reed communities. This is probably because cattail communities are more sensitive than rush and reed communities to environmental factors with regard to maintaining their vertical structure and therefore their suitability for breeding Marsh Harriers. For example, rising water levels, wind, the existence of a water current or amount of snowfall during the winter prior to breeding can destroy or crush the vegetation thus limiting or even preventing occupation by Marsh Harriers. In fact, vegetation height is one of the main factors determining the occupation of a wetland. The protection given by greater vegetation cover has been positively related to productivity in the Marsh Harrier, though not to brood size (Villarán Citation2000).
Water conditions that determine the input of water from irrigation favour the presence of certain vegetation communities over others. Thus, prolonged flooding is frequent in wetlands receiving surplus irrigation water, and this causes the rush-meadow to act as a low-lying helophytic community, and also at times favours the greater colonizing potential of the cattail community. This process is also favoured by eutrophication (Newman et al. Citation1998). In the study area, cattail communities have colonized almost all of the wetlands that receive water input from irrigation (in comparison with only 40% in wetlands with a natural regime) and reed communities are only present in wetlands with a natural regime. Moreover, it has been pointed out that agricultural and livestock activities in the surrounding areas or the wetlands themselves, and their consequent eutrophication and siltation, are amongst the principal threats to the richness and diversity of water plants (Del Pozo et al. Citation2012).
Although the entire study area is dedicated to crop production, which is much more intensive in irrigated areas, human disturbance measured as distance to paths, roads and populations, was not a factor affecting wetland occupation. This is in spite of the fact that human disturbance (direct persecution, agro-pastoral activities and lead-poisoning) is a key factor in wetland occupation in many of the areas in Europe where Marsh Harriers are found and can affect different aspects during the breeding period such as breeding effort, nest defense or provision of prey for offspring (Fernández & Azkona Citation1993, Íñigo & Atienza Citation2003, Stanevicius Citation2004).
Many birds of prey are habitat specialists and given their position at the top of the food chain, have traditionally been considered indicators of habitat quality (Sergio et al. Citation2008). Nevertheless, the rapid recovery of the Marsh Harrier population and its use of artificial wetlands and intensively farmed habitats have led to the validity of this species as a biological indicator to be questioned (Cardador et al. Citation2011). Generally speaking, specialist species show a preference for habitats that are relatively homogenous in space and time; they have low dispersal capacity and limited capacity to respond to environmental stochasticity (Kassen Citation2002, Sol et al. Citation2002, Tripet et al. Citation2002). The results obtained in this study seem to indicate that the Marsh Harrier is a species highly adapted to intensive farming and the profound alterations that it has caused in the area. Nevertheless, as occurs in other specialist species, its geographical range has not extended significantly despite the increase in population, and it continues to show some preference for nesting habitats that maintain certain natural conditions (Devictor et al. Citation2008).
Despite the high population density in the study area, distance to the nearest occupied wetland was an important factor in occupation, in contrast to other regions in the Iberian Peninsula (Cardador et al. Citation2011) where availability of wetlands is lower. This situation, together with the fact that wetland availability is still high, would suggest that we are not dealing with a population at carrying capacity. Also, the number of pairs nesting in cereal crops is insignificant (only one in 2005): the use of nesting sites other than helophytic vegetation, often close to occupied wetlands, has been used as an argument to confirm wetland saturation (Traverso et al. Citation2003) or the lack of suitable habitat (Martínez et al. Citation1996).
The Marsh Harrier nesting population has recovered significantly throughout its range. The recovery process has taken place very rapidly in Spain and Europe where it is one of the populations of migrating species that has increased most rapidly in the last three decades (Vickery et al. Citation2014). In Spain, between 1990 and 2006, the number of breeding pairs tripled, and in the study area, which has one of the largest breeding populations in Spain, the population has increased tenfold; in fact, it has increased from 5% of the breeding population in Spain in 1990 to almost 10% (Molina & Martínez Citation2008). But in spite of the great plasticity in breeding habitat choice, in a scenario of climate change and extreme meteorological phenomena (drought), many wetlands with a natural regime will probably lose their suitability as nesting areas in more years. Additionally, although wetland vegetation has adapted to alternating periods of drought and flooding, the foreseeable increase in periods of drought (due to climate change) may affect floristic composition, as is occurring in many Mediterranean areas (Radford et al. Citation2011), and this may in turn affect availability of breeding habitats for Marsh Harriers.
ACKNOWLEDGEMENTS
Our thanks to Marcelino de la Cruz and Marcos Méndez for their help with the statistical analysis and Soraya de Elera and Lorenzo Miguélez for their help during the Marsh Harrier surveys. Emilio de la Calzada provided data on historical census and Catherine Martin helped with language.
REFERENCES
- Arbeiter, S. & Tegetmeyer, C. 2011. Home range and habitat use by Aquatic Warblers Acrocephalus paludicola on their wintering grounds in Northwestern Senegal. Acta Ornithol. 46: 117–126. doi: 10.3161/000164511X625883
- Benoit, L.K. & Askins, R.A. 1999. Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19: 194–208. doi: 10.1007/BF03161749
- BirdLife International. 2004. Birds in Europe: Population Estimates, Trends and Conservation Status. BirdLife Conservation Series, no 12. BirdLife International, Cambridge.
- Burke, M.J.W. & Grime, J.P. 1996. An experimental study of plant community invasibility. Ecology 77: 776–790. doi: 10.2307/2265501
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer-Verlag, Berlin.
- Cardador, L., Carrete, M. & Mañosa, S. 2011. Can intensive agricultural landscapes favour some raptor species? The Marsh harrier in north-eastern Spain. Anim. Conserv. 14: 382–390. doi: 10.1111/j.1469-1795.2011.00449.x
- Chamberlain, D.E., Fuller, R.J., Bunce, R.G.H., Duckworth, J.C. & Shrubb, M. 2000. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J. Appl. Ecol. 37: 771–788. doi: 10.1046/j.1365-2664.2000.00548.x
- Cramp, S. & Simmons, K.E.L. (eds) 1980. Handbook of the Birds of Europe, the Middle East and North Africa, Vol. II. Oxford University Press, Oxford.
- Del Pozo, R., Fernández-Aláez, M. & Fernández-Aláez, C. 2012. Composición de las comunidades de macrófitos y establecimiento del estado de conservación de charcas y lagunas de la Depresión del Duero (Noroeste de España) en base a criterios botánicos. Limnetica 31: 47–58.
- Devictor, V., Julliard, R., Jiguet, F. & Couvet, D. 2008. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117: 507–514. doi: 10.1111/j.0030-1299.2008.16215.x
- Dijkstra, C. & Zijlstra, M. 1997. Reproduction of the Marsh Harrier Circus aeruginosus in recent land reclamations in the Netherlands. Ardea 85: 37–50.
- Donald, P.F., Green, R.E. & Heath, M.F. 2001. Agricultural intensification and the collapse of Europe's farmland bird populations. Proc. Roy. Soc. Lond. 268: 25–29. doi: 10.1098/rspb.2000.1325
- Fernández, C. & Azkona, P. 1993. Human disturbance affects parental care of Marsh Harriers and nutritional status of nestlings. J. Wildlife Manage. 57: 602–608. doi: 10.2307/3809289
- Fernández, C. & Azkona, P. 1994. Aerial food transfer as a demand behavior in the marsh Harrier. J. Field. Ornithol. 65: 109–114.
- Galatowitsch, S.M., Anderson, N.O. & Ascher, P.D. 1999. Invasiveness in wetland plants in temperate North America. Wetlands 19: 733–755. doi: 10.1007/BF03161781
- García, J., Ramos, L.A. & Vázquez, X. 2008. Atlas de las aves reproductoras de León. Diputación de León, León.
- Iñigo, A. & Atienza, J.C. 2003. Censo de la población reproductora y caracterización del hábitat del aguilucho lagunero occidental (Circus aeruginosus) en la comunidad de Madrid. Año 2002. Anuario Ornitológico de Madrid 2002: 92–103.
- Jubete, F. 2003. Aguilucho lagunero, Circus aeruginosus. In Martí, R. & del Moral, J.C. (eds) Atlas de las aves reproductoras de España, 174–175. Dirección General de Conservación de la Naturaleza–Sociedad Española de Ornitología, Madrid.
- Kassen, R. 2002. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 15: 173–190. doi: 10.1046/j.1420-9101.2002.00377.x
- Keddy, P.A. 2010. Wetlands Ecology. Cambridge University Press, Cambridge.
- Martínez, F.J., de la Calzada, E. & Otero, M. 1990. El Aguilucho Lagunero Circus aeruginosus en la provincia de León. Tierras de León 79–80: 83–95.
- Martínez, F., Ortega, A. & Jubete, F. 1996. Situación actual del aguilucho lagunero (Circus aeruginosus) en España. Reproducción e invernada. In Muntaner, J. & Mayol, J. (eds) Biología y conservación de las Rapaces Mediterráneas, 1994, 451–458. Monografías, no 4 SEO, Madrid.
- Molina, B. & Martínez, F. 2008. El aguilucho lagunero en España. Población en 2006 y método de censo. SEO/BirdLife, Madrid.
- Newman, S., Schuette, J., Grace, J.B., Rutchey, K., Fontaine, T. Reddy, K.R. & Peitrucha, M. 1998. Factors influencing cattail abundance in the northern Everglades. Aquat. Bot. 60: 265–280. doi: 10.1016/S0304-3770(97)00089-2
- Olden, J.D., Poff, N.L., Douglas, M.B., Douglas, M.E. & Fausch, K.D. 2004. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19: 18–24. doi: 10.1016/j.tree.2003.09.010
- Radford, E.A., Catullo, G. & de Montmollin, B. (eds) 2011. Important Plant Areas of the South and East Mediterranean Region: Priority Sites for Conservation. IUCN, Gland and Málaga.
- R Core Team. 2014. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. URL http://www.R-project.org/
- Runhaar, J., van Gool, C.R. & Groen, C.L.G. 1996. Impact of hydrological changes on nature conservation areas in The Netherlands. Biol. Conserv. 76: 269–276. doi: 10.1016/0006-3207(95)00119-0
- Sergio, F., Caro, T., Brown, D., Clucas, B., Hunter, J., Ketchum, J., McHugh, K. & Hiraldo, F. 2008. Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Ann. Rev. Ecol. Evol. Syst. 39: 1–19. doi: 10.1146/annurev.ecolsys.39.110707.173545
- Sol, D., Timmermans, S. & Lefebvre, L. 2002. Behavioural flexibility and invasion success in birds. Anim. Behav. 63: 495–502. doi: 10.1006/anbe.2001.1953
- Stanevicius, V. 2004. Nest site selection by Marsh harrier (Circus aeruginosus) in the shore belt of helophytes on large lakes. Acta Zoologica Lituanica 14: 47–53. doi: 10.1080/13921657.2004.10512591
- Sternalski, A., Mougeot, F. & Bretagnolle, V. 2012. Adaptive significance of permanent female mimicry in a bird of prey. Biol. Lett. 8: 167–170. doi: 10.1098/rsbl.2011.0914
- Sternalski, A., Blanc, J.-F., Augiron, S., Rocheteau, V. & Bretagnolle, V. 2013. Comparative breeding performance of Marsh Harriers Circus aeruginosus along a gradient of land-use intensification and implications for population management. Ibis 155: 55–67. doi: 10.1111/ibi.12003
- Traverso, J.M., Martínez, F., López & Salmerón, F. 2003. Población reproductora e invernante de aguilucho lagunero occidental (Circus aeruginosus) en Madrid. Año 2002. Anuario ornitológico de Madrid 2002: 104–109.
- Tripet, F., Christe, P. & Møller, A.P. 2002. The importance of host spatial distribution for parasite specialization and speciation: comparative study of bird leas (Siphonapter: Ceratphyllidae). J. Anim. Ecol. 71: 735–748. doi: 10.1046/j.1365-2656.2002.00639.x
- Tucker, G.M. & Evans, M.I. 1997. Habitats for Birds in Europe: A Conservation Strategy for the Wider Environment. Birdlife International, Cambridge.
- Vickery, J.A., Ewing, S.R., Smith, K.W., Pain, D.J., Bairlein, F., Škorpilová, J. & Gregory, R.D. 2014. The decline of Afro-Palaearctic migrants and an assessment of potential causes. Ibis 156: 1–22. doi: 10.1111/ibi.12118
- Villarán, A. 2000. Parámetros reproductivos del aguilucho lagunero occidental Circus aeruginosus en el sur de Madrid. Ecología 14: 285–290.
- Weller, M.W. 1999. Wetland Birds. Cambridge University Press, Cambridge.
- Witkowski, J. 1989. Breeding biology and ecology of the Marsh Harrier (Circus aeruginosus) in the Barycz Valley, Poland. Acta Ornithol. 25: 223–320.
- Woo, I. & Zedler, J.B. 2002. Can nutrients alone shift a sedge meadow towards dominance by the invasive Typha x glauca? Wetlands 22: 509–521. doi: 10.1672/0277-5212(2002)022[0509:CNASAS]2.0.CO;2
