Abstract
Capsule Corn Buntings nested in tall dense grasses and cereals, selecting for sward density over height, and cereal fields with high weed scores.
Aims To measure the vegetation attributes of fields selected as nest sites by Corn Buntings, to inform the design of safe nesting habitat measures in agri-environment schemes for this rapidly declining ground-nesting passerine.
Methods Nesting activity was monitored across 32 farms in eastern Scotland during 2004–09. Land use and singing males were mapped to measure habitat availability for nesting females within their mate's territory, and crop swards measured. Effects of sward characteristics on field selection for nesting, and seasonal variation in crop use, were modelled.
Results Nests (95% of 580) were mainly in cereals and grasses. Taller denser swards were selected, with sward density a stronger predictor of field use than sward height, and cereal field use strongly associated with high weed scores. Variation in sward structure between crop types, and changes due to crop maturation or harvesting, largely explained seasonal patterns in crop use.
Conclusion Conservation measures targeting nesting Corn Buntings should provide uncut or late-cut grasses or cereals 30–100 cm tall with a dense ground layer of weeds or crop vegetation.
Selecting a safe nest site is critical for birds, as exposure to adverse weather or discovery by a predator could threaten the survival of adults, eggs or chicks. Ground-nesting birds are especially vulnerable, being accessible to a wider range of predators than arboreal nesters (Weidinger Citation2002). Adaptations by ground-nesters to minimize predation risk include colonial nesting, selection of less accessible nest sites such as rock ledges or holes, and camouflage or concealment within vegetation. The strategy used largely determines the preferred nesting habitat, and nest site selection is often a trade-off between concealment and detection of potential predators (Gotmark et al. Citation1995). Nesting on bare ground or in short vegetation allows early visual detection of an approaching predator, enabling the incubating bird to leave the nest in good time, often followed by distraction to lure the predator away. Examples of European farmland species that use this strategy include Northern Lapwing Vanellus vanellus, Eurasian Stone-curlew Burhinus oedicnemus and Eurasian Skylark Alauda arvensis (Wilson et al. Citation1997, Green et al. Citation2000, Sheldon et al. Citation2005). Others, such as Grey Partridge Perdix perdix, Corncrake Crex crex and Little Bustard Tetrax tetrax rely mainly on plumage crypsis, sitting tight to avoid detection as a predator passes, so they nest in dense vegetation that offers good concealment (Rands Citation1986, Green & Stowe Citation1993, Bretagnolle et al. Citation2011).
In growing crops, sward structure can vary greatly over a few weeks, with applications of inorganic fertilizers accelerating growth, herbicide use desiccating weed vegetation, and mowing or harvesting causing immediate loss of height. Consequently, changes in sward structure are much more rapid than in natural grasslands (Vickery et al. Citation2001), altering habitat conditions for nesting birds very quickly, and intensive agricultural management of crops and grassland therefore poses challenges. Increased losses of nests, young and adults to mowing or harvesting have caused population declines of many ground-nesting European farmland birds such as Corncrake, Whinchat Saxicola rubetra and Little Bustard (Green & Stowe Citation1993, Grüebler et al. Citation2008, Bretagnolle et al. Citation2011), whilst a trend towards autumn-sown crops that create tall, dense homogenous swards earlier in the breeding season has reduced the reproductive success of Northern Lapwing, Eurasian Stone-curlew, Eurasian Skylark and Western Yellow Wagtail Motacilla flava flavissima (Wilson et al. Citation2005). In the latter cases, rapid crop growth prevents nesting attempts or obliges birds to nest in sub-optimal sites with high predation risk (Wilson et al. Citation1997, Donald et al. Citation2002, Gilroy et al. Citation2011). Effective conservation for crop-nesting species therefore requires within-field agri-environment measures that provide suitable vegetation structure, combined with control over the timing of agricultural operations (Green et al. Citation1997, Citation2000, Bretagnolle et al. Citation2011).
Here we consider a crop-nesting farmland bird, the Corn Bunting Emberiza calandra, which is territorial during the breeding season and combines camouflage with concealment for nest protection. Corn Bunting numbers declined by almost two-thirds across 20 European countries between 1980 and 2012 (PECBMS Citation2014), driven in part by increased nest losses due to agricultural intensification, including earlier and more frequent crop harvests (Brickle & Harper Citation2002). Late onset of breeding, and frequent second-brood attempts, make this species especially vulnerable to in-field farming operations (Crick et al. Citation1994, Perkins et al. Citation2013). The Corn Bunting is therefore a species of high conservation concern, and although interventions to delay crop harvest or leave patches uncut have proved successful (Perkins et al. Citation2011, Setchfield et al. Citation2012), a better understanding of the vegetation attributes of fields selected for nesting, and mechanisms explaining their selection, will improve the design of such measures.
Previously, we have shown that crop associations of territorial male Corn Buntings in eastern Scotland change as the breeding season progresses, with autumn-sown Barley Hordeum vulgare, forage grasses and spring-sown cereals all favoured early in the breeding season, but territories dominated by autumn-sown crops often abandoned from July onwards (Perkins et al. Citation2012). Following from that work, and using more study sites, here we hypothesized that crop selection by nesting females would also change as the breeding season progressed. Although breeding habitat selection by male and female Corn Buntings at the territory scale is not independent, the female undertakes nest building and incubation alone (Hartley & Shepherd Citation1994a) and is therefore the sex most likely to select the nest location within the male's territory. Hartley & Shepherd's (Citation1995) findings that polygyny was apparently due to a random female settlement pattern amongst the nesting habitat supports this conclusion. Incubating female Corn Buntings tend to flush at the last moment when approached by humans (Macdonald Citation1965, Brickle Citation1998), suggesting a preference for swards that give greater concealment from predators (Evans Citation2004). We therefore predicted that females would select to nest in crops with dense swards rather than short, sparse ones. Secondly, we predicted that changes in sward structure would explain observed seasonal variation in crop selection by nesting females, from autumn-sown cereals and forage grasses at the start of the breeding season to spring-sown crops later on. To further inform the design of agri-environment options, we also measured nest location distances to the nearest field edge.
METHODS
Study sites
Data collection took place at 32 farms (covering 1674–4377 ha per year) during 2004–09 in 4 regions of eastern Scotland (Aberdeenshire, 20 farms, centred approximately on 57°16ʹN 02°10ʹW; Angus, 4 farms, 56°34ʹN 02°34ʹW; Fife, 5 farms, 56°13ʹN 02°45ʹW and Inverness-shire, 3 farms, 57°29ʹN 03°57ʹW). Fields were classified as contiguous patches of the same crop or habitat type, and were mapped each year on each farm. Farmers grew autumn- and spring-sown cereals (mostly Barley and Wheat Triticum aestivum), Oilseed Rape Brassica napus, vegetables (root crops and legumes) and grass mown for silage or hay, or grazed by cattle, sheep, horses and pigs. Some fields were left uncultivated as rough grass or set-aside, and some spring-sown cereals remained unsprayed and unharvested as agri-environment measures to provide over-winter seed for birds (1–4% each year), or were sown as a cereal-legume mixture for early harvesting as arable silage (0–3% each year).
Nest monitoring
We visited farms at least weekly throughout May to early September, and repeated observations of territorial males led to most females being located. Male Corn Buntings are often polygynous (Hartley et al. Citation1995), so we always checked for additional females. We monitored nesting activity associated with all territorial males, except on three farms where high densities made this impossible. Here, monitoring focused on males whose territories included forage grasses as part of a related study (Perkins et al. Citation2013), but this is unlikely to have biased our results as other nesting habitats were also available. Nests were found by observing female behaviour (nest building, entering or leaving a nest, or feeding chicks), with frequent checks thereafter until failure or fledging. Nests themselves were visited only once, to ring chicks, to minimize the risk of predation or desertion caused by leaving a trail of scent or trampled vegetation to the nest. Instead, nest status (eggs, chicks, fledged or failed) was determined by prolonged observation (usually >1 hour) of the pair's behaviour. Some nests failed or fledged before they could be found, but we consider it likely that approximately 70–80% of nests within monitored territories were located.
Our observations of female behaviour allowed estimation of first egg dates (FED) from the mid-point of possible date ranges. We assumed a 12-day incubation following a 3-day laying period (clutch size = 4), a brood period of 9–13 days (Snow & Perrins Citation1998), and flight capability at 15 days. Thus, for a female observed nest building on 1 June, entering or leaving a nest and adjudged to be incubating on 9 June, and feeding chicks in the nest on 20 June, using the nest building and incubation observations, the FED range is 1–7 June, whereas back-calculation from the chick feeding observation gives it as 23 May–5 June. Combining both calculations, the FED range is 1–5 June, with a mid-point estimated FED of 3 June. In some cases, nests (n = 86, 15% of total) were visited to ring chicks, when back-calculation from recorded chick age confirmed the accuracy of our FED estimates (Fig. S1, see Supplemental data).
Measuring nesting habitat availability
Habitat availability for each nesting female was determined by land use within the territory of her mate, defined by a circle of 150 m radius (area = 7.02 ha) around the mapped position of the male. We chose this radius because 95% of nests lay within 150 m of the male's main song-post. Where two or more territory circles overlapped, we redrew the boundary along the mid-point of the area of overlap. Each year's territory and land use map was overlaid to determine the area of each crop type in each territory (now referred to as a ‘habitat patch’), and in the rare instances where males changed territory location between nesting attempts (n = 12), habitat availability was recalculated accordingly. Finally, we measured the distance from the mapped position of each nest to the nearest field boundary, for comparison with a set of random points. All digital mapping used MAPINFO PROFESSIONAL version 6.
Sward measurements
Crop swards were measured 1–3 times during May–August (n = 266 measurements in May–mid-June; 284 mid-June–mid-July; 264 mid-July–August) in a sample of forage grass and cereal fields on each farm (n = 89 fields of forage grasses; 238 spring-sown cereals and 85 autumn-sown cereals), with the aim of measuring all such fields available for nesting within each Corn Bunting territory. Some fields with other crop types were also measured (18 fields of rough grass/set-aside; 7 vegetables; 12 pastures and 3 newly sown grasses).
Initially, we used ten measuring points per field, but later reduced this to five to allow more fields to be measured. Resampling showed that using ten and five points per field gave very similar results (Fig. S2, see Supplemental data). At each sampling point, we took 3 measurements 30 cm apart of sward height (cm), one of weed score, and one of sward density at ground level as an index of nest concealment afforded by crop and weed vegetation. For all measurements, we used a 1 m cane marked at 10 cm intervals with 1 cm bands of coloured tape, laid flat on the ground to score weeds and density. Weed score was the number of 10 cm sections (0–10 scale) with a weed plant (dicotyledonous weeds – i.e. all non-crop plants, excluding grasses) within 5 cm of the stick. Density score was the number of coloured tape markers visible (0–10 scale) through the crop from a height of 1.5 m. To aid later interpretation of results, the inverse of the density score was used in data analyses (i.e. 0 = minimum nest concealment; 10 = maximum nest concealment). For cereals, we also recorded the stage of crop development using a simplified version of the Zadok's scoring system (Simmons et al. Citation1995): 0 = germination; 1 = seedling development; 2 = tillering; 3 = stem elongation; 4 = boot (growing head enclosed by flag leaf sheath); 5 = head emergence; 6 = flowering; 7 = milk development in kernel; 8 = dough development in kernel; 9 = ripening kernel.
Data analysis
We used scatter plots to demonstrate how crop sward characteristics varied during the growing season. We plotted height and density against date for each of the main crop types measured, and for cereals and forage grasses, we also plotted height against density to show how the relationship varied between these crop types. Finally, for the three main cereal types (spring-sown Barley, autumn-sown Barley and autumn-sown Wheat), we plotted Zadok's score against date.
To analyse seasonal variation in crop selection for nesting, we modelled the probability of habitat patch selection by a nesting female (response variable habitatpatch = selected, 1 or not selected, 0) as a function of croptype (fixed effect categorical variable with 8 levels – see ) and the interaction term croptype*FED (covariate). For this, we used a generalized linear mixed model (GLMM, using the SAS GLIMMIX procedure) with a logit-link function and binomial error distribution, and specified ln size (ha) of the habitat patch as an ‘offset’ variable. To control for spatial blocking and the repeated measures structure of the data (multiple habitat patches available per nesting attempt, nests per territory and territories per farm), we fitted farm, and nested within this, the habitatpatch*territory and territory*farm interactions as categorical random effects. The latter term had a zero covariance parameter estimate so was not retained in subsequent models. We also fitted year as a fixed categorical effect to control for temporal auto-correlation and annual weather effects on crop growth and timing of nesting. We used the Kenward–Roger method to calculate denominator degrees of freedom for tests of fixed effects (Littell et al. Citation1996).
Table 1. Parameter estimates and standard errors of predictors fitted in the GLMM; response variable habitatpatch (selected for nesting, 1 or not selected, 0) = croptype + croptype*FED, with farm and habitatpatch*territory as random effects, and ln size of habitat patch as an offset. N = 577 nesting selection events (i.e. response variable value = 1) out of 1529 trials. Parameter estimates are relative to the intercept (reference crop = PAS, and year = 2009). Random farm parameter estimate = 0.053 ± 0.047 se, habitatpatch*territory = 0.423 ± 0.160.
Finally, we analysed the effect of sward characteristics on nest habitat selection. Inter-correlations between the sward variables height and density were moderately strong (grass fields, rs = 0.620; cereal fields, rs = 0.516), so we modelled their effect on field selection separately. As before, we used GLMMs with a logit-link function and binomial error distribution to model the probability of field selection by a nesting female (response variable fieldpatch = selected, 1 or not selected, 0), and specified ln size (ha) of the field patch as an ‘offset’ variable. Fixed effects in the models were height and weeds (model a), and density (model b), and to test for residual crop type effects, fieldtype (a 2-level categorical variable – cereal, 1 or grass, 2), and the interactions fieldtype*height and fieldtype*weeds (model a), or fieldtype*density (model b). We also fitted period (3-levels – early, mid and late season, corresponding to date of sward measurement and FED of nests) and year as fixed categorical effects to check for seasonal or between-year variation in sward type selection. We also tested year* and period* interaction terms with each of the sward variables, none of which were significant and therefore not retained in the models. Random categorical effects were farm, and nested within this, the fieldpatch*territory interaction. Then, for cereals only, we fitted a third model as above, but the fixed effects were Zadokscore, period and year.
RESULTS
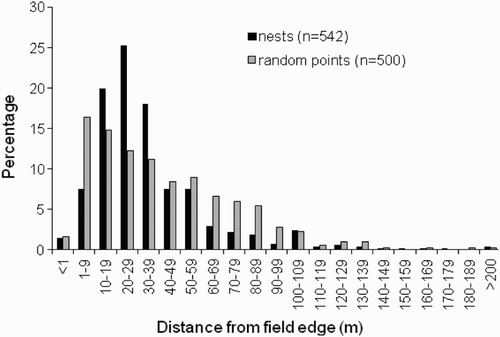
We located 580 nests, of which 93% were in arable or grass crops, the remainder being in other grassy habitats such as uncultivated fields and margins. The mean distance from a nest to the nearest field boundary was 33 m (sd = 28 m, range = 0–230 m). Most nests (72%) were <40 m, but only 9% were <10 m, leaving 63% between 10 and 39 m of a boundary (). Just 20% of nests were 50 m or more from a field boundary. For comparison, 56% of 500 randomly selected points in the same fields were <40 m from the nearest boundary, 18% were <10 m, 38% between 10 and 39 m, and 35% were at least 50 m from the field edge ().
Figure 1. Frequency distribution of nest distances from nearest field boundary, compared with randomly selected points within the same fields.
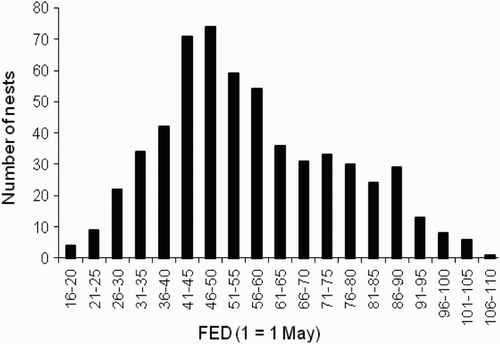
Females laid clutches between 17 May and 16 August, peaking in the second and third weeks of June (). The latest date for an active nest (13-day old chicks) was 9 September.
Sward characteristics of crop types
During May–early June, forage grasses (typically 20–60 cm) and autumn-sown cereals (60–80 cm) were the tallest crops and most spring-sown cereals were <30 cm (). Throughout June–mid-July, spring cereal heights increased rapidly to approximately 80 cm before declining from early August as crops ripened. Autumn cereal heights showed little change, and forage grass heights declined from mid-June due to harvesting of some crops, and swards partially collapsing in others. Sward heights of rough grass/set-aside varied considerably (10–90 cm), but tended to increase over time.
Figure 3. Scatter plots showing seasonal variation in sward height and density of grass and cereal fields. (a) Forage grasses height; (b) forage grass density; (c) autumn-sown cereals height; (d) autumn-sown cereals density; (e) spring-sown cereals height; (f) spring-sown cereals density; (g) rough grass/set-aside height; (h) rough grass/set-aside density. The solid line plots the mean predicted value for each date, and dotted lines the 95% confidence limits (using the SAS GPLOT procedure, specifying a cubic regression equation). Vertical lines sub-divide the plots by period (early, mid and late season).
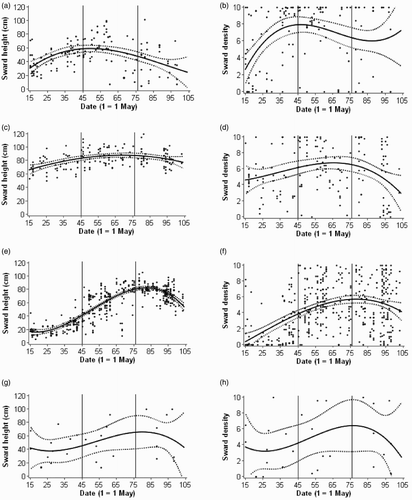
Density values varied greatly between individual fields, but overall, variation between crop types and seasonal changes reflected those of sward height (). Few spring cereals had a density value >4 during May–early June, in contrast to autumn cereals (mostly 4–6), and the densest crop type, forage grasses (6–8). Density and height were more strongly correlated in forage grasses than cereals, so sward growth from 20 to 40 to 60 cm tall, for example, gave a bigger density increase in forage grasses (from 2 to 6 to 8) than in cereals (from 1.5 to 4 to 4.5) (Fig. S3, see Supplemental data).
Weed scores were significantly higher in spring-sown than autumn-sown cereals throughout the breeding season (Kruskal–Wallis test, P < 0.001), with mean values of 5.6, 4.0 and 3.9 in spring-sown cereals in early, mid and late season, respectively, compared to corresponding mean values of 1.5, 1.7, and 1.3 in autumn-sown cereals. Weed scores also differed between grass field types in May–early June (Kruskal–Wallis test, P = 0.014), with a mean value of 4.2 in forage grasses compared to 6.8 in rough grass/set-aside.
Finally, the seasonal pattern of Zadok's score differed between the three main cereal types (Fig. S4, see Supplemental data). Autumn-sown Barley matured earliest, with average values of 5–6 during May–early June, compared with 2–5 for autumn-sown Wheat and 1–3 for spring-sown Barley. Green, part-ripe grains (Zadok's score 7) were available earliest in autumn Barley (mid-June–early July), followed by autumn Wheat (late June–July) and spring Barley (mid–late July).
Crop selection for nesting
Overall, nests were distributed non-randomly with respect to the availability of each crop type (F1508 = 9.34; P < 0.001). Most nests were in spring-sown cereals (288 nests; 50%), forage grasses (129; 22%), autumn-sown cereals (67; 12%) and other types of grass such as non-rotational set-aside, field margins and newly sown grass (66; 11%). Crop types used for nesting also varied seasonally, with five significant croptype*FED interactions (). The probability of forage grasses and pasture within a territory being used for nesting declined as the season progressed, but use of spring cereals, vegetables and newly sown grass increased (). In early season (before 10 June), probability of use for nesting was higher in forage grasses (0.6–0.8) than all other crops (<0.4), whilst from mid-June onwards, spring cereals (0.4–0.7) had the highest probability of use, except for the rare crop type newly sown grass. After mid-July, autumn cereals and forage grasses had very low probability of use (both <0.2).
Figure 4. Seasonal variation in probability of crop use for nesting, fitted using the GLMM; response variable habitatpatch (selected for nesting, 1 or not selected, 0) = croptype + croptype*FED, with farm and habitatpatch*territory as random effects, and ln size of habitat patch as an offset. (a) Crops widespread and often used for nesting and (b) crops scarce or used infrequently for nesting. * = significant seasonal trend (P < 0.05 for croptype*FED effect in ).
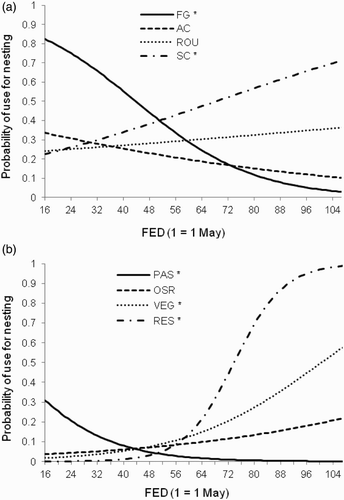
Due to land use differences between regions (less grass and more vegetables in Fife/Angus), a much higher proportion of the 125 nests in Fife/Angus were in cereals (81%) and vegetables (9%), with, by contrast to Aberdeenshire/Inverness-shire, very few nests in grass (9%).
Sward characteristics selected for nesting
Probability of field use for nesting was positively associated with sward height (F584 = 5.58, P = 0.019) and sward density (F584 = 26.99, P < 0.001), whilst a significant fieldtype*weeds effect (F584 = 19.73, P < 0.001) revealed that high weed scores attracted nesting females to cereals, but that there was a weak negative effect in grasses (, ). Raising the weed score from 2 to 8 increased the probability of cereal field use for nesting fourfold for any given crop height (e.g. from 0.06 to 0.26 for 40 cm, and 0.10 to 0.42 for 60 cm; a), but reduced the probability of grass field use by almost 40% (e.g. from 0.50 to 0.31 for 40 cm, and 0.65 to 0.41 for 60 cm; b). There was no significant difference between cereals and grasses with respect to female attraction to taller swards (fieldtype*height, F584 = 0.88, P = 0.349), or to denser swards (fieldtype*density, F611 = 0.81, P = 0.367), although the effect of sward height was stronger in cereals. An increase in sward height from 20 to 60 cm raised the probability of nesting in grass fields by almost two-thirds, whereas it almost trebled the probability of nesting in cereal fields (a, b). This level of increase in sward height was typically associated with an increase in sward density from 2 to 8 in grass fields, whereas in cereals an increase to 100 cm was usually required to achieve a similar magnitude of increase in sward density (Fig. S3, see Supplemental data). In both field types, probability of use for nesting was approximately 3–4 times greater in fields with a density score of 8 (0.44 and 0.57 in cereals and grass, respectively) than in fields with a density score of just 2 (0.11 and 0.21 in cereals and grass, respectively; c). Finally, probability of cereal field use was negatively associated with Zadok's score (F387.7 = 8.04, P = 0.005, slope parameter estimate = −0.306 ± 0.108 se).
Figure 5. Fitted relationship between crop sward characteristics and the probability of field use for nesting, using GLMMs: response variable fieldpatch selected (1) or not (0) = sward variables + fieldtype + fieldtype*sward variable interaction terms + year + period, with farm and fieldpatch*territory as random effects, and ln size of field patch as an offset. (a) Height and weeds, cereal fields; (b) height and weeds, grass fields; (c) density, cereal and grass fields. Each line represents a different value for weeds. Year is set to 2007 and period to mid. N = 123 nesting selection events out of 430 trials in cereal fields, and 78 nesting selection events out of 167 trials in grass fields.
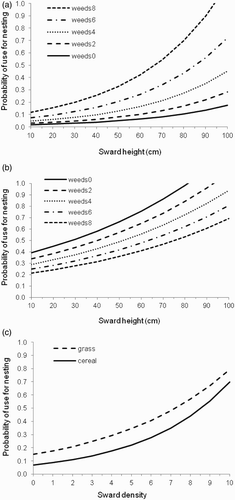
Table 2. Parameter estimates and standard errors of predictors fitted in the GLMMs; response variable fieldpatch selected (1) or not (0) = sward variables + fieldtype + fieldtype*sward variable interaction terms + year + period, with farm and fieldpatch*territory as random effects, and ln size of field patch as an offset. N = 123 nesting selection events out of 430 trials in cereal fields, and 78 nesting selection events out of 167 trials in grass fields. Parameter estimates are relative to the intercept (reference fieldtype = Grass, year = 2008, period = Mid). Random farm parameter estimate = 0.067 ± 0.105 se, fieldpatch*territory = 0.533 ± 0.236.
DISCUSSION
Seasonal variation in crop use
Corn Buntings laid clutches over a 3-month period (mid-May–mid-August) and crop use for nesting varied seasonally. During May to early June, almost two-thirds of nests were in forage grasses and autumn-sown cereals, despite these crops covering less than one-third of the study area. The probability of Corn Buntings selecting forage grasses then declined by half between late May and late June, whilst selection of spring cereals, vegetables and newly sown grass increased. By mid-July to August, two-thirds of nesting attempts were initiated in spring cereal fields, which covered only one-third of the study area. Use of set-aside and rough grass (4% of study area) did not vary seasonally, with approximately 8% of nests throughout.
The seasonal pattern of crop use for nesting reflected similar changes in habitat associations previously reported for territorial male Corn Buntings (Perkins et al. Citation2012). Mid-season habitat switching has also been reported in other multiple-brooded ground-nesting passerines, notably Eurasian Skylark, Woodlark Lullula arborea, Western Yellow Wagtail and Yellowhammer Emberiza citrinella (Wilson et al. Citation1997, Chamberlain et al. Citation1999, Bradbury et al. Citation2000, Brambilla & Rubolini Citation2009, Gilroy et al. Citation2010).
Sward types selected
We predicted that Corn Buntings would select fields with tall, dense swards for nesting. This was indeed the case, and is consistent with Hartley et al. (Citation1995) who found that Corn Buntings nested mainly beneath Hogweed Heracleum sphondylium plants which provided nest concealment in a landscape otherwise dominated by short-grazed grass swards and sparse, late-sown cereals. The closely related Yellowhammer, Ortolan Bunting Emberiza hortulana and Common Reed Bunting Emberiza schoeniclus also favour dense swards for nesting, but usually select field edge habitats such as ditchbanks (Bradbury et al. Citation2000, Brickle & Peach Citation2004, Berg Citation2008, Douglas et al. Citation2009).
In both cereals and grasses, Corn Buntings showed stronger selection for high sward density than for sward height, suggesting that swards need not be particularly tall provided there is sufficient vegetation to conceal nests. Swards of 30–40 cm height frequently had density scores ≥6 and attracted nesting Corn Buntings, especially in forage grasses. Indeed, the tallest crops, autumn-sown Oilseed Rape (1–2 m), were rarely used, as elsewhere are other tall crops such as Field Beans Vicia faba and Maize Zea mays (Gillings & Watts Citation1997, Hustings Citation1997). The dense canopy of these crops may hinder access for nesting adults, whilst at ground level an open structure easily accessible to mammalian predators may also offer little nest concealment, leading to high predation rates (Gilroy et al. Citation2011, Kirby et al. Citation2012).
Female Corn Buntings also selected to nest in cereal fields with high weed scores, consistent with habitat selection by both sexes at the territory scale (Perkins et al. Citation2012). Arable flora hosts many invertebrates essential for rearing chicks (Wilson et al. Citation1999, Brickle et al. Citation2000), and furthermore, can provide nest concealment by forming a dense ground layer within crops. This was often evident in spring-sown cereals without herbicide use or prior to spraying, where almost no bare ground was visible, and nests were difficult to see without parting the vegetation. Visual concealment can reduce nest predation in ground-nesting passerines (Weidinger Citation2002), although many mammalian predators use scent to locate nests (Davis Citation2005, Schüttler et al. Citation2009). However, dense weeds may also conceal odours from incubating females or chicks, and inhibit predator movements through a crop. A negative association with weeds in grass fields was likely due to a strong preference for nesting in the intensively managed ryegrass swards of forage grasses, which had higher density scores than rough grass or set-aside. Selection of cereals with lower Zadok's scores suggests a preference for nesting in growing crops that have not reached senescence, but may also reflect a preference for spring-sown crops which tended to be weedier than those sown in autumn.
Sward structure effects on seasonal crop use
As we predicted, variation in sward structure between crop types, and changes due to maturation or harvesting, largely explained the seasonal pattern of crop use by nesting Corn Buntings. From May to early June, Corn Buntings selected forage grasses and autumn-sown cereals because these crops had the densest swards. By contrast, spring-sown cereals were short and sparse at this time, offering little nest cover. However, the rapid growth of spring cereals and their weed flora during June to mid-July explained their increased use by nesting Corn Buntings. At the same time, mowing (to approximately 5 cm height) made forage grasses unsuitable for nesting. By mid-July to mid-August, senescence and harvesting of autumn cereals had also begun, so almost all new nests were in spring-sown crops. Eurasian Skylarks also respond to changes in sward structure by switching crops, albeit with an earlier breeding season reflecting the species’ preference for shorter, sparser swards than Corn Buntings (Wilson et al. Citation1997, Chamberlain et al. Citation1999, Hiron et al. Citation2012). By contrast, a mid-season habitat shift by Woodlarks is driven by changes in food availability, due to crop harvest reducing invertebrate abundance at lowland sites coinciding with increased insect activity in upland grasslands as temperatures rise (Brambilla & Rubolini Citation2009).
On UK farmland, the Corn Bunting is one of the latest nesting birds. The egg-laying period in our study (earliest annual FED 17 May to 4 June; latest 29 July to 16 August) was similar to other UK studies (Yom-Tov Citation1992, Hartley & Shepherd Citation1994b, Brickle & Harper Citation2002, Setchfield et al. Citation2012), although longer than for most. Factors potentially controlling the onset of breeding include availability of nest sites, food for females gaining body condition prior to laying eggs, or food for chicks later on (Newton Citation1998). Brickle & Harper (Citation2002) found that the presence of unripe grain better predicted the onset of breeding than crop height, and suggested food availability for females (ripening grains or associated invertebrates) could be the main trigger. In our study, however, Corn Buntings began nesting up to one month prior to cereals bearing ripening grains. Although we cannot discount the role of food, especially as first broods often coincided with newly available unripe grain, an important food for Corn Bunting chicks and fledglings (Watson Citation1992, Brickle & Harper Citation1999), it seems likely that sward densities in growing crops dictate the onset of nesting.
Effects of agricultural intensification on sward structure and crop use
Many of the species that created dense weed cover within cereal crops (e.g. Corn Spurrey Spergula arvensis, Chickweed Stellaria media, Fat Hen Chenopodium album, Knotgrass Polygonum aviculare, Bugloss Anchusa arvensis, Charlock Sinapis arvensis and Pineappleweed Matricaria discoidea), and also host many invertebrates (Marshall et al. Citation2003), are easily controlled by herbicides and have declined greatly in Britain during the last century (Preston et al. Citation2002). Their loss from cereal fields as agriculture has intensified could have been a major driver of Corn Bunting population declines, as demonstrated by the recent decline to extinction of one of our study populations coinciding with reduced weediness of cereal crops (Perkins et al. Citation2012). As well as reductions in chick-food invertebrates, another potential mechanism is that cleaner, weed-free crops are now less attractive to nesting Corn Buntings than the denser swards of grass habitats whose early cutting dates present high risks of nest loss (e.g. forage grasses and set-aside). This, along with an advancement of harvest dates, may have contributed to a fourfold rise from 10% to 43% of reported nest failures caused by agricultural operations between 1948–70 and 1971–91 (Crick et al. Citation1994). Widespread nesting in forage grasses in our study is a good example, as agri-environment measures to delay mowing were essential for these nests to succeed (Perkins et al. Citation2013).
Recommendations for agri-environment measures
With good targeting, agri-environment schemes can provide safe and attractive nesting habitats for Corn Buntings and reverse population declines (Perkins et al. Citation2011). We have shown that Corn Buntings select cereal or grass swards 30–100 cm tall, with a dense ground layer of vegetation that gives good nest concealment. Current UK schemes could deliver such swards via management options that restrict herbicide use in cereals or delay mowing of forage grasses. The former could allow ‘desireable’ plants, such as those listed earlier, to grow whilst selectively spraying ‘problem’ weeds such as Docks Rumex spp. and Thistles Cirsium spp. (Jones & Smith Citation2007). In two separate trials, Corn Buntings nesting in low-input cereals (Setchfield et al. Citation2012) and meadows with delayed mowing (Perkins et al. Citation2013) both had higher breeding success than those nesting in conventional cereal and forage grass crops, respectively. Combined deployment of these measures on the same farm would likely be even more effective, by safeguarding first nests in meadows and providing weed-rich late season cereal swards for some females to rear a second brood.
Alternatively, agri-environment options to create bespoke small-scale habitat patches may be more acceptable to farmers than manipulations to commercial crops (Butler et al. Citation2007). These include beetle-banks or grass margins within or surrounding arable fields, but our data suggest that they must be at least 10–30 m wide to attract nesting Corn Buntings regularly, and that swards must be denser than the adjacent crop. Margins sown with nectar flower mixtures also attract territorial Corn Buntings (Burgess et al. Citation2015), and could provide nesting habitat if swards are sufficiently tall and dense. However, in current UK schemes the maximum width for most field margin options is 6 m, although wider margins may be available from 2015 in new schemes and CAP ‘greening’ measures. Furthermore, the area required for 30 m margins around the entire perimeters of fields may not be viable either (e.g. 34% of a 10-ha field). In the present study, Corn Buntings frequently nested in ‘Unharvested Crops’, an agri-environment option for providing over-winter seed food for birds (see Perkins et al. Citation2008). With low or no use of herbicides, these 1–2 ha patches replicate a low-input spring cereal crop with dense weed growth, and inclusion of grasses or Clovers Trifolium spp. as a sown component (akin to ‘under-sowing’ in traditional cereal/grass crop rotations) gives even denser ground cover, increasing their attractiveness to nesting Corn Buntings. Such adaptations to existing agri-environment measures are valuable, because by delivering multiple objectives (in this case, winter seed food and safe nesting habitats), they improve the cost-effectiveness of schemes.
SUPPLEMENTAL DATA
Supplementary online Figures S1–S4 plotting first egg dates (S1) and sward measurements (S2) derived from alternative methods, the relationship between sward height and density in forage grasses and cereals (S3), and the seasonal pattern of crop maturity in different cereal types (S4).
Supplementary material
Download MS Word (355.5 KB)ACKNOWLEDGEMENTS
We thank Amanda Biggins, Ken Bruce, Alan Bull, Steven Coyne, Richard Firmin, Clive McKay, John McMahon, Kathleen Sinclair and Adam Watson for their assistance with fieldwork, and John Deag for valuable comments on the manuscript. We are grateful to farmers and landowners in the study area for their cooperation and interest.
REFERENCES
- Berg, A. 2008. Habitat selection and reproductive success of Ortolan Buntings Emberiza hortulana on farmland in central Sweden – the importance of habitat heterogeneity. Ibis 150: 565–573. doi: 10.1111/j.1474-919X.2008.00825.x
- Bradbury, R.B., Kyrkos, A., Morris, A.J., Clark, S.C., Perkins, A.J. & Wilson, J.D. 2000. Habitat associations and breeding success of yellowhammers on lowland farmland. J. Appl. Ecol. 37: 789–805. doi: 10.1046/j.1365-2664.2000.00552.x
- Brambilla, M. & Rubolini, D. 2009. Intra-seasonal changes in distribution and habitat associations of a multi-brooded bird species: implications for conservation planning. Anim. Conserv. 12: 71–77. doi: 10.1111/j.1469-1795.2008.00226.x
- Bretagnolle, V., Villers, A., Denonfoux, L., Cornulier, T., Inchausti, P. & Badenhausser, I. 2011. Rapid recovery of a depleted population of Little Bustards Tetrax tetrax following provision of alfalfa through an agri-environment scheme. Ibis 153: 4–13. doi: 10.1111/j.1474-919X.2010.01092.x
- Brickle, N.W. 1998. The effect of agricultural intensification on the decline of the Corn Bunting Miliaria calandra. PhD Thesis, University of Sussex.
- Brickle, N.W. & Harper, D.G.C. 1999. Diet of nestling Corn Buntings Miliaria calandra in southern England examined by compositional analysis of faeces. Bird Study 46: 319–329. doi: 10.1080/00063659909461145
- Brickle, N.W. & Harper, D.G.C. 2002. Agricultural intensification and the timing of breeding of Corn Buntings Miliaria calandra. Bird Study 49: 219–228. doi: 10.1080/00063650209461269
- Brickle, N.W. & Peach, W.J. 2004. The breeding ecology of Reed Buntings Emberiza schoeniclus in farmland and wetland habitats in lowland England. Ibis 146: 69–77. doi: 10.1111/j.1474-919X.2004.00349.x
- Brickle, N.W., Harper, D.G.C., Aebischer, N.J. & Cockayne, S.H. 2000. Effects of agricultural intensification on the breeding success of corn buntings Miliaria calandra. J. Appl. Ecol. 37: 742–755. doi: 10.1046/j.1365-2664.2000.00542.x
- Burgess, M.D., Bright, J.A., Morris, A.J., Field, R.H., Grice, P.V., Cooke, A.I. & Peach, W. 2015. Influence of agri-environment scheme options on territory settlement by Yellowhammer (Emberiza citronella) and Corn Bunting (Emberiza calandra). J. Ornithol. 156: 153–163. doi: 10.1007/s10336-014-1113-1
- Butler, S.J., Vickery, J.A. & Norris, K. 2007. Farmland biodiversity and the footprint of agriculture. Science 315: 381–384. doi: 10.1126/science.1136607
- Chamberlain, D.E., Wilson, A.M., Browne, S.J. & Vickery, J.A. 1999. Effects of habitat type and management on the abundance of skylarks in the breeding season. J. Appl. Ecol. 36: 856–870. doi: 10.1046/j.1365-2664.1999.00453.x
- Crick, H.Q.P., Dudley, C., Evans, A.D. & Smith, K.W. 1994. Causes of nest failure among buntings in the UK. Bird Study 41: 88–94. doi: 10.1080/00063659409477203
- Davis, S.K. 2005. Nest-site selection patterns and the influence of vegetation on nest survival of mixed-grass prairie passerines. Condor 107: 605–616. doi: 10.1650/0010-5422(2005)107[0605:NSPATI]2.0.CO;2
- Donald, P.F., Evans, A.D., Muirhead, L.B., Buckingham, D.L., Kirby, W.B. & Schmitt, S.I.A. 2002. Survival rates, causes of failure and productivity of Skylark Alauda arvensis nests in lowland farmland. Ibis 144: 652–664. doi: 10.1046/j.1474-919X.2002.00101.x
- Douglas, D.J.T., Vickery, J.A. & Benton, T.G. 2009. Improving the value of field margins as foraging habitat for farmland birds. J. Appl. Ecol. 46: 353–362. doi: 10.1111/j.1365-2664.2009.01613.x
- Evans, K. 2004. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146: 1–13. doi: 10.1111/j.1474-919X.2004.00231.x
- Gillings, S. & Watts, P.N. 1997. Habitat selection and distribution of corn buntings Miliaria calandra in the Lincolnshire Fens. In Donald, P.F. & Aebischer, N.J. (eds.) The Ecology and Conservation of Corn Buntings Miliaria Calandra, 139–150. Joint Nature Conservation Committee, Peterborough.
- Gilroy, J.J., Anderson, G.Q.A., Grice, P.V., Vickery, J.A. & Sutherland, W.J. 2010. Mid-season shifts in the habitat associations of Yellow Wagtails Motacilla flava breeding in arable farmland. Ibis 152: 90–104. doi: 10.1111/j.1474-919X.2009.00988.x
- Gilroy, J.J., Anderson, G.Q.A., Vickery, J.A., Grice, P.V. & Sutherland, W.J. 2011. Identifying mismatches between habitat selection and habitat quality in a ground-nesting farmland bird. Anim. Conserv. 14: 620–629. doi: 10.1111/j.1469-1795.2011.00480.x
- Gotmark, F., Blomqvist, D., Johansson, O.C. & Bergkvist, J. 1995. Nest site selection: a trade-off between concealment and view of the surroundings? J. Avian. Biol. 26: 305–312. doi: 10.2307/3677045
- Green, R.E. & Stowe, T.J. 1993. The decline of the Corncrake in Britain and Ireland in relation to habitat change. J. Appl. Ecol. 30: 689–695. doi: 10.2307/2404247
- Green, R.E., Tyler, G.A., Stowe, T.J. & Newton, A.V. 1997. A simulation model of the effect of mowing of agricultural grassland on the breeding success of the corncrake Crex crex. J. Zool. 243: 81–115. doi: 10.1111/j.1469-7998.1997.tb05758.x
- Green, R.E., Tyler, G.A. & Bowden, C.G.R. 2000. Habitat selection, ranging behaviour and diet of the stone curlew (Burhinus oedicnemus) in southern England. J. Zool. 250: 161–183. doi: 10.1111/j.1469-7998.2000.tb01067.x
- Grüebler, M.U., Schuler, H., Müller, M., Spaar, R., Horch, P. & Naef-Daenzer, B. 2008. Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird. Biol. Conserv. 141: 3040–3049. doi: 10.1016/j.biocon.2008.09.008
- Hartley, I.R. & Shepherd, M. 1994a. Female reproductive success, provisioning of nestlings and polygyny in Corn Buntings. Anim. Behav. 48: 717–725. doi: 10.1006/anbe.1994.1290
- Hartley, I.R. & Shepherd, M. 1994b. Nesting success in relation to timing of breeding in the Corn Bunting on North Uist. Ardea 82: 173–184.
- Hartley, I.R. & Shepherd, M. 1995. A random female settlement pattern can explain polygyny in the Corn Bunting. Anim. Behav. 49: 1111–1118. doi: 10.1006/anbe.1995.0139
- Hartley, I.R., Shepherd, M. & Thompson, D.B.A. 1995. Habitat selection and polygyny in breeding Corn Buntings Miliaria calandra. Ibis 137: 508–514. doi: 10.1111/j.1474-919X.1995.tb03260.x
- Hiron, M., Berg, A. & Pärt, T. 2012. Do skylarks prefer autumn sown cereals? Effects of agricultural land use, region and time in the breeding season on density. Agric. Ecosyst. Environ. 150: 82–90. doi: 10.1016/j.agee.2012.01.007
- Hustings, F. 1997. The decline of the corn bunting Miliaria calandra in The Netherlands. In Donald, P.F. & Aebischer, N.J. (eds.), The Ecology and Conservation of Corn Buntings Miliaria Calandra, 42–51. Joint Nature Conservation Committee, Peterborough.
- Jones, N.E. & Smith, B.M. 2007. Effects of selective herbicide treatment, row width and spring cultivation on weed and arthropod communities in winter wheat. Aspects. Appl. Biol. 81: 39–46.
- Kirby, W.B., Anderson, G.Q.A., Grice, P.V., Soanes, L., Thompson, C. & Peach, W.J. 2012. Breeding ecology of Yellow Wagtails Motacilla flava in an arable landscape dominated by autumn-sown crops. Bird Study 59: 383–393. doi: 10.1080/00063657.2012.715136
- Littell, R.C., Milliken, G.A., Stroup, W.W. & Wolfinger, R.D. 1996. SAS System for Mixed Models. SAS Institute Inc., Cary, NC.
- Macdonald, D. 1965. Notes on the Corn Bunting in Sutherland. Scott. Birds 3: 235–246.
- Marshall, E.J.P., Brown, V.K., Boatman, N.D., Lutman, P.J.W., Squire, G.R. & Ward, L.K. 2003. The role of weeds in supporting biological diversity within crop fields. Weed. Res. 48: 77–89. doi: 10.1046/j.1365-3180.2003.00326.x
- Newton, I. 1998. Population Limitation in Birds. Academic Press, London.
- PECBMS. 2014. Trends of Common Birds in Europe, 2014 Update. CSO/RSPB, Prague. http://www.ebcc.info/pecbm.html. (Accessed September 2014).
- Perkins, A.J., Maggs, H.E. & Wilson, J.D. 2008. Winter bird use of seed-rich habitats in agri-environment schemes. Agric. Ecosyst. Environ. 126: 189–194. doi: 10.1016/j.agee.2008.01.022
- Perkins, A.J., Maggs, H.E., Watson, A. & Wilson, J.D. 2011. Adaptive management and targeting of agri-environment schemes does benefit biodiversity: a case study of the corn bunting Emberiza calandra. J. Appl. Ecol. 48: 514–522. doi: 10.1111/j.1365-2664.2011.01958.x
- Perkins, A.J., Watson, A., Maggs, H.E. & Wilson, J.D. 2012. Conservation insights from changing associations between habitat, territory distribution and mating system of Corn Buntings Emberiza calandra over a 20-year population decline. Ibis 154: 601–615. doi: 10.1111/j.1474-919X.2012.01246.x
- Perkins, A.J., Maggs, H.E., Wilson, J.D. & Watson, A. 2013. Delayed mowing increases corn bunting Emberiza calandra nest success in an agri-environment scheme trial. Agric. Ecosyst. Environ. 181: 80–89. doi: 10.1016/j.agee.2013.09.010
- Preston, C.D., Pearman, D.A. & Dines, T.D. (eds) 2002. New Atlas of the British and Irish Flora. Oxford University Press, New York, NY.
- Rands, M.R.W. 1986. Effect of hedgerow characteristics on partridge breeding densities. J. Appl. Ecol. 23: 479–487. doi: 10.2307/2404030
- Schüttler, E., Klenke, R., McGehee, S., Rozzi, R. & Jax, K. 2009. Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol. Conserv. 142: 1450–1460. doi: 10.1016/j.biocon.2009.02.013
- Setchfield, R.P., Mucklow, C., Davey, A., Bradter, U. & Anderson, G.Q.A. 2012. An agri-environment option boosts productivity of Corn Buntings Emberiza calandra in the UK. Ibis 154: 235–247. doi: 10.1111/j.1474-919X.2011.01207.x
- Sheldon, R.D., Chaney, K. & Tyler, G.A. 2005. Factors affecting nest-site choice by Northern Lapwing Vanellus vanellus within arable fields: the importance of crop structure. Wader Study Group Bull. 108: 47–52.
- Simmons, S.R., Oelke, E.A. & Anderson, P.M. 1995. Growth and Development Guide for Spring Wheat. University of Minnesota, St Paul, MN.
- Snow, D.W. & Perrins, C.M. (eds) 1998. The Birds of the Western Palearctic, Concise Edition, Vol. 2. Oxford University Press, New York, NY.
- Vickery, J.A., Tallowin, J.R., Feber, R.E., Asteraki, E.J., Atkinson, P.W., Fuller, R.J. & Brown, V.K. 2001. The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. J. Appl. Ecol. 38: 647–664. doi: 10.1046/j.1365-2664.2001.00626.x
- Watson, A. 1992. Unripe grain, a major food for young finch-like birds. Scott. Birds 16: 287.
- Weidinger, K. 2002. Interactive effects of concealment, parental behaviour and predators on the survival of open passerine nests. J. Anim. Ecol. 71: 424–437. doi: 10.1046/j.1365-2656.2002.00611.x
- Wilson, J.D., Evans, J., Browne, S.J. & King, J.R. 1997. Territory distribution and breeding success of skylarks Alauda arvensis on organic and intensive farmland in southern England. J. Appl. Ecol. 34: 1462–1478. doi: 10.2307/2405262
- Wilson, J.D., Morris, A.J., Arroyo, B.E., Clark, S.C. & Bradbury, R.B. 1999. A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric. Ecosyst. Environ. 75: 13–30. doi: 10.1016/S0167-8809(99)00064-X
- Wilson, J.D., Whittingham, M.J. & Bradbury, R.B. 2005. The management of crop structure: a general approach to reversing the impacts of agricultural intensification on birds? Ibis 147: 453–463. doi: 10.1111/j.1474-919x.2005.00440.x
- Yom-Tov, Y. 1992. Clutch size and laying dates of three species of buntings Emberiza in England. Bird Study 39: 111–114. doi: 10.1080/00063659209477106