Abstract
Capsule Red-spotted Bluethroats Luscinia s. svecica from two European breeding populations spent the boreal winter on the Indian sub-continent.
Aim Tracking the migration of Red-spotted Bluethroats from Europe to the hitherto unknown non-breeding areas and back.
Methods Light-level geolocators were deployed on male Bluethroats at breeding sites in the Czech Republic (n = 10) and in Norway (n = 30). Recorded light intensity data were used to estimate the locations of non-breeding sites and migration phenology during the annual cycle.
Results Bluethroats spent the boreal winter in India (n = 3) and Pakistan (n = 1), on average more than 6000 km from their breeding areas. Autumn migration started in August (n = 1) or early September (n = 2), and lasted for 26–74 days. Spring migration commenced on 8 and 9 April (n = 2) and lasted for about a month. During both autumn and spring migration, birds stopped over two or three times for more than 3 days.
Conclusion This study for the first time showed where Red-spotted Bluethroats from European breeding populations stay during the boreal winter. This seems to be the first time that a passerine bird has been tracked along the Indo-European flyway.
Most long-distance migratory birds breeding in Europe spend the non-breeding period in Africa, whereas only a few species migrate to Asia (Alerstam Citation1990, Ellegren & Staav Citation1990, Newton Citation2008). Much of our basic knowledge about these bird migration systems comes from the recoveries of ringed birds (Wernham et al. Citation2002). However, in many species or populations there is an extremely low ring recovery rate (Webster et al. Citation2002), for instance because the birds spend the non-breeding period in sparsely populated areas. This is problematic for the interpretation of ring recovery patterns, potentially resulting in geographically biased pictures of migratory routes and non-breeding areas (Wernham & Siriwardena Citation2002).
The Bluethroat Luscinia svecica is a widely distributed Palearctic passerine in which ten subspecies are recognized (Gill & Donsker Citation2015). Of these, the nominate subspecies L. s. svecica (hereafter Red-spotted Bluethroat) has the widest and most northern breeding distribution, ranging from Norway throughout Asia to western Alaska and with a small population extending further south to mountain areas in central Europe (del Hoyo et al. Citation2005). During the non-breeding season, this subspecies occurs from the Sahel region in West Africa and eastwards to South-east China (Cramp Citation1988), but nothing is known about how the various breeding populations are distributed in the non-breeding areas.
The Red-spotted Bluethroat has been regarded as one of the best documented examples of a bird migrating from Europe to Asia (Ellegren & Staav Citation1990). Nevertheless, the movements of this subspecies are ambiguous and open to debate (Staav Citation1975, Ellegren & Staav Citation1990, Ellegren & Wallin Citation1991, Andersen & Gylseth Citation1992). A particularly intriguing issue is that some existing long-distance ring recoveries could indicate that the birds breeding in Fennoscandia exhibit a dual migration strategy in which some move to Pakistan or even further south-east, and a smaller proportion moves towards the south or south-west to winter in tropical Africa (Staav Citation1975, Cramp Citation1988, Bakken et al. Citation2006, Fransson & Hall-Karlsson Citation2008, Valkama et al. Citation2014). Stable isotope analyses demonstrated that birds breeding in Scandinavia show uniform isotopic profiles from non-breeding sites but could not determine if these sites were located in South Asia, Africa or both (Hellgren et al. Citation2008). Also, since ring recoveries are completely lacking from the period December–February they can provide no information on where Red-spotted Bluethroats spend the boreal winter (Bakken et al. Citation2006, Fransson & Hall-Karlsson Citation2008, Valkama et al. Citation2014) and the issue about dual migration remains unresolved. If a dual migration strategy exists, one might expect birds from the most south-western part of the distribution to be the most likely candidates for migrating to Africa, due to the shorter distance between breeding areas and presumed non-breeding areas (Staav Citation1975).
In cases like the Red-spotted Bluethroat where ringing recoveries produce incomplete or mixed results, direct tracking should provide essential information on individual birds’ movements (Bridge et al. Citation2011, Fiedler Citation2011). We used light-level geolocators to track the migration of Red-spotted Bluethroats from two well separated European breeding populations representing the western-most part of this subspecies’ global breeding range (Cramp Citation1988). Although geolocation by light does not yield tracking data with high precision (Lisovski et al. Citation2012) such data are sufficiently accurate to give us insight into the migration schedules and non-breeding areas of birds that are moving thousands of kilometres (Bridge et al. Citation2011). The aim of this study was to identify the areas where European Red-spotted Bluethroats spend the boreal winter and to estimate their migration routes and migration phenology.
MATERIALS AND METHODS
We studied Red-spotted Bluethroats breeding in the Czech Republic and in Norway. The Czech study area is situated at 1400 m a.s.l. in the Krkonoše Mountains, (50°44ʹN, 15°42ʹE) where Bluethroats nested in alpine wet meadows and peat bogs partially covered by scrub of Dwarf Pine Pinus mugo and solitaire Norway Spruces Picea abies. This breeding population was established during the 1970s and has been subject to thorough population studies since 1989 (Pavel & Chutný Citation2007, VP & BH unpubl. data). The average annual population size during 1988–2014 was 26 males and 17 females, where both unmated and socially polygynous males occurred regularly. Bluethroats typically arrive in the Krkonoše Mountains from the first days of May and until late June. Males in their second calendar year tend to arrive later than older males. In autumn, birds leave about one month after finishing breeding, a period which is spent moulting in or near the breeding area. Date of departure depends on breeding phenology, as birds that finish breeding early or fail to breed probably leave the area in August, while birds with replacement clutches finish breeding in mid-August and probably do not depart until mid-September (VP & BH unpubl. data). The Norwegian study area is located in the Isdalen Valley, Eidfjord municipality, in the Hardangervidda mountains (60°27ʹN, 7°18ʹE, approximately 850 m a.s.l.) which is part of the main Norwegian breeding distribution of the species (Gjershaug et al. Citation1994). The Bluethroat habitat in Isdalen predominately consisted of open forest interspersed with mires and scrub, where sub-Alpine Birch Betula pubescens tortuosa, willows Salix spp. and Junipers Juniperus communis dominated the taller vegetation. In spring, Bluethroats appear on migration in the lowland of Sweden and Norway from late April and later in the alpine breeding areas (www.artsobservasjoner.no, Svanberg & Waldenström Citation2011). Observations in the Isdalen area in 2005 and 2006 indicated that Bluethroats arrived around 10–12 May (A.T. Mjøs, pers. comm.). Thus, the breeding phenology in our study population is most likely comparable to other Bluethroat populations in Fennoscandia which have been studied extensively. For instance, in the Jotunheimen mountains north-east of Isdalen, male Bluethroats typically arrive from mid-May, and egg-laying is quite synchronous during the first two weeks of June (Lifjeld et al. Citation2005). This is similar to the breeding phenology recorded in Swedish Lapland (Ellegren Citation1990). Most adult Bluethroats moult in the breeding area, although some move to the Scandinavian lowlands to moult (Ellegren & Staav Citation1990). In Scandinavia, autumn migration normally takes place from late August, peaks in the first week of September (Staav Citation1975, Ellegren Citation1990), and is positively correlated with timing of breeding which typically depends on the timing of spring thaw in the mountains (Ellegren Citation1990).
By using mist nets and play-back of song, we trapped 10 adult Bluethroat males in the Krkonoše Mountains during 4 May–15 June 2012, and 30 adult males in Isdalen during 7–13 June 2012. Birds in the Czech Republic were ringed with a metal ring and a plastic colour ring, whereas the Norwegian birds were ringed with a metal ring only. Geolocators (model SOI-GDL2.0, manufacturer: Swiss Ornithological Institute) were attached to the back of birds by a flexible leg-loop harness (Rappole & Tipton Citation1991) made of 1 mm thick VMQ silicone o-rings with elastomere hardness of 60° shore A (Johanssen AG, Switzerland). Leg-loop span was calculated from an allometric function (Naef-Daenzer Citation2007) assuming a body mass of 17 g. The weight of geolocators including harness was 0.62 ± 0.01 g (sd), i.e. about 3.7% of the tagged birds’ body mass (mean body masses, the Krkonoše Mountains: 16.9 ± 0.68 g, n = 10; Isdalen: 16.7 ± 0.76 g, n = 26). Geolocators measured light intensity and stored such data every 5 minutes. All devices were equipped with a 5 mm light guide (stalk) to reduce shading by feathers.
If geolocators have a negative impact on the tagged birds this might be reflected by relatively low return rates, altered body masses, altered behaviour and reduced mating and/or reproductive success (Arlt et al. Citation2013, Scandolara et al. Citation2014). In the Czech study site, the return rate of geolocator birds (4/10, 40%) was comparable with that of colour-ringed only males in 2010–2013 (8/17, 47%; 12/24, 50%; and 8/17; 47%; BCH & VP unpubl. data; χ2 = 0.11, df = 3, P = 0.99). In Norway, 17% (5/30) of the geolocator birds returned and this is similar to the 20% (sample size unknown) which has been found in Bluethroats elsewhere in Norway (Bakken et al. Citation2006; χ2 = 0.21, df = 1, P = 0.65). To test for effects of geolocators on body mass we pooled the data from our two study sites since there were no population differences in body mass (data also including non-experimental males from several years; the Krkonoše Mountains mean = 16.8 ± 0.9 g, n = 35, Isdalen mean = 16.7 ± 0.7 g, n = 34; t67 = 0.91, P = 0.37). Body mass at the time when devices were removed (16.9 ± 0.9 g) did not differ from the mass recorded at the time of deployment one year earlier (16.8 ± 0.9 g; paired t-test: t8 = 0.07, P = 0.95). Nor did body mass differ between recaptured males with geolocators (n = 9) and other males (n = 60; t67 = 0.26, P = 0.80). Moreover, we checked if birds returning with geolocators had longer wings than those that did not return. To reduce errors due to different recorders taking the measurments in the two populations, we analysed these data separately but did not find any differences in wing length in either the Krkonoše Mountains (returned, mean = 75.8 ± 3.1 mm, n = 4; not returned, mean: 78.3 ± 4.9 mm, n = 6, t8 = 0.89, P = 0.40) or in Isdalen (returned, mean = 77.8 ± 1.9 mm, n = 5; not returned: mean = 77.8 ± 1.1 mm, n = 25, t28 = 0.02, P = 0.98). Except for some slight feather abrasion on the back just at the site where the device had been positioned (comparable to description in Lislevand & Hahn Citation2013), and also some crust formation on the skin in this area, the device or harness had not caused wounds or any other visible damage to feathers or skin. In spring, the four tagged Czech birds were first recorded in the study area 11–12 May 2013, which was not later than non-tagged birds (median arrival date = 12 May, range 6 May–23 June, n = 15). All four tagged birds were breeding and three of them were paired with two females and had two nests. We do not have any detailed information on breeding behaviour in the Norwegian birds, although all five were singing actively, similarly to non-experimental birds, prior to re-capture.
Five of the nine recovered devices produced useful light data. One of these stopped functioning in September while the Norwegian bird was still in or near the breeding area, leaving a sample of two birds from each population with useful data on movements and non-breeding areas. Light data from migration periods were of mixed quality but one logger from the Czech Republic provided data for the whole annual cycle, single loggers from each population provided data from autumn and winter months, while one logger from Norway only provided useful data from the winter months and spring migration. The light data quality was often poor during migration periods which made it hard to estimate complete migratory tracks. These problems were partly, but not exclusively, caused by bird movements during the equinox periods when estimates of latitudes are highly error prone (Lisovski et al. Citation2012). Another source of problems more specific for Bluethroats may be related to the behaviour of this species, where birds are known for their preference for skulking in dense vegetation where light conditions are obscured (Fudickar et al. Citation2012, Lisovski et al. Citation2012). Nevertheless, our results should show a true picture of migration in the species since the timing of departure and arrival in the tracked Bluethroats fits well with what is already known from the study areas (see above). Also, estimated positions of stopover areas fit well with the easternmost ring recoveries in Scandinavian Bluethroats (Bakken et al. Citation2006, Fransson & Hall-Karlsson Citation2008, Valkama et al. Citation2014).
We analysed the light-level data according to the procedure described by Lisovski and Hahn (Citation2012) by using the R package ‘GeoLight’, version 1.03 (Lisovski et al. Citation2013). In order to filter outlying sun events, i.e. affected by shading, we used the ‘loessFilter’ function with k = 3 interquartile ranges. Stationary periods were determined using the ‘changeLight’ function with a change probability q = 0.8 and a minimum stationary period of 3 days (see Lisovski et al. Citation2013 for function details). In one bird, the change probability q was adjusted to 0.9 due to lack of useful data in autumn-early winter. We used Hill–Ekstrom calibration, i.e. stepwise minimization of variance in latitudinal data (Lisovski et al. Citation2012), to determine sites where birds were resident during the non-breeding period, while stopover-site positions were calculated using sun elevation angels (SEA) derived from in-habitat calibration at the breeding sites. Hill–Ekstrom calibration failed in stopover sites due to high variation in recorded day/night lengths and short duration of the stopover periods. The resulting SEAs for sites of non-breeding residency were much higher in the two devices used in Norway than in the Czech ones (13.7 and 14.7 vs. 3.4 and 4.4, respectively) due to very low recorded light intensities in the former. Positions estimated during stationary periods are shown on maps as median values with interquartile ranges calculated after filtering out latitudinal positions above 60°N and south of the Equator. Migration distances are calculated as loxodromic distances. Travel time (in days) is here defined as the number of days actually spent moving (i.e. excluding stopover/stationary periods) between the breeding site and a non-breeding residency site, whereas migration time is the total number of days from the departure of the breeding area until arrival in the non-breeding residency area. Similarly, travel speed (km/day) is defined as the distance travelled divided by the number of days with actual movement, whereas migration speed (km/day) is the distance travelled divided by the total number of days spent on migration, also including stopover periods. Mean values are reported with standard deviations throughout the text.
RESULTS
Migration data and estimated positions for stopover and non-breeding residency sites are summarized in and . One Czech bird (CZ-1, ) provided a full-year track. Three birds spent the boreal winter in India and one bird at the border between India and Pakistan, on average 6314 ± 451 km from their European breeding areas. As indicated in , which shows the longitudinal shifts over the year in the four birds (latitudes could often not be reliably estimated; see Methods), autumn migration started in August or early September. The bird CZ-1 spent a total of 38 days (14 October–22 November) at an unknown site, presumably at the border between Afghanistan and Pakistan (around 68°E, latitude not detectable), before moving on to the main Indian non-breeding residency site. Moreover, one bird from each breeding population stopped over twice for 5–9 days at unknown sites () and arrived in India 5 October (CZ-2) and 30 September (NO-1). In autumn, the mean estimated travel speed was 309 ± 256 km/day (n = 3) and the mean migration speed was 151 ± 92 km/day (n = 3; ). Birds stayed >40 to >172 days in the final non-breeding residency area (), but exact information about the duration of this period was only available from bird CZ-1. The non-breeding residency sites of the four birds was located 579–1499 km away from each other (mean = 914 ± 327 km, n = 6 comparisons).
Figure 1. Individual migration of four Red-spotted Bluethroats from breeding sites in the Czech Republic (CZ) and Norway (NO; yellow dots) to their non-breeding stationary sites on the Indian sub-continent (blue symbols). Stopovers in autumn (n = 3 birds) are indicated by red symbols whereas spring stopovers (n = 2 birds) are shown in green. When positions could be estimated from the data median longitudes and latitudes are plotted with interquartile ranges (crosses). Shaded red and green areas indicate the interquartile ranges of longitudes for autumn and spring stopover sites, respectively, when latitude could not be reliably estimated.
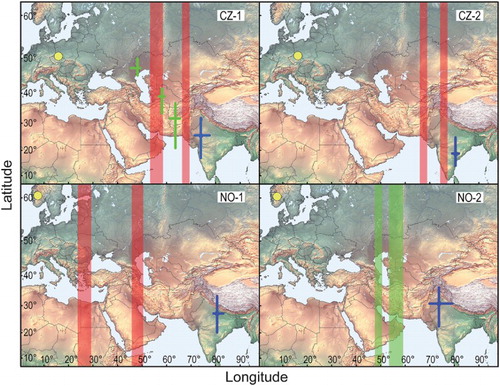
Figure 2. Changes in estimated longitudes over the annual cycle in four individual Red-spotted Bluethroats from the Czech Republic (CZ) and Norway (NO), as estimated from geolocator data. Longitudes are shown as five days moving averages and dotted lines indicate uncertain data.
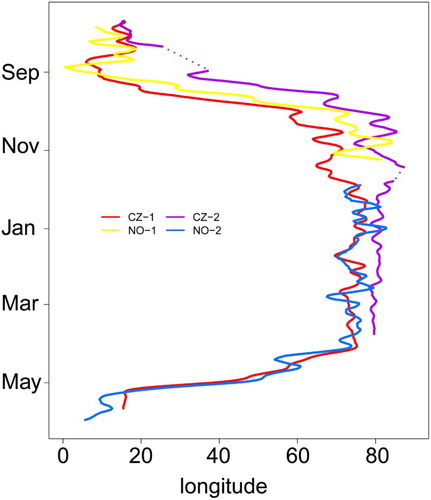
Table 1. Migration in four individual Red-spotted Bluethroat males breeding in the Czech Republic (CZ) and in southern Norway (NO). Migration distance is the loxodromic distance between the estimated non-breeding residency area on the Indian sub-continent and the known breeding area. Stopovers are defined as periods when birds were stationary for more than three days. Travel rates were calculated as the direct distance divided by the number of days with actual movement, whereas migration rate also included stopover periods. Except for bird CZ-1 which yielded data for a full annual cycle, data are incomplete with regard to migration periods and periods of residency. Note that stopover time in bird CZ-1 includes a 38 days long stay in an unknown pre-wintering site at 68°E (arrived 14 October).
In two birds with available data, spring migration commenced 8 April (CZ-1) and 9 April (NO-2). These birds were estimated to arrive in the breeding areas on 5 May and 11 May, respectively. The bird CZ-1 stopped three times en route, in Afghanistan (9–14 April), in central Turkmenistan (15–22 April) and near the Volga River in the western Kazakhstan/Russia border area (23 April–1 May). Two stationary periods were estimated for NO-2 in spring (10–16 April and 23 April–5 May) but geographical positions are uncertain (). The mean estimated spring travel speed was 444 ± 132 km/day (n = 2), whereas the mean migration speed was 196 ± 22 km/day (n = 2).
DISCUSSION
This study confirmed that Bluethroats breeding in the Czech Republic and in Norway migrate along the Indo-European flyway. For the first time, we showed that Bluethroats of the nominate subspecies breeding in Europe spend the boreal winter on the Indian sub-continent. Although the sample is small and thus cannot be expected to give a full overview of non-breeding areas in the studied populations, the present work represents an important contribution to the long-lasting debate on where European Red-spotted Bluethroats reside during the winter months (Staav Citation1975, Ellegren & Staav Citation1990, Ellegren & Wallin Citation1991, Andersen & Gylseth Citation1992, Hellgren et al. Citation2008). Although still a common bird species, the Bluethroat population in Fennoscandia is declining (Lehikoinen et al. Citation2014). Tracks of migration, as we have provided here, may become increasingly important for understanding such population changes in the future (Faaborg et al. Citation2010).
We only have data from adult males, so our sample may be biased. Migration distances are known to often differ within species in relation to sex or age (Newton Citation2008), and there is a possibility that female Bluethroats or young birds choose different migration strategies and use other non-breeding areas than the males studied here. Sex and age differences in the timing of migration are known in Bluethroats from lowland Scandinavia and our Czech study site both in autumn and spring (Ellegren Citation1991, Pavel & Chutný Citation2007, Svanberg & Waldenström Citation2011). However, sex-specific separation of non-breeding areas are not indicated in the literature about winter distribution in Africa (Cramp Citation1988, Urban et al. Citation1997, del Hoyo et al. Citation2005), and juvenile birds on autumn migration in Sweden migrate towards east-southeast (Ellegren & Wallin Citation1991) as one should expect if they follow the same migratory paths as the males in this study.
If Red-spotted Bluethroats from Western Europe do not migrate to Africa, how could we explain the ring recoveries indicating a southern direction of migration? Importantly, compared with the majority of recoveries which indicate an eastern migratory direction, ring recoveries towards the south or south-west are few (approximately ten of several hundred; Bakken et al. Citation2006, Fransson & Hall-Karlsson Citation2008, Valkama et al. Citation2014). One possibility is that these few records involve different subspecies (for instance L. s. cyanecula or L. s. volgae), or hybrids between the nominate and other subspecies (such hybridization is known from our Czech study area; own unpubl. data), because some of the birds in question were ringed during migration when subspecies cannot be easily distinguished (Bakken et al. Citation2006). Alternatively, such ring recoveries may represent birds that went astray and ended up as vagrants in areas where they do not normally occur (Alerstam Citation1990). A third possibility is that only a fraction of the West-European population migrates to Africa and that a larger sample would be needed to detect these birds. Since we studied birds from western European populations which should presumably be most likely to migrate to Africa (Staav Citation1975), and still found no evidence for a migration direction towards south-west, this weakens the hypothesis that European Red-spotted Bluethroats winter in Africa. Although L. s. cyanecula seems to be generally more common in the west and Red-spotted Bluethroat relatively more common in the east, the winter distribution of the different subspecies in Africa is confused and should be subject to further studies (Cramp Citation1988). In fact, little is known about migration in all subspecies of the Bluethroat and our data is only resolving a part of this presumably complex picture.
In addition to revealing where the Red-spotted Bluethroats spend the boreal winter, this study yields new information about the use of stopover sites and sites of non-breeding residency in the species, and about speed of migration. The known stopover sites were located near a route crossing the Caucasus and the Caspian Sea. Compared to the Palearctic-African migration system, in which many species and populations cross the Mediterranean Sea and the Sahara desert, little is known about migratory strategies in relation to ecological barriers for birds migrating through these areas on their way to the Indian sub-continent (Dolnik Citation1990). Some studies have suggested that an arid belt of western Central Asia just east of the Caspian Sea constitutes such an important barrier for birds migrating from west towards south-east (Bulyuk & Chernetsov Citation2005, Chernetsov et al. Citation2007). This would be especially important for Scandinavian Bluethroats since the arid areas are situated along a path between NW Europe and India. Further studies should be made to elucidate if Bluethroats and other species moving between Europe and SE Asia make detours around these barriers.
To conclude, this study is the first to show an Indian non-breeding area of Red-spotted Bluethroats from breeding populations in Europe. To our knowledge, this is in fact the first time that movements of a passerine bird are tracked from its European breeding haunts to its non-breeding areas in Asia. The findings of this study significantly improve our understanding of both the migration and non-breeding behaviour in Bluethroats and of the little studied Indo-European bird migration system.
ACKNOWLEDGEMENTS
We thank Kåre Kyllingstad and Fred Marius Svendsen for invaluable help with the field work in Norway and Alf Tore Mjøs for sharing information about Bluethroats in Isdalen.
Additional information
Funding
REFERENCES
- Alerstam, T. 1990. Bird Migration. Cambridge University Press, Cambridge.
- Andersen, G.S. & Gylseth, P. 1992. The migration routes of the Bluethroat. Luscinia s. svecica. Ornis Svecica 2: 91–92.
- Arlt, D., Low, M. & Pärt, T. 2013. Effect of geolocators on migration and subsequent breeding performance of a long-distance passerine migrant. PLoS ONE 8: e82316. doi:10.1371/journal.pone.0082316.
- Bakken, V., Runde, O. & Tjørve, E. 2006. Norwegian Bird Ringing Atlas, Vol. 2. Stavanger Museum, Stavanger. ( In Norwegian with English summary).
- Bridge, E.S., Thorup, K., Bowlin, M., Chilson, P.B., Diehl, R.H., Fléron, R.W., Hartl, P., Kays, R., Kelly, J.F., Robinson, W.D. & Wikelski, N. 2011. Technology on the move: recent and forthcoming innovations for tracking migratory birds. Bioscience 61: 689–698. doi: 10.1525/bio.2011.61.9.7
- Bulyuk, V.N. & Chernetsov, N. 2005. Nocturnal passage of passerines in Western Kazakhstan in autumn. J. Arid Environ. 61: 603–607. doi: 10.1016/j.jaridenv.2004.10.013
- Chernetsov, N., Bulyuk, V.N. & Ktitorov, P. 2007. Migratory stopovers of passerines in an oasis at the crossroads of the African and Indian flyways. Ringing Migr. 23: 243–251. doi: 10.1080/03078698.2007.9674372
- Cramp, S. (ed) 1988. The Birds of the Western Palearctic. Vol. 5. Tyrant Flycatchers to Thrushes. Oxford University Press, Oxford.
- del Hoyo, J., Elliott, A. & Christie, D.A. (eds) 2005. Handbook of the Birds of the World. Vol. 10. Cuckoo-shrikes to Thrushes. Lynx Edicions, Barcelona.
- Dolnik, V.R. 1990. Bird migration across arid and mountainous regions of the Middle Asia and Kasakhstan. In Gwinner, E. (ed.) Bird Migration – Physiology and Ecophysiology, 368–386. Springer-Verlag, Berlin.
- Ellegren, H. 1990. Timing of autumn migration in Bluethroats Luscinia s. svecica depends on timing of breeding. Ornis Fenn. 67: 13–17.
- Ellegren, H. 1991. Stopover ecology of autumn migrating Bluethroats Luscinia s. svecica in relation to age and sex. Ornis Scand. 22: 340–348. doi: 10.2307/3676506
- Ellegren, H. & Staav, R. 1990. The migration of the Bluethroat Luscina s. svecica: a recovery analysis based on birds ringed in Sweden and Finland. Vår Fågelvärld 49: 323–336.
- Ellegren, H. & Wallin, K. 1991. Autumn migrating Bluethroats Luscinia s. svecica orient in an eastsoutheasterly direction at Gävle, East Sweden. Ornis Svecica 1: 47–50.
- Faaborg, J., Holmes, R.T., Anders, A.D., Bildstein, K.L., Dugger, K.M., Gauthreaux, S.A. Jr., Heglund, P., Hobson, K.A., Jahn, A.E., Johnson, D.H., Latta, S.C., Levey, D.J., Marra, P.P., Merkord, C.L., Nol, E., Rothstein, S.I., Sherry, T.W., Scott Sillett, T., Thompson III, F. R. & Warnock, N. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20: 398–418. doi: 10.1890/09-0397.1
- Fiedler, W. 2011. New technologies for monitoring bird migration and behaviour. Ringing Migr. 24: 175–179. doi: 10.1080/03078698.2009.9674389
- Fransson, T. & Hall-Karlsson, S. 2008. Swedish Bird Ringing Atlas, Vol. 3. The Swedish Museum of Natural History & Swedish Ornithological Society, Stockholm. ( In Swedish with English summary).
- Fudickar, A.M., Wikelski, M. & Partecke, J. 2012. Tracking migratory songbirds: accuracy of light-level loggers (geolocators) in forest habitats. Methods Ecol. Evol. 3: 47–52. doi: 10.1111/j.2041-210X.2011.00136.x
- Gill, F. & Donsker, D. (eds) 2015. IOC World Bird List (v 5.1). doi:10.14344/IOC.ML.5.1.
- Gjershaug, J.O., Thingstad, P.G., Eldøy, S. & Byrkjeland, S. (eds) 1994. Norsk fugleatlas. Hekkefuglenes utbredelse og bestandsstatus i Norge. Norsk Ornitologisk Forening, Klæbu. ( In Norwegian).
- Hellgren, O., Bensch, S., Hobson, K.A. & Lindström, Å. 2008. Population structure and migratory directions of Scandinavian bluethroats Luscinia svecica – a molecular, morphological and stable isotope analysis. Ecography 31: 95–103. doi: 10.1111/j.2007.0906-7590.05258.x
- Lehikoinen, A., Green, M., Husby, M., Kålås, J.A. & Lindström, Å. 2014. Common montane birds are declining in northern Europe. J. Avian Biol. 45: 3–14. doi: 10.1111/j.1600-048X.2013.00177.x
- Lifjeld, J., Johnsen, A. & Petitguyot, T. 2005. Egg-size variation in the bluethroat (Luscinia s. svecica): constraints and adaptation. J. Ornithol. 146: 249–256. doi: 10.1007/s10336-005-0086-5
- Lislevand, T. & Hahn, S. 2013. Effects of geolocator deployment by using flexible leg loop harnesses in a small wader. Wader Study Group Bull. 120: 108–113.
- Lisovski, S. & Hahn, S. 2012. GeoLight – Processing and analysing light-based geolocator data in R. Methods Ecol. Evol. 3: 1055–1059. doi: 10.1111/j.2041-210X.2012.00248.x
- Lisovski, S., Hewson, C.M., Klaassen, R.H.G., Korner-Nievergelt, F., Kristensen, M.W. & Hahn, S. 2012. Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol. Evol. 3: 603–612. doi: 10.1111/j.2041-210X.2012.00185.x
- Lisovski, S., Bauer, S. & Emmenegger, T. 2013. Package ‘GeoLight’. Analysis of light based geolocator data. www.cran.r-project.org.
- Naef-Daenzer, B. 2007. An allometric function to fit leg-loop harnesses to terrestrial birds. J. Avian Biol. 38: 404–407. doi: 10.1111/j.2007.0908-8857.03863.x
- Newton, I. 2008. The Migration Ecology of Birds. Academic Press, London.
- Pavel, V. & Chutný, B. 2007. Population trends of the bluethroat (Luscinia s. svecica) in the Giant Mountains. Opera Corcontica 44: 557–565. ( In Czech).
- Rappole, J.H. & Tipton, A.R. 1991. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62: 335–337.
- Scandolara, C., Rubolini, D., Ambrosini, R., Caprioli, M., Hahn, S., Liechti, F., Romano, A., Romano, M., Sicurella, B. & Saino, N. 2014. Impact of miniaturized geolocators on barn swallow Hirundo rustica fitness traits. J. Avian Biol. 45: 417–423. doi: 10.1111/jav.00412
- Staav, R. 1975. Migration in Nordic Bluethroats. Luscinia s. svecica. Vår Fågelvärld 34: 212–220.
- Svanberg, S. & Waldenström, J. 2011. Population fluctuations and timing of spring migration of the Scandinavian Bluethroat Luscinia svecica svecica at Ottenby Bird Observatory, Sweden, 1955–2008. Ornis Svecica 21: 92–100.
- Urban, E., Fry, C.H. & Keith, S. 1997. The Birds of Africa, Vol. 5: Thrushes to Puffback Flycatchers. Academic Press, San Diego.
- Valkama, J., Saurola, P., Lehikoinen, A., Lehikoinen, E., Piha, M., Sola, P. & Velmala, W. 2014. The Finnish Bird Ringing Atlas. Vol. 2. Finnish Museum of Natural History and Ministry of Environment, Helsinki. ( In Finnish with English summary).
- Webster, M.S., Marra, P.P., Haig, S.M., Bensch, S. & Holmes, R.T. 2002. Links between worlds: unraveling migratory connectivity. Tree 17: 76–83.
- Wernham, C. & Siriwardena, G. 2002. Analysis and interpretation of the ring recovery data. In Wernham, C., Toms, M., Marchant, J., Clark, J., Siriwardena, G. & Baillie, S. (eds) The Migration Atlas. Movements of the Birds of Britain and Ireland, 44–69. T. & A. D. Poyser, London.
- Wernham, C., Toms, M., Marchant, J., Clark, J., Siriwardena, G. & Baillie, S. (eds) 2002. The Migration Atlas. Movements of the Birds of Britain and Ireland. T. & A. D. Poyser, London.
