Abstract
Capsule Abundance monitoring data suggest that the short-term response of breeding birds to recent warming in Great Britain has been range expansion, caused by poleward shifts of leading range margins and no significant shifts of trailing range margins.
Aims To quantify latitudinal and elevational shifts of breeding bird populations in Great Britain and test for differential shifts in range margins during a period of warming (1994–2009).
Methods We modelled the population density of 80 species as a smooth function of latitude, longitude, elevation and year. Reference points on the distribution curve were used to describe latitudinal and elevational shifts.
Results Across species, poleward shifts in the leading range margin were greater than in the range-centre. The trailing range margin was largely static, providing evidence for significant range expansion. The magnitude of latitudinal range shift lagged behind the equivalent shift in temperature, suggesting that species may be accumulating a climatic debt. There was no evidence for consistent elevational shifts.
Conclusion Contrary to the generally expected long-term consequences of climate change of range contraction, we show that the short-term response to recent warming has been range expansion. This suggests the mechanisms of short-term and long-term consequences of climate change may differ.
Temperature is a key driver of species’ distributions, which are expected to shift poleward and upward in response to global warming (Thomas & Lennon Citation1999, Parmesan & Yohe Citation2003). Changes in species’ distributions are predicted to cause range contractions, alterations in community composition and increased extinction risk (Thomas et al. Citation2006, Bellard et al. Citation2012). Recent poleward range shifts have been widely documented (Parmesan et al. Citation1999, Thomas & Lennon Citation1999, Root et al. Citation2003, Hickling et al. Citation2006, Hitch & Leberg Citation2007, Devictor et al. Citation2008, Zuckerberg et al. Citation2009, Gillings et al. Citation2015) and related to the degree of warming (Chen et al. Citation2011). Elevational range shifts have also been observed but, in most cases, have been less marked than expected or inconsistent between species, potentially because of the importance of other factors, such as precipitation, habitat modification or transient reduction in competition (Tingley et al. Citation2009, Lenoir et al. Citation2010, Chen et al. Citation2011).
There is some evidence that shifts in range margins may differ between leading (cool) and trailing (warm) range margins (Parmesan et al. Citation1999, Thomas & Lennon Citation1999), which may reflect differential physiological responses to warming at different margins, or variation in the ecological processes that underpin the expansion of the leading margin and contraction of the trailing margin. For example, the warmest range margins of terrestrial ectotherms appear limited by water availability, temperature extremes and biotic interactions rather than direct physiological limits (Sunday et al. Citation2012). However, it is unclear whether this differentiation applies to terrestrial endotherms, such as birds. Most studies of range change have been based on species’ occurrence from atlas-type data collected over large areas (Gibbons et al. Citation2007), which makes the results sensitive to spatial variation in detectability and to the choice of thresholds for transforming modelled probabilities of occurrence into predictions of presence and absence (Nenzén & Araújo Citation2011). In occurrence data, colonizations may be easier to detect than extinctions (Thomas et al. Citation2006), whilst variation in sampling effort, both spatially and temporally, may also lead to potential bias in the estimates of range shifts (Kujala et al. Citation2013), as can heterogeneity in detectability. All of this means that at least some of the previously inferred variation in the rate of shift in leading and trailing range margins may be due to methodological artefacts. However, new methodological approaches using standardized biological monitoring data (Maggini et al. Citation2011, Virkkala & Lehikoinen Citation2014, Tayleur et al. Citation2015) provide an opportunity to examine this robustly. We apply these methods to examine shifts in range margins of breeding birds in Great Britain. In common with previous analyses of range shift in temperate environments (Thomas & Lennon Citation1999, Brommer, Citation2004, Hitch & Leberg Citation2007, Devictor et al. Citation2008, Citation2012, Zuckerberg et al. Citation2009), we focused on north–south and elevational gradients, particularly as in Great Britain the north–south temperature gradient is an order of magnitude greater than the east–west gradient.
We used standardized monitoring data of bird abundances in Great Britain from 1994 to 2009 to empirically quantify range shifts whilst explicitly accounting for variation in detection probability. We tested whether the magnitude of shifts in range margins varied between the trailing and leading range-edge, as well as the species’ range-centre. We also tested whether the rate of any change matched that expected from the degree of warming, as any lag in biotic response may indicate limits to the ability of a species to respond to rapid climate change (Devictor et al. Citation2008, Citation2012). This paper therefore summarizes the short-term consequences of recent warming on the range extent and abundance of species that have experienced significant climate change (Devictor et al. Citation2012).
MATERIAL AND METHODS
Quantifying temperature change
We used three key temperature variables to describe potential temperature effects on bird populations: mean annual temperature (a measure of general warmth related to plant growing seasons and other ecological processes), mean minimum monthly winter temperature (a measure of winter cold) and mean maximum monthly summer temperature (a measure of summer heat), each measured on a 5-km grid across the whole of Great Britain from 1994 to 2009 (Perry et al. Citation2009). Given strong correlations between these variables over time and space, we performed a principal component analysis (PCA) on the data across the whole study area and period, using the correlation matrix approach. The PCA allowed us to describe the overall pattern of warming using a single axis. The spatial variation in the temperature trend over time, and the extent to which it represents a geographical shift in climate, was described by modelling the PCA temperature axis within a linear model as a function of northing, easting, elevation, year, and the interactions between year and each of the other variables.
Survey data and distance sampling analysis
We used data from the Breeding Bird Survey (BBS), an extensive volunteer survey used to monitor bird populations in the UK from 1994. The BBS is undertaken on a stratified random sample of 1-km squares (2456 squares year−1 on average). The distribution of all BBS squares surveyed in the study period is shown in Fig. S1. Each 1-km square is sampled by surveying two 1-km line transects which are surveyed twice from April to July and bird abundance recorded in three distance bands (0–25 m, 25–100 m, 100 m+). To account for potential heterogeneity in detectability, we used a distance sampling approach (Buckland et al. Citation2001) and fitted half normal distributions to count data with visit (early or late) and habitat variables as covariates (Renwick et al. Citation2012). Habitat was described by a categorical variable indicating the main habitat type for each 200-m transect section (categories were: woodland, scrubland, semi-natural grassland/marsh, heathland and bogs, farmland, human sites, water bodies, coastal and inland rock; Gregory & Bashford Citation1996).
Heterogeneity in detectability across habitats may lead to biased conclusions if not accounted for, as species range shifts may cause changes in habitat occupancy. We did not include year when modelling detectability as previous work has shown that the degree of such temporal variation is insufficient to affect long-term trends, such as those reported here (Newson et al. Citation2013). The square-level estimates of detectability, expressed as probabilities of detecting each individual given that it is present in the sampled area, were used as an offset in the species density models, as described below. We considered all 80 native species recorded from a mean of at least 100 squares year−1 during a period of warming from 1994 to 2009. We used this threshold as initial simulations suggested our methods were only sensitive enough to identify changes for species which were observed in at least 100 squares year−1. Because we focused on latitudinal shifts, we excluded data from Northern Ireland, where sample size was also very small in the first few years.
Modelling range shifts
Generalized additive models (GAMs) were used to model the population density of each species, as a smooth function of northing, easting, elevation and year. The response variable was the number of individuals, of a given species, observed in each 1-km square in each year. Heterogeneity in detectability was accounted for by including the log of square-level species-specific estimates of detection probability, from the distance sampling analysis, as an offset. The observations were weighted by the inverse of the sampling effort within each region to account for spatial variation in the coverage of BBS squares across the country. Including year in the smooth term together with the spatial coordinates allowed us to model spatial variability in the temporal trend of each species. We set the maximum degrees of freedom for the four-dimensional smooth to 16, to avoid over-fitting and to keep the relationships unimodal. Species latitudinal (or elevational) distributions were defined as the marginal latitudinal (or elevational) smooth, holding the other spatial coordinates at their average over the whole sample, weighted by the relative density of the species in different areas, to ensure the change in the reference points were along the gradient of interest. Up to five reference points were located along both latitudinal and elevational distributions (). The optimum point (OP) was located at peak density, leading- and trailing-outer points were defined as the locations at which the modelled density was the peak density * exp(−2), and leading- and trailing-central points were the locations at which the modelled density was the peak density * exp(−0.5) (Heegaard Citation2002, Maggini et al. Citation2011). For a normal distribution, the central points are approximately situated where the slope of the modelled species density as a function of latitude or elevation is the greatest. The outer points are situated at a distance from the OP which is approximately twice as large as the distance between the central and the OP.
Figure 1. Density distribution for nuthatch (a, b) and curlew (c, d). The solid line shows the modelled 1994 distribution and the dashed line shows the modelled 2009 distribution, in relation to (a, c) northing and (b, d) elevation, with the relevant reference points added (OP, optimum point; SOP, Southern Outer Point; SCP, Southern Central Point; NCP, Northern Central Point; NOP, Northern Outer Point; LCP, Lower Central Point; UCP, Upper Central Point and UOP, Upper Outer Point). Nuthatch shows evidence for a northwards expansion in northern outer and central points, but no evidence for any elevational shift, although densities have increased. Curlew shows evidence for an upward shift in optimum, lower and upper central points, but no evidence for any latitudinal shift. Its densities have declined.
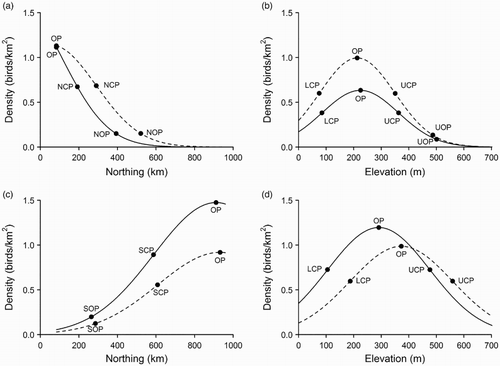
We used the model to quantify the shift in each reference point from 1994 to 2009. Estimates of shift between these two years were based upon the results from the GAM smooths, and therefore were a smoothed function of the data from the whole period of the study. Data were bootstrapped (n = 200) and the models fitted to each bootstrap sample, to quantify the error in the location of each reference point and estimate 95% confidence intervals of the shift in each reference point. Our modelling approach was dependent upon relationships between latitude and abundance modelled within the UK, but yet many species have extensive distributions further north into Scandinavia, or south, into France. This means that for most species we were only able to fit curves that covered part of the species’ latitude–density relationship, and therefore that described only a subset of the maximum of five reference points possible (). Reference points for individual species were only included in the analyses if that point was within Great Britain in both time periods, and not restricted by the geographical area covered. For example, in the case of nuthatch (), the lack of shift in the latitudinal OP was not included in the analysis because this was at the southern edge of the area covered, suggesting that the true optimum may be further south, in France. This ensures that the results reported are for species whose range boundaries or range centroids are modelled to occur within the UK.
It is possible that, as a result of the data from the UK only covering part of a species’ latitudinal range (), our models of latitude–density relationships may be erroneous for some species. This is most likely to affect southerly distributed species whose ranges extend into continental Europe. For this reason we conducted a broad comparison of the locations of reference points described above, with reference points visually estimated from European distribution data (Hagemeijer & Blair Citation1997). This comparison showed similar distributions for the vast majority of species. Two species displayed contrasting distributions between the two data sets, but their removal did not affect our results (Supplementary Material).
SUMMARIZING RANGE SHIFT
Range shift was estimated from these data in a number of different ways. Firstly, we calculated the average shift of each reference point across all species. The 95% confidence intervals were estimated by calculating the average shift across species for each of the bootstrap resamples and the 2.5% and 97.5% quantiles of the bootstrap distribution taken as the lower and upper confidence limits. These distributions of estimated shifts therefore describe the mean shift in distribution across all species, whilst any differences in shift between reference points may be used to infer changes in the latitudinal or elevational extent in the range of an average species. However, given that only part of the geographical range of each species is found in the UK, there is variation in the composition of species that contribute to the estimates of shift of the different references points. Therefore, we secondly, calculated the difference between the shifts of the two reference points of each species that were furthest apart, in order to provide an alternative estimate of the extent of range expansion or contraction within the UK, for each species.
The magnitude of range shift will vary with population trend (Thomas & Lennon Citation1999, Brommer Citation2004, Zuckerberg et al. Citation2009). Therefore, thirdly, in order to account for the influence of population trends on the degree of shift between species, and test for differences between reference points, species-specific estimates of latitudinal and elevational shift of each reference point were modelled as a function of the logarithm of national population trend from 1995 to 2009 from the BBS data. National long-term population trends are routinely calculated every year from smoothed population indices and expressed as the ratio of the smoothed index in the penultimate year to the smoothed index in the second year (Risely et al. Citation2011). The intercept at population trend = 0 provides an estimate of shift in each reference point for a stable population (Thomas & Lennon Citation1999). Thus we modelled shift as a function of reference point, population trend, and the interaction between reference point and population trend to test for any evidence for differential rates of shift in different reference points that would be indicative of either range expansion or contraction. In order to check whether possible differential shifts were related to spatial variation in temperature trends, a likelihood ratio test was used to assess whether the inclusion of temperature trend at each reference point improved the fit of the model. Data were weighted by the inverse of the standard error of shifts, to give greatest weight to more reliable estimates. We used a mixed model with species specified as a random effect to account for the non-independence of multiple points from the same species, and the fact that different species contributed data to different reference points by virtue of their distribution within Britain.
Combined, these different approaches provide an assessment of the changes in range distributions that have occurred in Britain during 15 years of warming, by accounting for spatial variation in the distribution of species, and inter-specific variation in population trends.
As any discrepancy between the rate of species distribution shift and the degree of warming (climate debt) may be indicative of limited ability of the species to cope with long-term climate change (Devictor et al. Citation2008), we calculated the climate debt for each species, defined as the difference between the temperature shift, calculated specifically for the species, and the observed shift of the OP reference point (optimum) of the species’ distribution. The species-specific temperature shift was calculated firstly by assessing the value of the PCA axis that described temperatures at the latitude or elevation where the OP for the species was located in 1994. To assess a reference point along a latitudinal (or elevational) gradient, we held the other spatial coordinates at their average over the whole sample, weighted by the relative density of the species. For example, a northerly distributed species would have the elevation fixed to the mean elevation within its range, which would be higher than the average elevation calculated for a southerly distributed species. We then assessed the latitude or elevation where the same value of the PCA axis was located in 2009. The difference between the two values of latitude or elevation was the species-specific temperature shift, or distance the species range would need to travel to maintain the optimum reference point in the same climate.
RESULTS
Temperature change
During the study period (1994–2009) Great Britain experienced an average temperature increase of 0.59 ± 0.27°C, equivalent to a mean annual temperature increase of 0.039 ± 0.018°C year-1 (mean ± se, from a linear model). The first axis of the PCA described 76% of the variance in the three temperature variables (Table S1). The average increase in annual temperature across Great Britain was equivalent to a 108 ± 2 km (mean ± se) northward shift in isotherms or 63 ± 3 m upward shift over 1994–2009 (Fig. S2).
Species’ range shifts
There was considerable species-specific variation in the extent of range shift documented, but northwards shifts in the Northern Outer Point (NOP) and the Northern Central Point (NCP) were more frequently detected than southwards shifts ( & S2). Across species, the average NOP shifted north by 48.8 km (95% confidence interval: 23.4–76.0; N = 17), equivalent to 3.3 km year−1, and the NCP by 42.3 km (23.9–63.2; N = 44). The northward shift in the OP of 21.4 km (−2.4–49.1; N = 45) exhibited a statistical distribution that only just overlapped zero, whilst the Southern Outer Point (SOP) and Southern Central Point (SCP) changed by only 3.8 km (−55.0–65.5; N = 22) and −0.6 km (−43.4–27.5; N = 7), respectively ().
Figure 2. Latitudinal shifts of reference points and temperatures. The black line (left axis) shows the distribution of bootstrapped median values of reference point latitudinal shifts across species. The grey line (right axis) shows the distribution of the corresponding estimated temperature shifts for each bootstrap replicate. The vertical dashes on the x-axis are the medians of the reference point shifts for each individual species.
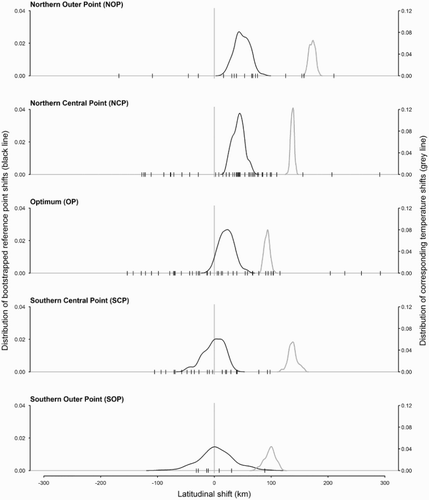
Table 1. Number of species with evidence for a significant northward or southward shift at different reference points. A significant result suggests the median difference between the two time points across all species is not zero. SOP, Southern Outer Point; SCP, Southern Central Point; OP, optimum point; NCP, Northern Central Point and NOP, Northern Outer Point.
When estimating shifts in the outer-most reference points for the 45 species where measures of two or more reference points could be made in both time periods, there was evidence for significant range expansion of 14.5 ± 4.3 km in 15 years (t = 3.33, N = 45, P = 0.0018). Thus, not only do northern reference points show a greater range shift than southern reference points, but within a species, the reference point closest to the northern (leading) range margin shifted northwards more than the reference point closest to the southern (trailing) range margin. Combined, these results provide strong evidence that the different reference points across a species’ distribution have become further apart through time.
Although there were more significant elevational shifts than expected by chance (38/175 tests), in contrast to the latitudinal shifts, there was no evidence for consistent directional elevational changes in distribution ( & S3). The average shift of reference points along the elevational gradient across species ranged between −9.7 m (−22.5–0.0; N = 48) for the OP, which suggested a significant downward shift across species, and 14.6 m (−13.3–44.2; N = 3) for the Lower Outer Point (LOP) ().
Figure 3. Elevational shifts of reference points and temperatures. The black line (left axis) shows the distribution of bootstrapped median values of reference point elevational shifts across species. The grey line (right axis) shows the distribution of the corresponding estimated temperature shifts for each bootstrap replicate. The vertical dashes on the x-axis are the medians of the reference point shift for each individual species.
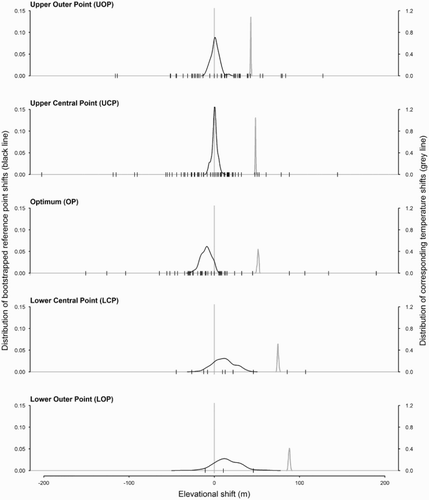
Table 2. Number of species with evidence for a significant upward or downward shift at different reference points. A significant result suggests the median difference between the two time points across all species is not zero. LOP, Lower Outer Point; LCP, Lower Central Point; OP, optimum point; UCP, Upper Central Point and UOP, Upper Outer Point.
Correcting for the effect of population trend on the strength and direction of reference point shift resulted in estimated significant northward shifts for a stable population in NOP of 50.1 ± 13.2 km, NCP of 32.2 ± 12.1 km and OP of 24.2 ± 12.0 km (), broadly similar to the previously reported estimates of shift above, while SOP and SCP did not show any significant shift (17.3 ± 14.1 km and 17.1 ± 12.7 km, respectively). The differential shifts in latitudinal reference points did not appear to be significantly related to the spatial variation in temperature trends, as inclusion of temperature trend at each reference point did not improve the fit of the model (likelihood ratio test: D = 1.446, P = 0.229). This suggests that the greater shifts of northern points compared to southern points were not caused by the greater degree of warming in the north of Great Britain (Fig. S2), but instead were likely due to birds responding more rapidly to warming. The significant interaction between population trend and reference point is indicative of differential shifts in distribution between reference points in response to large-scale population increases (). There was again no significant overall shift in any of the elevational reference points (Fig. S3).
Figure 4. Latitudinal shift of reference points plotted against population trend. Population trend is calculated as the logarithm of the ratio between the smoothed population index in 2009 (P1) and 1995 (P0). SOP, Southern Outer Point; SCP, Southern Central Point; OP, optimum point; NCP, Northern Central Point and NOP, Northern Outer Point.
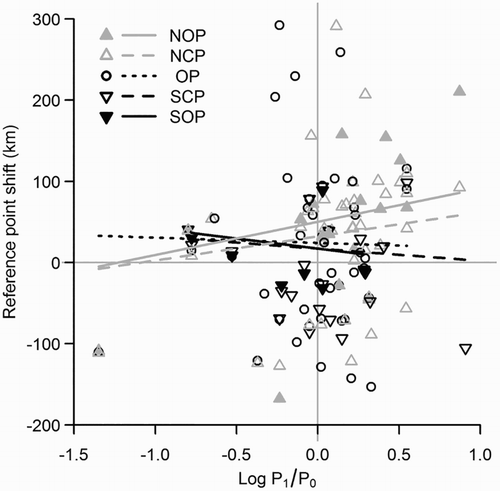
The degree of range shift has been less than that expected from the degree of warming; the NOP shift lagged on average 59 km behind the temperature shifts at the same locations and the OP 87 km behind.
DISCUSSION
Our results show an average poleward shift in the NOP of 3.3 km year−1, which is greater than the range shift detected in other avian studies (Thomas & Lennon Citation1999, Brommer Citation2004, Hitch & Leberg Citation2007, Zuckerberg et al. Citation2009, Gillings et al. Citation2015) and the rate of community composition change across Europe (Devictor et al. Citation2012). Crucially, by using a standardized approach that accounts for potential methodological biases which make it easier to detect colonization rather than extinction (Thomas et al. Citation2006, Maggini et al. Citation2011), and that have increased the uncertainty associated with previous estimates of range shift (Kujala et al. Citation2013), we are able to conclude that the latitudinal shift of northern reference points was greater than for central and southern reference points. Although our data are from only part of the geographical range of most species, these results are robust to whether they are based upon analysis of the mean shift in reference points observed across species, or comparing changes in the distribution of reference points within each species’ range. Thus, although the precise form of the latitude–density relationships for particular species may not fully reflect pan-European patterns due to our use of data from the UK only, this does not appear to have affected our results.
Our finding, that the leading latitudinal range limit of birds has responded proportionally more to warming than the trailing range margin is in concordance with observed shifts in the distribution of terrestrial ectotherms, where thermal tolerance limits the leading range margin, but is less-directly related to the trailing margin (Sunday et al. Citation2012). Such effects could occur because of varying physiological and ecological responses to warming at the leading and trailing edges of a species’ range (Araújo et al. Citation2013, Cahill et al. Citation2013). At the leading margin, warming may have relaxed cold-limits to species’ distributions, where populations may be physiologically limited by cold winter weather (Robinson et al. Citation2007, Pearce-Higgins et al. Citation2015), leading to a potentially rapid response. Conversely, there is increasing evidence that changes at trailing range margins may occur through biotic mechanisms of altered species’ interactions (Cahill et al. Citation2013, Ockendon et al. Citation2014), which are likely to occur over longer timescales. In addition, the island nature of the study area may slow the progression of biotic factors (e.g. competition) even further, due to limited dispersal across the sea. The net result of this contrast means that species’ geographical ranges have expanded by an average of 15 km over 15 years, based upon differential rates of movement in leading and trailing range margins, although with considerable variation between species.
The lack of consistent directional elevational shifts that we found is largely in agreement with previous studies on birds (Archaux Citation2004, Zuckerberg et al. Citation2009, Lenoir et al. Citation2010, Popy et al. Citation2010), although some recent studies have shown upward shifts in avian distributions in both temperate (Reif & Flousek Citation2012, Auer & King Citation2014) and tropical areas (Freeman & Freeman Citation2014) characterized by significant mountain ranges. Upward shifts in temperate areas have been shown to be related to species’ life-history traits, such as larger clutch size and narrow diet breadth (Auer & King Citation2014) and preference for open habitats (Reif & Flousek Citation2012). In our case, the lack of consistent upwards response in species’ distribution may be due to other potential constraints such as topography or habitat condition, which restrict species’ distributions in the UK uplands (Pearce-Higgins et al. Citation2009), or the effects of other climatic limiters on distribution that will also vary with elevation, such as precipitation (Tingley et al. Citation2012).
In common with other studies (Devictor et al. Citation2008, Citation2012), we have noted that the rate of shift in species’ distribution is much less than that expected from the rate of warming. Our observed 49 km northwards shift in NOP and 21 km shift in OP is much less than the 108 km northwards shift in temperature, whilst we noted no consistent change in elevational distribution (a 63 m upwards shift would have been expected on the basis of warming). Species and communities are now occupying warmer temperatures than they have done previously, and it has been suggested that this could have profound consequences on the ability of species to cope with climate change in the future (Devictor et al. Citation2008), although this remains largely untested.
These short-term responses to temperature increases of expanding geographical ranges and stable populations contrast with the expectation from model projections that species ranges are likely to contract in response to climate change (Thomas et al. Citation2004, Bellard et al. Citation2012) and this discrepancy may exist for a number of reasons. Firstly, although birds are regarded as among the taxa most threatened by climate change (Bellard et al. Citation2012), and therefore an appropriate choice for this study, by chance we may have focused on species and populations likely to benefit from climate change, thus making our results unrepresentative of wider projected impacts. Reference to other work shows this was not the case; the species included in this study are projected to suffer a mean 15.4% reduction in their European range extent under a HadCM3 B2 scenario (Huntley et al. Citation2007). These projections are further supported by evidence that climate change is driving changes in communities comprising these species (Devictor et al. Citation2008, Citation2012, Davey et al. Citation2012). Our results suggest these community changes may be caused by expanding leading range margins, rather than contracting trailing range margins, accounting for the observed increase in community diversity with increasing temperature (Davey et al. Citation2012). We did not describe distribution changes in species with fewer than 100 records year-1. If these rare species are more susceptible to climate change than those species included, then we may have under-estimated negative impacts of climate change on the bird community as a whole. There is also little evidence for this. Population changes in rare breeding birds in the UK are indeed related to recent climate change, but the effects are both positive and negative, depending on the species, rather than consistently negative (Green et al. Citation2008).
Secondly, it is possible that this finding may be an artefact of biases in the distribution of BBS squares, or our use of only data from the UK. There is a strong latitudinal gradient in the density of BBS squares, with a greater density of squares in the south than the north due to a greater number of surveyors (Risely et al. Citation2011). Whilst it is plausible that this could differentially affect our ability to describe range shifts in different parts of the UK, this should be through an increased error associated with northern range shifts relative to more precisely estimated shifts in the south. However, as it is the northern reference point shifts which were greatest in extent, and that differed most strongly from zero, this bias does not appear to have affected our results. Our data also include a relative under-representation of northern or upland species in Great Britain due to the latitudinal gradient in sampling effort. Although some of these species may be among the most vulnerable to climate change (Pearce-Higgins Citation2010), there is no evidence for a northwards shift in the southern range margins of four upland species included (Red Grouse Lagopus lagopus, Common Snipe Gallinago gallinago, Eurasian Curlew Numenius arquata and Meadow Pipit Anthus pratensis; Table S2). This suggests that our results are generalizable, although clearly a greater sampling of upland areas to improve our coverage of upland species would be desirable. It is also true that the majority of species covered by this study do not have true latitudinal range boundaries in the UK, with many extending south into continental Europe in particular. As a result, we were able to track changes in relatively few southern reference points compared to northern points. Nevertheless, for the 45 species for which at least two reference points were measured, there was a consistent divergence of about 1 km year−1 between the most northern and most southern reference points. Whilst it would be valuable to repeat our analysis with more extensive data from across Europe to confirm these conclusions, this would require additional analytical complexity due to the need to account for methodological differences between bird monitoring schemes in different countries. In the absence of such an analysis, we believe our conclusions appear robust to these potential methodological concerns.
Thirdly, other factors may be more important drivers of range extent. There is good evidence that this is the case for farmland birds (Eglington & Pearce-Higgins Citation2012), and this may apply to other species groups (Reif et al. Citation2010). However, the fact that the majority of species included do appear to have shifted at least their leading range margin in response to recent warming, suggests that they are sensitive to climate change to some degree.
Fourthly, contrasting effects of climate change may impact species over different timescales. If leading range margins are limited by direct effects of temperature, whilst warm range margins are limited by indirect effects, such as biotic interactions (Sunday et al. Citation2012), the former may happen relatively quickly, whilst depending on the nature of any such interaction, the latter may occur over years or decades. There is increasing evidence for the role of biotic interactions, such as variation in prey availability, predation rates and habitat modification as drivers of population responses to climate change (Cahill et al. Citation2013, Ockendon et al. Citation2014). Although some of these have been detected by studies operating over similar time-frames to our own, these cover perhaps a minority of species, and a longer time-scale may be required to detect other possible indirect responses to warming, particularly if associated with habitat modification. Our results may therefore suggest contrasting timescales over which the potential benefits and costs of climate change may be manifest.
Irrespective of the ultimate reason for the apparent lack of range contraction in response to recent warming, climate change impacts on biodiversity are of concern, largely due to the magnitude of future projected impacts (Thomas et al. Citation2004, Bellard et al. Citation2012), rather than current observations. These are primarily based on bioclimatic models which relate species’ distributions to a climatic niche, and project future changes in species’ range extent in response to projected climate change (Bellard et al. Citation2012). Previous studies have shown good correlations between observed trends or range shifts and those predicted by bioclimatic models (Araújo et al. Citation2005, Gregory et al. Citation2009, Jiguet et al. Citation2013, Virkkala et al. Citation2014a, Citation2014b). However, our results show that the relationship between climate and distribution may be more spatially heterogeneous than predicted by such models. For this reason we suggest that future projections of biodiversity loss based on modelled range shifts in response to climate change should be validated by evidence that they are supported by recent trends. In the long term, we advocate that more dynamic models should be developed to better account for differential ecological processes operating at leading and trailing range margins.
Our relatively short-term observations should not be taken to mean that long-term climate change will not cause range contraction in many species. Instead, they emphasize the need to ensure that the long-term monitoring is in place to detect, document and correctly attribute future impacts on biodiversity. Providing the evidence to support (or refute) the contention that climate change is the threat to biodiversity which statistical models suggest, will be a key challenge for ecologists in the years ahead.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed at 10.1080/00063657.2015.1089835.
Supplementary material
Download MS Word (455 KB)ACKNOWLEDGEMENTS
We are grateful to the BTO members and volunteers who have contributed to the BBS. The BBS is a Partnership between the BTO, Joint Nature Conservation Committee (on behalf of Natural Resources Wales, Natural England, Council for Nature Conservation and Countryside and Scottish Natural Heritage) and Royal Society for Protection of Birds. We are grateful to Mark Eaton and Deborah Procter for comments to an earlier draft of this manuscript.
FUNDING
This research was funded through the BBS partnership.
References
- Araújo, M.B., Pearson, R.G., Thuiller, W. & Erhard, M. 2005. Validation of species-climate impact models under climate change. Glob Change Biol. 11: 1504–1513. doi: 10.1111/j.1365-2486.2005.01000.x
- Araújo, M.B., Ferri-Yáñez, F., Bozinovic, F., Marquet, P.A., Valladares, F. & Chown, S.L. 2013. Heat freezes niche evolution. Ecol. Lett. 16: 1206–1219. doi: 10.1111/ele.12155
- Archaux, F. 2004. Breeding upwards when climate is becoming warmer: No bird response in the French Alps. Ibis 146: 138–144. doi: 10.1111/j.1474-919X.2004.00246.x
- Auer, S.K. & King, D.I. 2014. Ecological and life-history traits explain recent boundary shifts in elevation and latitude of western North American songbirds. Glob. Ecol. Biogeogr. 23: 867–875. doi: 10.1111/geb.12174
- Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15: 365–377. doi: 10.1111/j.1461-0248.2011.01736.x
- Brommer, J.E. 2004. The range margins of northern birds shift polewards. Ann. Zool. Fenn. 41: 391–397.
- Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L., Borchers, D.L., & Thomas, L. 2001. Introduction to Distance Sampling: Estimating Abundance of Biological Populations. Oxford University Press, Oxford.
- Cahill, A.E., Aiello-Lammens, M.E., Fisher-Reid, M.C., Hua, X.,Karanewsky, C.J., Ryu, H.Y., Sbeglia, G.C., Spagnolo, F., Waldron, J.B., Warsi, O. & Wiens, J.J. 2013. How does climate change cause extinction? Proc. R. Soc. B. 280: 20121890. doi: 10.1098/rspb.2012.1890
- Chen, I.-C., Hill, J.H., Ohlemüller, R., Roy, D.B. & Thomas, C.D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333: 1024–1026. doi: 10.1126/science.1206432
- Davey, C.M., Chamberlain, D.E., Newson, S.E., Noble, D.G. & Johnston, A. 2012. Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr. 21: 568–578. doi: 10.1111/j.1466-8238.2011.00693.x
- Devictor, V., Julliard, R., Couvet, D. & Jiguet, F. 2008. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. B. 275: 2743–2748. doi: 10.1098/rspb.2008.0878
- Devictor, V., van Swaay, C., Brereton, T., Brotons, L., Chamberlain, D., Heliola, J., Herrando, S., Julliard, R., Kuussaari, M., Lindstrom, A., Reif, J., Roy, D.B., Schweiger, O., Settele, J., Stefanescu, C., Van Strien, A., Van Turnhout, C., Vermouzek, Z., WallisDeVries, M., Wynhoff, I. & Jiguet, F. 2012. Differences in the climate debts of birds and butterflies at a continental scale. Nat. Clim. Change 2: 121–124. doi: 10.1038/nclimate1347
- Eglington, S.M. & Pearce-Higgins, J.W. 2012. Disentangling the relative importance of changes in climate and land-use intensity in driving recent bird population trends. PLoS ONE 7: e30407. doi: 10.1371/journal.pone.0030407
- Freeman, B.G. & Freeman, A.M.C. 2014. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. USA 111: 4490–4494. doi: 10.1073/pnas.1318190111
- Gibbons, D.W., Donald, P.F., Bauer, H.-G., Fornasari, L., & Dawson, I.K. 2007. Mapping avian distributions: the evolution of bird atlases. Bird Study 54: 324–334. doi: 10.1080/00063650709461492
- Gillings, S., Balmer, D.E., & Fuller, R.J. 2015. Directionality of recent bird distribution shifts and climate change in Great Britain. Glob. Change Biol. 21: 2155–2168. doi: 10.1111/gcb.12823
- Green, R.E., Collingham, Y.C., Willis, S.G., Gregory, R.D., Smith, K.W. & Huntley, B. 2008. Performance of climate envelope models in retrodicting recent changes in bird population size from observed climatic change. Biol. Lett. 4: 599–602. doi: 10.1098/rsbl.2008.0052
- Gregory, R.D. & Bashford, R.I. 1996. The Breeding Bird Survey 1994–1995. British Trust for Ornithology, Thetford.
- Gregory, R.D., Willis, S.G., Jiguet, F., Voříšek, P., Klvaňová, A., van Strien, A., Huntley, B., Collingham, Y.C., Couvet, D., & Green, R.E. 2009. An indicator of climatic change on European bird populations. PLoS ONE 4: e4678. doi: 10.1371/journal.pone.0004678
- Hagemeijer, W.J.M. & Blair, M.J. 1997. The EBCC Atlas of European Breeding Birds. Their Distribution and Abundance. T & A D Poyser, London.
- Heegaard, E. 2002. The outer border and central border for species-environmental relationships estimated by non-parametric generalised additive models. Ecol. Model. 157: 131–139. doi: 10.1016/S0304-3800(02)00191-6
- Hickling, R., Roy, D.B., Hill, J.K., Fox, R. & Thomas, C.D. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12: 450–455. doi: 10.1111/j.1365-2486.2006.01116.x
- Hitch, A.T. & Leberg, P.L. 2007. Breeding distributions of North American bird species moving north as a result of climate change. Conserv. Biol. 21: 534–539. doi: 10.1111/j.1523-1739.2006.00609.x
- Huntley, B., Green, R.E., Collingham, Y.C. & Willis, S.G. 2007. A Climatic Atlas of European Breeding Birds. Lynx Edicions, Barcelona.
- Jiguet, F., Barbet-Massin, M., Devictor, V., Jonzén, N. & Lindström, Å. 2013. Current population trends mirror forecasted changes in climatic suitability for Swedish breeding birds. Bird Study 60: 60–66. doi: 10.1080/00063657.2012.733337
- Kujala, H., Vepsäläinen, V., Zuckerberg, B. & Brommer, J.E. 2013. Range margin shifts of birds revisited – the role of spatiotemporally varying survey effort. Glob. Change Biol. 19: 420–430. doi: 10.1111/gcb.12042
- Lenoir, J., Gégout, J.-C., Guisan, A., Vittoz, P., Wohlgemuth, T., Zimmermann, N.E., Dullinger, S., Pauli, H., Willner, W. & Svenning, J.-C. 2010. Going against the flow: potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 33: 295–303.
- Maggini, R., Lehmann, A., Kéry, M., Schmid, H., Beniston, M., Jenni, L. & Zbinden, N. 2011. Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol. Model. 222: 21–32. doi: 10.1016/j.ecolmodel.2010.09.010
- Nenzén, H.K. & Araújo, M.B. 2011. Choice of threshold alters projections of species range shifts under climate change. Ecol. Model. 222: 3346–3354. doi: 10.1016/j.ecolmodel.2011.07.011
- Newson, S.E., Massimino, D., Johnston, A., Baillie, S.R. & Pearce-Higgins, J.W. 2013. Should we account for detectability in population trends? Bird Study 60: 384–390. doi: 10.1080/00063657.2013.805729
- Ockendon, N., Baker, D.J., Carr, J.A., White, E.C., Almond, R.E.A., Amano, T., Bertram, E., Bradbury, R.B., Bradley, C., Butchart, S.H.M., Doswald, N., Foden, W., Gill, D.J.C., Green, R.E., Sutherland, W.J., Tanner, E.V.J. & Pearce-Higgins, J.W. 2014. Mechanisms underpinning climate impacts on natural populations: altered species interactions are more important than direct effects. Glob. Change Biol. 20: 2221–2229. doi: 10.1111/gcb.12559
- Parmesan, C. & Yohe, G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. doi: 10.1038/nature01286
- Parmesan, C., Ryrholm, N., Stefanescu, C., Hill, J.K., Thomas, C.D., Descimon, H., Huntley, B., Kaila, L., Kullberg, J., Tammaru, T., Tennent, W.J., Thomas, J.A. & Warren, M. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399: 579–583. doi: 10.1038/21181
- Pearce-Higgins, J.W. 2010. Using diet to assess the sensitivity of northern and upland birds to climate change. Clim. Res. 45: 119–130. doi: 10.3354/cr00920
- Pearce-Higgins, J.W., Grant, M.C., Beale, C.M., Buchanan, G.M. & Sim, I.M.W. 2009. International importance and drivers of change of upland bird populations. In Bonn, A., Allott, T., Hubacek, K. & Stewart, J. (eds.) Drivers of Environmental Change in Uplands, 209–227. Routledge, Abingdon.
- Pearce-Higgins, J.W., Eglington, S.M., Martay, B. & Chamberlain, D.E. 2015. Drivers of climate change impacts on bird communities. J. Anim. Ecol. doi:10.1111/1365-2656.12364.
- Perry, M., Hollis, D. & Elms, M. 2009. The generation of daily gridded datasets of temperature and rainfall for the UK. Climate Memorandum No. 24. National Climate Information Centre, Met Office, Exeter.
- Popy, S., Bordignon, L., & Prodon, R. 2010. A weak upward elevational shift in the distributions of breeding birds in the Italian Alps. J. Biogeogr. 37: 57–67. doi: 10.1111/j.1365-2699.2009.02197.x
- Reif, J. & Flousek, J. 2012. The role of species’ ecological traits in climatically driven altitudinal range shifts of central European birds. Oikos 121: 1053–1060. doi: 10.1111/j.1600-0706.2011.20008.x
- Reif, J., Št'astný, K. & Bejček, V. 2010. Contrasting effects of climatic and habitat changes on birds with northern range limits in Central Europe as revealed by an analysis of breeding bird distribution in the Czech Republic. Acta Ornithol. 45: 83–90. doi: 10.3161/000164510X516128
- Renwick, A.R., Massimino, D., Newson, S.E., Chamberlain, D.E., Pearce-Higgins, J.W. & Johnston, A. 2012. Modelling changes in species’ abundance in response to projected climate change. Divers. Distrib. 18: 121–132. doi: 10.1111/j.1472-4642.2011.00827.x
- Risely, K., Renwick, A.R., Dadam, D., Eaton, M.A., Johnston, A., Baillie, S.R., Musgrove, A.J. & Noble, D.G. 2011. The Breeding Bird Survey 2010. BTO Research Report 597. British Trust for Ornithology, Thetford.
- Robinson, R.A., Baillie, S.R. & Crick, H.Q.P. 2007. Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149: 357–364. doi: 10.1111/j.1474-919X.2006.00648.x
- Root, T.L., Price, J.T., Hall, K.R., Schneider, S.H., Rosenzweig, C. & Pounds, J.A. 2003. Fingerprints of global warming on wild plants and animals. Nature 421: 57–60. doi: 10.1038/nature01333
- Sunday, J.M., Bates, A.E. & Dulvy, N.K. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2: 686–690. doi: 10.1038/nclimate1539
- Tayleur, C., Caplat, P., Massimino, D., Johnston, A., Jonzén, N., Smith, H.G., & Lindström, Å. 2015. Swedish birds are tracking temperature but not rainfall: evidence from a decade of abundance changes. Glob. Ecol. Biogeogr. 24: 859–872. doi:10.1111/geb.12308.
- Thomas, C.D. & Lennon, J.J. 1999. Birds extend their ranges northwards. Nature 399: 213. doi: 10.1038/20335
- Thomas, C.D., Cameron, A., Green, R.E., Bakkenes, M., Beaumont, L.J., Collingham, Y.C., Erasmus, B.F.N., de Siqueira, M.F., Grainger, A., Hannah, L., Hughes, L., Huntley, B., van Jaarsveld, A.S., Midgley, G.F., Miles, L., Ortega-Huerta, M.A., Peterson, A.T., Phillips, O.L. & Williams, S.E. 2004. Extinction risk from climate change. Nature 427: 145–148. doi: 10.1038/nature02121
- Thomas, C.D., Franco, A.M.A. & Hill, J.K. 2006. Range retractions and extinction in the face of climate warming. Trends. Ecol. Evol. 21: 415–416. doi: 10.1016/j.tree.2006.05.012
- Tingley, M.W., Monahan, W.B., Beissinger, S.R. & Moritz, C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 106: 19637–19643. doi: 10.1073/pnas.0901562106
- Tingley, M.W., Koo, M.S., Moritz, C., Rush, A.C. & Beissinger, S.R. 2012. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Change Biol. 18: 3279–3290. doi: 10.1111/j.1365-2486.2012.02784.x
- Virkkala, R. & Lehikoinen, A. 2014. Patterns of climate-induced density shifts of species: poleward shifts faster in northern boreal birds than in southern birds. Glob. Change Biol. 20: 2995–3003. doi: 10.1111/gcb.12573
- Virkkala, R., Heikkinen, R.K., Lehikoinen, A. & Valkama, J. 2014a. Matching trends between recent distributional changes of northern-boreal birds and species-climate model predictions. Biol. Conserv. 172: 124–127. doi: 10.1016/j.biocon.2014.01.041
- Virkkala, R., Pöyry, J., Heikkinen, R.K., Lehikoinen, A. & Valkama, J. 2014b. Protected areas alleviate climate change effects on northern bird species of conservation concern Ecol. Evol. 4: 2991–3003.
- Zuckerberg, B., Woods, A.M., Porter, W.F. 2009. Poleward shifts in breeding bird distributions in New York State. Glob. Change Biol. 15: 1866–1883. doi: 10.1111/j.1365-2486.2009.01878.x
