ABSTRACT
Capsule: We found high diet overlap and different uses of space and time between Moustached Warblers Acrocephalus melanopogon and Reed Warblers Acrocephalus scirpaceus breeding in sympatry at a marshland in Spain.
Aims: To study the degree of diet overlap between both species, their space use on a local scale and their breeding phenologies.
Methods: We studied the breeding phenologies of the two species by standardized ringing activity. Spatial distribution was investigated by point counts. We determined diet composition from emetic samples and we collected invertebrates by standardized sweep-netting to estimate food availability.
Results: Diet and prey selection were similar among species. Conversely, spatial overlap was relatively small (<50%) and breeding phenologies were not synchronized. Both food availability and the overall abundance of the two species increased throughout the breeding season.
Conclusion: The two species are potential competitors for food and the observed differences in spatial and temporal niches may represent a way to lower competition for trophic resources: Moustached Warblers could reduce competition by breeding early, while Reed Warblers could avoid settling in areas occupied by the other species.
Studying the degree of niche overlap among morphologically and ecologically similar species is crucial to understand their co-occurrence (Gonzalez-Solis et al. Citation1997, Vieira & Port Citation2007). In fact, the coexistence of similar species is often associated with niche differentiation strategies, which include differences in resource use and in spatial and temporal distribution (Begon et al. Citation2006). Determining the degree of overlap in resource use provides information about the possibility of interspecific competition, although large overlap does not automatically imply competition (Wiens Citation1977). In fact a resource may be abundant enough, or even overabundant, compared to the overall demand of co-existing species. More generally, the occurrence of competition is difficult to assess when using merely observational data (Begon et al. Citation2006), and distribution models can highlight problems in understanding the factors influencing co-occurrence of species (Morelli & Tryjanowski Citation2015).
In this work, we studied two Acrocephalus warblers in a Spanish marshland where they breed sympatrically: the Moustached Warbler Acrocephalus melanopogon and the Reed Warbler Acrocephalus scirpaceus. Sympatric breeding populations of Acrocephalus species have been the subject of several studies of their interactions (Catchpole Citation1978, Hoi et al. Citation1991, Honza et al. Citation1999). Despite this, as far as we know there are currently no studies that have combined the temporal dynamics of reproduction and food availability. Moustached Warblers and Reed Warblers are extremely similar in size (∼10 g) and both are insectivorous (Kennerley & Pearson Citation2010), but their migration strategy, reproduction timing and habitat specialization differ. South-west Mediterranean Moustached Warbler populations are sedentary or migrate over short distances, and start breeding earlier than the congeneric long-distance migrant species, with some females incubating as early as March (see our results and those of Castany Citation2003). This species has specialized breeding habitat preferences that require flooded reedbeds near open waters, and they prefer zones where Phragmites is mixed with other wetland plants like Typha, Scirpus, Juncus and Cladium (Castany Citation2003, Kennerley & Pearson Citation2010). The Reed Warbler is a long-distance migrant and winters in Africa, arriving back to Europe between March and May; in West Europe egg-laying begins in May (Kennerley & Pearson Citation2010). This warbler is a common and widespread breeding bird in European reedbeds, including those of limited extent (Kennerley & Pearson Citation2010). Flooded reedbeds are preferred, but breeding is also possible in drier habitats (Kennerley & Pearson Citation2010). In Spain, the Moustached Warbler breeds across a highly fragmented range, almost always in sympatry with the commoner Reed Warbler (Castany & Lopez Citation2006). As far as we know, the literature contains no detailed information about trophic niche overlap between the two species.
The aims of this work were to measure the trophic niche overlap between both species throughout the breeding season and to compare their spatial distributions and breeding phenologies in the study area. Given that potentially competing sympatric species are predicted to develop niche differentiation (Schoener Citation1974), we expected to find substantial differences in one or more of the investigated niche axes (trophic, spatial and temporal). To gain further insights into the coexistence of the two species, we used breeding phenology and arthropod abundance data to compare the temporal dynamics of food demand and food availability. We considered this species pair instead of a wider assemblage of sympatric reedbed-nesting insectivorous passerines because the remaining species (Great Reed Warbler Acrocephalus arundinaceus and Savi’s Warbler Locustella luscinioides) were scarcer at our study site, thus we could obtain only low sample sizes.
Methods
Data collection
Data collection took place at the Pego-Oliva Natural Park (38°52′N 0°04′W), located on the Mediterranean coast of Spain. This marshland includes wide reedbed areas (dominated by Phragmites australis and Typha angustifolia), water bodies and rice fields, and covers approximately 1250 ha (Urios et al. Citation1993). Except for the point counts carried out in April 2013 and April 2014, all the fieldwork took place between 23 February and 5 July 2012.
We investigated breeding phenology by standardized ringing activity in a part of the marshland (30 ha) where both species breed. In this area we established ten ringing stations and captured birds daily with six mist nets (10 m, 16-mm mesh) at one randomly selected station. Captures started 30 minutes before dawn and lasted 4 hours. Birds were ringed, aged, sexed whenever possible (Svensson Citation1992), measured, weighed and released. The development of brood patches in breeding females was also recorded using the code proposed in Clarabuch (Citation2000), and this information was used to estimate the higher food demand periods. Females with an evidently vascularized brood patch (codes 2 and 3, evident and maximum vascularization, respectively) were considered to be incubating eggs or rearing nestlings of up to 4–5 days old (Bailey Citation1952). According to Kennerley & Pearson (Citation2010), incubation and the period from hatching to fledging respectively last 14–15 and approximately 12 days in Moustached Warblers, and 9–12 and 10–12 days in Reed Warblers. Accordingly, evident brood patch vascularization lasts approximately 18–20 days for Moustached Warblers and 13–17 days for Reed Warblers (incubation + 4–5 days after hatching). Thus we estimated the weekly abundance of pairs rearing nestlings as the average of abundances of the females with vascularized brood patches that were recorded in the previous 3 weeks. We considered the whole period of nestling rearing as the maximum food demand time. In fact, birds often have the greatest breeding success if they produce nestlings when food availability is seasonally at its highest (Lack Citation1968, Perrins & McCleery Citation1989, van Noordwijk et al. Citation1995). Ringing data were also used to measure the weekly overall abundance (Moustached + Reed Warblers) of the two species; that is, the abundance of potential competing individuals that forage at the same time in the reedbed. The resulting trend widely fluctuated, so we calculated the centred moving averages (MA) over 3 weeks to obtain more reliable information. In both cases (incubating females and Moustached + Reed Warblers) weekly abundance of birds was calculated as N captured birds/N days (usually 7, sometimes less due to adverse weather conditions which did not allow ringing activity).
We collected information about food availability by sampling invertebrates once a week through sweep-netting (Ausden & Drake Citation2006). Sampling took place 4 hours after dawn along four transects, located in the same area where we performed the ringing activity and always in the same places. These transects consisted of hitting vegetation with the net ring from the bottom upwards and alternatively on both sides of the trail (Poulin et al. Citation2002). Along each transect (approximately 125 m long), vegetation was swept 125 times, with 500 hits in all. We adopted this method because it samples a wide variety of invertebrate taxa from the foraging substrate of our study species (Poulin & Lefebvre Citation1997, Poulin et al. Citation2002). Afterwards, collected invertebrates were identified to the level of order according to Barrientos (Citation2004) and were counted. Eggs, pupae and larvae were also counted, but were not taxonomically differentiated. A reference collection was created to facilitate prey identification (see below).
To study diet composition, during four periods (approximately monthly: 12–23 March, 10–30 April, 31 May–7 June and 2–5 July) birds captured when ringing were induced to regurgitate using apomorphine as an emetic agent (see Ceresa et al. Citation2014 for details). Each food sample was obtained from a different individual. Sampling periods lasted until we treated at least 15 individuals per species; from Moustached Warblers we did not always obtain this number of food samples because for this species the emetic was not highly effective (<70% of the treated individuals regurgitated, Ceresa et al. Citation2014). Overall, we obtained 44 food samples from Moustached Warblers and 48 from Reed Warblers. During the first sampling period, Reed Warblers were still absent in the study area. Thus we were able to sample only Moustached Warblers. According to the capture–recapture analysis and body mass change, we found no evidence for deleterious effects of this treatment on birds (Ceresa et al. Citation2014). We examined food samples under a stereo microscope and identified prey to the level of order according to Barrientos (Citation2004) and Shiel et al. (Citation1997), and also the reference collection created with the invertebrates sampled in the study area (see above). Save a few intact bodies, most samples were represented by prey fragments. Therefore, the minimum number of individuals of each prey type was calculated by counting body parts (Carlisle & Holberton Citation2006, Orłowski & Karg Citation2013).
In order to assess the possible spatial segregation of the study species, we performed point counts (Gibbons & Gregory Citation2006) at 72 stations in 2013 (11–17 April) and 58 in 2014 (16 and18 April). Counting stations were chosen from across the entire marshland by systematic selection. Each station was surveyed one time, the census lasted 10 minutes per station and the distance between each detected bird and the observer was recorded.
Statistical analysis
We calculated weekly food availability with the weighed abundance index (WAI) used by Poulin & Lefebvre (Citation1997):where pi is the proportion of prey group (order) i in the birds’ diet; xij is the number of prey of group i sweep-netted on date j; and yi is the number of prey from group i sweep-netted during the whole sampling period. Birds’ food sampling was not carried out weekly, unlike sweep-netting. Thus the xij of each week was associated with the pi obtained from the chronologically closer food sampling.
With the diet composition and invertebrate sampling data, we assessed prey selection using Jacobs’ index of selectivity (S; Jacobs Citation1974). It was calculated as S = r − p/(r + p − 2rp), where r is the proportion of a prey type in diet and p is the proportion of that prey type in the environment. S begins at −1 (prey available in the environment, but never consumed) and comes close to 1 (the proportion of a given prey type in diet is far larger than in the environment). We tested the independence between prey availability and consumption using Fisher’s exact test. To avoid proliferation of categories, we excluded the prey taxa sampled extremely rarely in the environment from this analysis (<5 individuals in the entire study period). To measure diet overlap, we calculated Pianka’s overlap index (Citation1973) using package pgirmess 1.5.9 (Giradoux Citation2014), in R 3.1.1 (R Core Team Citation2014). This index ranges between 0 (totally different diets) and 1 (identical diets). We also measured diet specialization with the Berger–Parker index of dominance (d), calculated as d = Nmax/N, where Nmax is the number of individuals of the most abundant prey type; N is the total number of individuals in the sample (Berger & Parker Citation1970). Prey selection, niche overlap and diet specialization were calculated for the entire study period and per food sampling period.
Radial distances from the point transects were used to estimate density using program Distance 6.0 (Thomas et al. Citation2009). There were no observations closer than 5 m, thus zero distance was set at that point. This was probably due to the presence of the observer in the habitat. Data were right-truncated at a maximum distance of 50 m for Moustached Warblers and of 60 m for Reed Warblers. Data were grouped into 10 m wide intervals. In order to estimate the detection function, the half normal, uniform and hazard rate keys were used with the cosine, simple polynomial and hermite polynomial as series adjustments. The model that best fitted the data was selected using the Akaike Information Criteria (AIC). The model with the lowest AIC was chosen to obtain estimates (Burnham & Anderson Citation2002). The detection probability was estimated with all the combined data, while density and encounter rates were estimated per sample. The pooled estimate of density was made from the sample estimates treated as replicates. Afterwards we used Spearman rank correlation to assess the possible association between the densities of both species. The points without contacts were excluded from the analysis.
Results
Diet composition, prey selection and food niche overlap
The diet of both species exclusively included arthropods, which belonged to the orders Araneida, Coleoptera, Diptera, Hemiptera, Hymenoptera and (only in Reed Warblers’ diet) Orthoptera (). Coleoptera and Hymenoptera were the most widely consumed prey and jointly represented 76.6% of Moustached Warblers’ diet and 74.4% of Reed Warblers’ diet. By studying prey choice over the entire study period, we found a clear selection for almost all prey types, and also similarly between both warbler species (). In fact both species positively selected Coleoptera, Araneida and Hymenoptera, and negatively selected Hemiptera and Diptera. In all these cases, the difference between the proportion of prey type in the diet and in the environment was significant. When considering the single food sampling periods, prey preferences were less pronounced and not significant in some cases (), possibly also because of the smaller sample size, but were still similar between both species. Eight other invertebrate categories (Gasteropoda, Glomerida, Lepidoptera, Odonata, Parasitiformes, Thysanoptera, Larvae and Eggs) were sampled in the environment, but were not encountered in food samples. In such cases, S always corresponds to −1. However, the proportions of these categories in the diet and in the environment were never significantly different. Thus their absence in food samples cannot be certainly ascribed to negative selection. According to the Pianka index, the trophic niche overlap was very large during all the food sampling periods (April = 0.934; May/June = 0.984; July = 0.995), and also during the whole study period (0.998). Diet specialization calculated over the entire data set was similar between the species (Moustached Warblers: d = 0.387; Reed Warblers: d = 0.384), but diverged slightly throughout the breeding season (). For both species, specialization reached its maximum in July because of the large proportion of Coleoptera found in diets.
Figure 1. Prey selection of (a) Moustached and (b) Reed Warblers during the 2012 breeding season according to Jacob’s index of selectivity (S). Asterisks mark the cases when the proportion of a prey type in diet and in the environment significantly differed according to Fisher’s exact test. Only the prey types represented in diet are shown; for the other categories S = −1 and Fisher’s exact test was never significant.
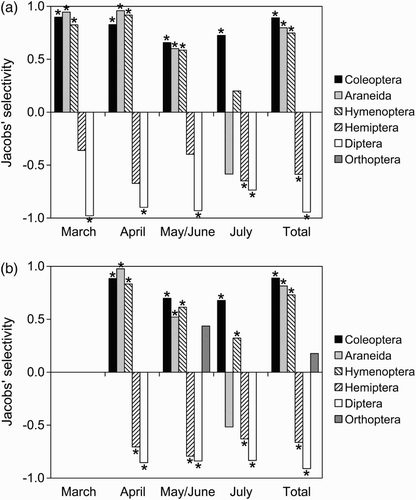
Figure 2. Diet specialization of Moustached (solid line) and Reed (dashed line) Warblers during the 2012 breeding season according to the Berger–Parker index of dominance (d).
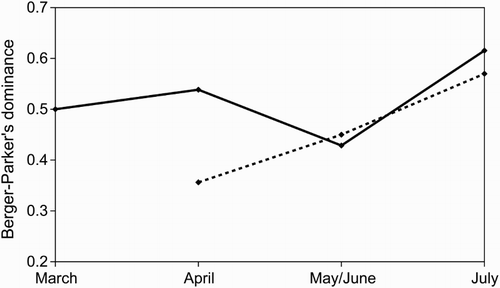
Table 1. Number of individuals and relative frequencies of the prey types in the food samples of Moustached (N = 44) and Reed (N = 48) Warblers collected during the 2012 breeding season. For Reed Warblers, we did not collect samples in March because this species was absent from the study area.
Temporal patterns of food availability and food demand
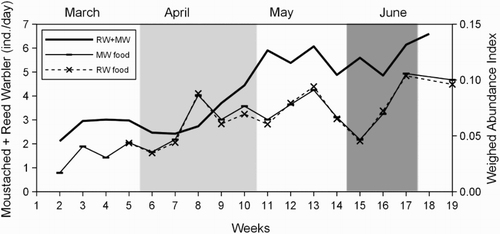
The breeding phenology data () allowed us to measure the temporal mismatch of the reproduction stages between the study species: the first incubating female was captured 7 weeks earlier for Moustached Warblers than for Reed Warblers and the first capture of a fledged young occurred 6 weeks earlier for Moustached Warblers than for Reed Warblers. The highest food demand period (nestlings rearing, see Methods) was estimated to be April for Moustached Warblers and approximately 5–25 June for Reed Warblers (). As a result of this temporal difference, when adult Reed Warblers reared nestlings, both adults and an increasing number of young Moustached Warblers foraged in the reedbed. Furthermore, the higher food demand of Reed Warblers coincided with a second lower food demand peak of Moustached Warblers () due to the latter species’ substitution/second clutches. Food availability, estimated with the WAI, was similar between both species (which is not surprising given the similar diets), and increased with fluctuations throughout the breeding season (). For both species, the greatest food abundance was reached late in June. For Moustached Warblers, the highest food demand occurred when food availability rose (WAI range = 0.036–0.086), but was still below the maxima reached late in June (WAI = 0.106) and at the beginning of July (WAI = 0.100). The overall abundance of the potential competitors calculated for the corresponding weeks (6–10, ) ranged between 2.5 and 4.4 individuals per day (MA, see Methods), which was lower than for the following months, but still rapidly increased (). For Reed Warblers, the WAI ranged between 0.045 and 0.104 during the highest food demand period, including greatest recorded availability, but the abundance of potential competitors was also high (MA range = 4.9–6.1 ind./day; ).
Figure 3. Weekly abundance of females with vascularized brood patches and young individuals of Moustached (MW) and Reed (RW) Warblers during the 2012 breeding season.
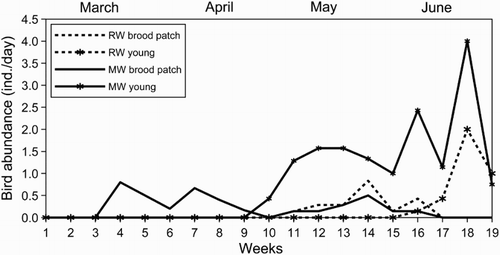
Figure 4. Abundance estimation of breeding pairs rearing nestlings of Moustached (MW) and Reed (RW) Warblers during the 2012 breeding season, calculated as the averaged abundance of females with evidently vascularized brood patches recorded in the previous 3 weeks, and food availability for the two species according to the WAI.
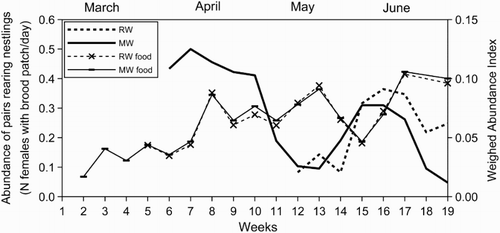
Figure 5. Weekly overall abundance of Moustached and Reed Warblers (RW + MW) during the 2012 breeding season (the solid line represents MA over 3 weeks), higher food demand periods of Moustached and Reed Warblers (light grey area and dark grey area, respectively) identified on the basis of brood patch data, and food availability for the two species according to the WAI.
Spatial segregation
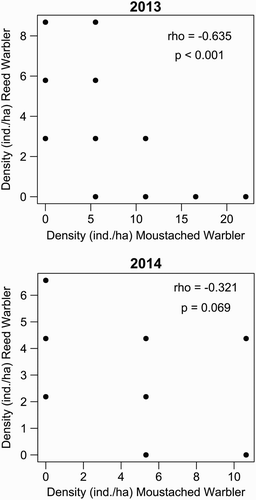
During the census of 2013, we recorded Moustached Warblers at 40 points, Reed Warblers at 33 points and the two species overlapped at 12 points. Thus 30.0% of Moustached Warbler occurrences overlapped spatially with Reed Warblers, and vice versa in 36.4% of cases. In 2014, we recorded Moustached Warblers at 20 points, Reed Warblers at 23 points and the two species overlapped at 9 points; that is, in 45.0% and 39.1% of the cases, respectively. A significant negative association between the densities of the two species was found in 2013 but not 2014 (2013: rho = −0.635, P < 0.001; 2014: rho = −0.321, P = 0.069; ).
Discussion
Our results indicate a large trophic niche overlap and similar prey choice between the Moustached and Reed Warblers. These findings confirm that the two species are potential competitors for food. The low sample size should be taken into account when considering these results, especially for the single food sampling periods. The negative association between densities of Moustached and Reed Warblers in 2013 and the degree of spatial overlap (<50%) indicate a clear difference in the spatial distribution of both species in our study area. Given the greater habitat specialization of Moustached Warblers (see the references in the first section), its occurrence in only one part of the census points occupied by Reed Warblers is not surprising. Yet Reed Warblers were also contacted only in one part (<40%) of the Moustached Warblers’ occurrence points. Reed Warblers are an Acrocephalus with eclectic habitat requirements (see the first section). Thus its absence from most areas occupied by Moustached Warblers, and consequently from wide reedbed areas (suitable habitat), needs explaining. We also observed a partial temporal segregation of breeding between both species, and the most important periods for the chick rearing of Moustached and Reed Warblers did not overlap. Thus, as predicted, we found evidence of niche differentiation among two potentially competing species.
Given these elements, we suggest that a small spatial and temporal overlap may be a response to interspecific competition for food. According to this scenario, Moustached Warblers reduced competition by breeding early, and partly avoided the higher food demand period of Reed Warblers. As a further possible advantage, the early fledged juveniles of Moustached Warblers did not have to compete with young Reed Warblers for several weeks (, weeks 10–15). In addition, early breeding implies the possibility to carry out a substitution/second clutch (). The breeding phenology of Moustached Warblers varies notably among years and breeding sites, thus it is likely to be influenced by inter-annual climate variability and local factors (Castany & Lopez Citation2006 and references therein). The advantages that stem from lower competition may also influence the breeding phenology of Moustached Warblers and help explain the early breeding of this species. Concerning Reed Warblers, individuals arriving from Africa would be advantaged from settling preferably in those areas not previously occupied by Moustached Warblers, because they would experience less competition for food during nestling rearing.
A large trophic niche overlap does not necessarily imply interspecific competition, and no direct evidence was obtained that food availability was scarce compared to the demand observed during the study period, or at least part of it. An appropriate way to assess the occurrence of interspecific competition among our study species would be to compare their niche dimensions in breeding sites of sympatry and allopatry (Begon et al. Citation2006). For example, detailed data about habitat preferences of both species in our study area and in allopatry sites may have allowed us to distinguish the effects of their co-occurrence from those of the habitat features on the observed spatial patterns. Conversely, starting from our data it is possible to draw conclusions only about niche overlap. According to our results, it is possible to identify periods when competition was more likely to occur based on high food demand and fluctuations in food availability (see weeks 15–16, & ). As a result of inter-annual climate variability, such fluctuations are likely to occur not only during one breeding season, but also between years, with competition being more likely in years with lower food availability. However, competition for food was unlikely to occur if food availability at our study site was higher than the overall demand throughout the breeding season. After studying several wetlands in south France, Poulin et al. (Citation2002) found a significant positive correlation between the abundance of breeding reedbed-nesting passerines and food (arthropods) availability. This result does not support the scenario where food in Mediterranean reedbeds is generally overabundant compared with the demand of reedbed-nesting passerines because, in this case, food availability should not influence bird abundance. In other European regions, researchers who studied Acrocephalus warblers found no clear influence of food abundance on breeding success (Bibby & Thomas Citation1985) or attributed low importance to this factor in territorial spacing (Hoi et al. Citation1991). Other researchers who studied these species found possible signs of competition: Castany (Citation2003) reported a surprisingly low breeding density of Reed Warblers in a Spanish marshland that hosts a large population of Moustached Warblers. This author hypothesized that such a low density could be explained by the competition of both Moustached and Great Reed Warblers; Castany (Citation2003) and Poulin et al. (Citation2002) reported Reed Warblers to be common in areas that are not optimal for other species, such as dry reedbed zones. Thus this species’ eclectic habitat requirements may enable it to occupy the reedbed areas that other species avoid, which would reduce interspecific competition.
Factors other than interspecific competition for food may explain the segregation we observed. Some authors have reported interspecific aggressive interactions (interference competition, Begon et al. Citation2006) among Acrocephalus warblers, but the meaning and effects of such behaviours have not been clarified (Murray Citation1971, Catchpole Citation1978, Hoi et al. Citation1991). In a Central Europe marshland, Moustached Warblers bred in a limited area within the wider spatial range of Reed Warblers, and no dominant relationship between the two species was found (Hoi et al. Citation1991). While conducting fieldwork in areas where the two species co-occur, we noticed no interactions to support the existence of interference competition. However, we did not carry out specific observations or experiments to study aggressive interactions. While studying Phylloscopus warblers, Bourski & Forstmeier (Citation2000) suggested that some species may simply avoid areas occupied by the congeneric competitors that arrived earlier. Given the apparent lack of aggressive interactions, they also suggested the term ‘territorial avoidance' as being more correct than ‘territorialism'.
A further possible explanation has been suggested by Hoi et al. (Citation1991): territorial spacing and differences in the breeding times between Acrocephalus warblers may operate to reduce nest predation. As we did not collect data on this issue, we were unable to evaluate this possibility in our study.
By way of conclusion, we found a large trophic niche overlap at our study site between Moustached and Reed Warblers throughout the breeding season. Conversely, we found differences in space and time use between both species. We suggest that these differences may be a response to interspecific competition for food. The need to reduce competition may also help explain the early breeding of Moustached Warblers. Although we found no direct evidence for the occurrence of competition, we identified periods when interspecific competition was more likely to occur given high food demand and fluctuations in food availability. Other factors, such as interspecific aggressive interactions and nest predation, may also help determine the observed spatial and temporal patterns.
Acknowledgements
We are grateful to G. Assandri, D. Beneyto, M. Delandés, P. Lucio, M. Marín, M. Morganti, R. Oliver, R. Piculo, E. Pons, R. Sánchez-Serrano and D. Vidal for their help with the fieldwork, and to S. Añó and N. Ibañez for their help in counting and classifying invertebrates. We would like to thank the authorities of the Marjal de Pego-Oliva Natural Park and the ‘Servei de Conservació de la Biodiversitat de la Generalitat Valenciana’ for providing the facilities to work in protected areas, and also the relevant permits. We thank Helen Warburton for reviewing the English language.
Additional information
Funding
References
- Ausden, M. & Drake, M. 2006. Invertebrates. In Sutherland, W.J. (eds.) Ecological Census Techniques: A Handbook, 214–249. Cambridge University Press, Cambridge.
- Bailey, R.E. 1952. The incubation patch of passerine birds. Condor 54: 121–136.
- Barrientos, J.A. 2004. Curso práctico de entomología. Universitat Autonòma de Barcelona, Barcelona.
- Begon, M., Townsend, C.R. & Harper, J.L. 2006. Ecology: From Individual to Ecosystems, 4th edn. Blackwell Publishing, Oxford.
- Berger, W.H. & Parker, F.L. 1970. Diversity of planktonic foraminifera in deep sea sediments. Science 168: 1345–1347.
- Bibby, C.J. & Thomas, D.K. 1985. Breeding and diets of the Reed Warbler at a rich and a poor site. Bird Study 32: 19–31.
- Bourski, O.V. & Forstmeier, W. 2000. Does interspecific competition affect territorial distribution of birds? A long-term study on Siberian Phylloscopus warblers. Oikos 88: 341–350.
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. Springer Verlag, New York.
- Carlisle, J.D. & Holberton, R.L. 2006. Relative efficiency of fecal versus regurgitated samples for assessing diet and the deleterious effects of a tartar on migratory birds. J. Field Ornithol. 77: 126–135.
- Castany, J. 2003. El carricerín real (Acrocephalus melanopogon) en el P. N. del Prat de Cabanes-Torreblanca. Doctoral Thesis. University of Valencia.
- Castany, J. & Lopez, G. 2006. El carricerín real en España. I Censo Nacional (2005). SEO/Birdlife, Madrid.
- Catchpole, C.K. 1978. Interspecific territorialism and competition in Acrocephalus warblers as revealed by playback experiments in areas of sympatry and allopatry. Anim. Behav. 26: 1072–1080.
- Ceresa, F., Belda, E.J. & Monrós, J.S. 2014. Apomorphine as an emetic for insectivorous songbirds: effectiveness and post-release effects on survival and mass change. J. Field Ornithol. 85: 213–220.
- Clarabuch, O. 2000. El estudio del ave en mano. In Pinilla, J. (ed.) Manual para el anillamiento científico de aves, 73–98. SEO/BirdLife, Madrid.
- Gibbons, D.W. & Gregory, R.D. 2006. Birds. In Sutherland, W.J. (eds.) Ecological Census Techniques: A Handbook, 308–350. Cambridge University Press, Cambridge.
- Giradoux, P. 2014. Package ‘pgirmess’ 1.5.9. Data analysis in ecology. http://cran.r-project.org/web/packages/pgirmess/index.html
- Gonzalez-Solis, J., Oro, D., Jover, L., Ruiz, X. & Pedrocchi, V. 1997. Trophic niche width and overlap of two sympatric gulls in the southwestern mediterranean. Oecologia 112: 75–80.
- Hoi, H., Eichler, T. & Dittami, J. 1991. Territorial spacing and interspecific competition in three species of reed warblers. Oecologia 87: 443–448.
- Honza, M., Moksnes, A., Roskraft, E. & Øien, I.J. 1999. Effect of great reed warbler Acrocephalus arundinaceus on the reproductive tactics of the reed warbler A. scirpaceus. Ibis 141: 489–493.
- Jacobs, J. 1974. Quantitative measurements of food selection: a modification of the forage ratio and Ivlev's electivity index. Oecologia 14: 413–417.
- Kennerley, P. & Pearson, D. 2010. Reed and Bush Warblers. Christopher Helm Publishers Ltd, London.
- Lack, D. 1968. Ecological Adaptation for Breeding in Birds. Methuen, London.
- Morelli, F. & Tryjanowski, P. 2015. No species is an island: testing the effects of biotic interactions on models of avian niche occupation. Ecol. Evol. 5: 759–768.
- Murray, B.G. 1971. The ecological consequences of interspecific territorial bahavior in birds. Ecology 52: 414–423.
- van Noordwijk, A.J., McCleery, R.H. & Perrins, C.M. 1995. Selection for the timing of great tit (Parus major) breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64: 451–458.
- Orłowski, G. & Karg, J. 2013. Diet breadth and overlap in three sympatric aerial insectivorous birds at the same location. Bird Study 60: 475–483.
- Perrins, C.M. & McCleery, R.H. 1989. Laying dates and clutch size in the Great Tit. Wilson Bull. 101: 236–253.
- Pianka, E.G. 1973. The structure of lizard communities. Annu. Rev. Ecol. Syst. 4: 53–74.
- Poulin, B. & Lefebvre, G. 1997. Estimation of arthropods available to birds: effect of trapping technique, prey distribution, and bird diet. J. Field Ornithol. 68: 426–442.
- Poulin, B., Lefebvre, G. & Mauchamp, A. 2002. Habitat requirements of passerines and reedbed management in southern France. Biol. Conserv. 107: 315–325.
- R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing http://www.R-project.org
- Schoener, T.W. 1974. Resource partitioning in ecological communities. Science 185: 27–39.
- Shiel, C., McAney, C., Sullivan, C. & Fairley, J. 1997. Identification of Arthropod Fragments in Bat Droppings. Mammal Society, London.
- Svensson, L. 1992. Identification Guide to European Passerines, 4th edn. Svensson, Stockholm.
- Thomas, L., Laake, J.L., Rexstad, E., Strindberg, S., Marques, F.F.C., Buckland, S.T., Borchers, D.L., Anderson, D.R., Burnham, K.P., Burt, M.L., Hedley, S.L., Pollard, J.H., Bishop, J.R.B. & Marques, T.A. 2009. Distance 6.0. Release ‘2’. – Research Unit for Wildlife Population Assessment, University of St. Andrews, UK. http://ruwpa.st-and.ac.uk/distance/
- Urios, V., Donat, P. & Viñals, M.J. 1993. La marjal de Pego-Oliva. Instituto de estudios Comarcales de la marina Alta, Valencia.
- Vieira, E.M. & Port, D. 2007. Niche overlap and resource partitioning between two sympatric fox species in southern Brazil. J. Zool. 272: 57–63.
- Wiens, J.A. 1977. On competition and variable environments. Am. Sci. 65: 590–597.