ABSTRACT
Capsule: Mediterranean Yellow-legged Gulls mate assortatively according to carotenoid-based colouration but not in relation to size.
Positive assortative mating, defined as the propensity of phenotypically similar individuals to mate with each other, is well documented in birds. Birds appear to select mates based on several traits related to body size, such as body mass (Chardine & Morris Citation1989), tarsus length (Helfenstein et al. Citation2004), wing or tail length (Boland Citation2004) and bill size (Wagner Citation1999). Mate choice may also be influenced by melanin or carotenoid-based colouration, such as bill and eye colouration (Massaro et al. Citation2003), plumage colouration (Safran & McGraw Citation2004) and the size of coloured plumage patches (Masello & Quillfeldt Citation2003). In addition, assortative mating for body condition (Bortolotti & Iko Citation1992), age (Black & Owen Citation1995) and social rank (Rintamäki et al. Citation1998) also occurs.
The Yellow-legged Gull Larus michahellis is a socially monogamous seabird which exhibits biparental care. Adults appear to be sexually monomorphic, although males do tend to be slightly larger than females (Arizaga et al. Citation2008, Galarza et al. Citation2008, Hammouda & Selmi Citation2013). Moreover, adults of both sexes have carotenoid-based colouration on their legs, eye rings, gapes and bills (e.g. red bill spots) (Cramp & Simmons Citation1983). However, mating patterns in this species are poorly described, and it is unknown whether gulls mate assortatively for morphology.
In this study, we analysed evidence for assortative mating for body size and colouration in the Mediterranean race of the Yellow-legged Gull Larus michahellis michahellis. Birds might be expected to pair with larger sized mates because many fitness traits are positively correlated with size (Widemo & Saether Citation1999). Indeed, larger females may be more fecund, and larger males may be more efficient at territory defense. Furthermore, birds might mate assortatively for carotenoid-based colouration features because such features can be influenced by foraging ability, metabolic processes and general health, making them good potential signals of phenotypic quality (Olson & Owens Citation1998).
Data were collected from two colonies of Yellow-legged Gulls located in the Gulf of Gabès in southeastern Tunisia (Sfax salina: 34°42′28″N 10°45′02″E and Djerba island: 33°39′10″N 10°58′59″E). Randomly selected nests were marked with small wooden stakes, which were placed nearby. These nests were checked every 1–2 days until all the eggs had been laid and incubation had been initiated; the laying date of the first egg in each nest was then noted. Subsequently, we captured as many of the incubating parents as possible by means of noose-carpet traps placed on the nests. Each bird was then marked with a patch of paint on the head to avoid resampling, and we measured head length, bill length, bill depth, tarsus length and middle toe length using a digital caliper (± 0.01 mm). Wing length was measured with a ruler (± 1 mm), and body mass was determined (± 20 g) with a spring scale (Pesola, Baar, Switzerland). For bilateral traits, the right and left sides were measured and the mean values were calculated. Measurements were repeated three times by the same observer; the mean values were calculated and used in the statistical analyses.
The head of each bird was photographed in profile against a standardized white background using a digital camera (Nikon Coolpix S6000); a red colour standard and a millimetre scale bar were also included in the photograph. The distance from the lens to the bill was kept constant (20 cm). Adobe® Photoshop® was used to assess the hues of the bill, the red bill spot, the gape and the orbital rings in the digital images. Hue measurements were repeated three times, and the average values were used. To account for possible differences among the photos, the hue data were calibrated using the hue values of the standardized colours. Before the birds were released, a 1 ml blood sample was taken from their brachial veins using a sterile syringe. The blood sample was immediately transferred to a heparinized tube and maintained in a cooler at 4°C. In the laboratory, the blood samples were used to sex the birds employing the methodology described in Griffiths et al. (Citation1998).
Body mass and head length were used to calculate a scaled mass index (SMI), which is a measure of body condition. We used the approach described by Peig & Green (Citation2009). Principal component analysis (PCA) was conducted to see if the colouration features could be grouped into a smaller number of independent factors. Using Spearman’s rank correlation, we determined whether the morphometric traits and colouration features were correlated with laying date, which was defined as the number of days it took a given bird to lay after the first recorded laying date for the colony. We also assessed morphometric traits and colouration feature correlations within mated pairs using a paired t-test. Prior to conducting the analysis, the assumption of normality for within-pair differences was verified (Shapiro–Wilk test: P > 0.05 for all characters). Then, we used Pearson’s product–moment correlation to determine whether mated birds had correlated morphometric traits and/or colouration features. All the statistical tests and analyses were performed using SAS (SAS Institute Citation1999).
In total, we captured and sexed 81 birds (39 males and 42 females). Within the birds captured, there were 16 breeding pairs. Complete morphometric data were collected for all the breeding pairs, but data on colouration features were obtained for only 12 of them (). In both sexes, morphometric traits and colouration features were uncorrelated with laying date (Spearman correlation: P > 0.05). Within pairs, males were significantly larger than females for all the morphometric traits (). However, mated pairs did not have correlated morphometric traits (). Mated birds did not differ significantly in their colouration features (). The PCA grouped the colouration features into two independent factors that accounted for a total of 91% of the original variance. PC1 (eigenvalue = 2.56; explained variance = 65%) was positively correlated with red bill spot hue (r = 0.935, n = 12, P < 0.0001), gape hue (r = 0.901, n = 12, P < 0.0001) and orbital ring hue (r = 0.935, n = 12, P < 0.0001). However, PC2 (eigenvalue = 1.05; explained variance = 26%) was positively correlated with bill hue (r = 0.973, n = 12, P < 0.0001). Neither PC1 nor PC2 differed significantly between the sexes (). However, mated birds had significantly correlated colouration features and PC1 and PC2 scores (, ).
Figure 1. Within-pair correlations of the principal components derived from the colouration features of Yellow-legged Gulls. Dashed lines indicate equality within mated pairs.
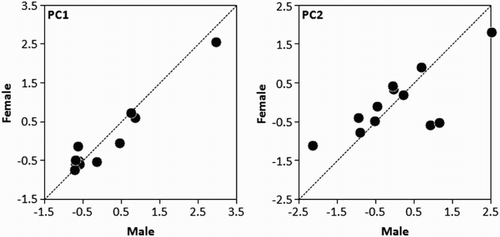
Table 1. Statistics for the morphometric traits and colouration features of Yellow-legged Gulls, and the results of the within-pair comparisons.
Table 2. Within-pair correlations of morphometric traits and colouration features of Yellow-legged Gulls.
Our results showed significant within-pair size dimorphism; males were consistently larger than their female mates. This finding is consistent with results obtained for various populations of the same subspecies in the Mediterranean Basin (e.g. Bosch Citation1996, Aguirre et al. Citation2009). However, we found no evidence for assortative mating by body size. None of the size traits we examined were correlated between mates. Similar results have been obtained in other seabird species, including the Cory’s Shearwater Calonectris borealis (Mougin Citation2000) and the Short-tailed Shearwater Puffinus tenuirostris (Carey Citation2011). However, contrasting patterns have been observed in some more closely related species, such as the Herring Gull Larus argentatus, the Lesser Black-backed Gull Larus fuscus (Harris & Hope-Jones Citation1969) and the Black-legged Kittiwake Rissa tridactyla (Helfenstein et al. Citation2004).
We did find evidence of assortative mating for carotenoid-based colouration features. Within mated pairs, birds had similarly coloured bills, gapes and orbital rings. This pattern could simply reflect an age effect, if bill, gape and orbital ring colour vary with age, which could mean that Yellow-legged Gulls mate assortatively for age. However, there is little support for an age effect since laying date, often a proxy for age in gull species (Sydeman et al. Citation1991), was not correlated with the morphometric traits or colouration features examined here. That said, given our small sample size, we believe that more extensive data obtained from marked birds of known ages could help verify whether or not the observed mating patterns reflect an age effect. Alternatively, colouration-based assortative mating could be the result of similar mate preferences in males and females. Indeed, in birds, carotenoid-based colouration is known to honestly signal quality (Pike et al. Citation2007) and may be evaluated by prospective mates (Andersson Citation1994). Carotenoids cannot be synthesized and must thus be obtained from food (McGraw Citation2006). Consequently, the expression and maintenance of carotenoid-based colouration often entail costs that only higher quality birds can afford (Zahavi & Zahavi Citation1997). In addition, in gulls, the red bill spot has been found to reliably reflect a bird’s feeding abilities (Morales et al. Citation2009), which may directly benefit that bird’s mate.
In conclusion, our results suggest that carotenoid-based colouration plays a more important role in mate choice than does body size in Mediterranean Yellow-legged Gulls. However, these results might have been impacted by the study’s small sample size. Moreover, our data are descriptive in nature and reveal nothing about potential underlying processes. We thus believe that additional studies involving larger data sets obtained from marked birds and field experiments are necessary to clarify mate choice mechanisms in this species.
Acknowledgements
We thank A. Kabaou and M.A. Chokri who helped in data collection. We also thank Dr Ian Hartley, the editor, and two anonymous reviewers for their valuable comments on a previous version of the manuscript. This work complies with Tunisian law; authorizations for bird and egg sampling were obtained from the Forest Service of the Tunisian Ministry of Agriculture (permit reference: 518-28/02/2009).
References
- Aguirre, J.I., Arana, P. & Antonio, M.T. 2009. Testing effectiveness of discriminant functions to sex different populations of Mediterranean Yellow-legged Gulls Larus michahellis michahellis. Ardeola 56: 281–286.
- Andersson, M. (ed.) 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
- Arizaga, J., Aldalur, A., Herrero, A. & Galicia, D. 2008. Sex differentiation of Yellow-legged Gull (Larus michahellis lusitanius): the use of biometrics, bill morphometrics and wing tip coloration. Waterbirds 31: 211–219. doi: 10.1675/1524-4695(2008)31[211:SDOYGL]2.0.CO;2
- Black, J.M. & Owen, M. 1995. Reproductive performance and assortative pairing in relation to age in barnacle geese. J. Anim. Ecol. 64: 234–244. doi: 10.2307/5758
- Boland, C.R.J. 2004. Assortative mating by tail streamer length in Red-tailed Tropicbirds Phaethon rubricauda breeding in the Coral Sea. Ibis 146: 687–690. doi: 10.1111/j.1474-919x.2004.00310.x
- Bortolotti, G.R. & Iko, W.M. 1992. Non-random pairing in American kestrels: mate choice versus intra-sexual competition. Anim. Behav. 44: 811–821. doi: 10.1016/S0003-3472(05)80577-9
- Bosch, M. 1996. Sexual size dimorphism and determination of sex in Yellow-legged Gulls. J. Field Ornith. 67: 534–541.
- Carey, M.J. 2011. Sexual size dimorphism, within-pair comparisons and assortative mating in the short-tailed shearwater (Puffinus tenuirostris). Notornis 58: 8–16.
- Chardine, J.W. & Morris, R.D. 1989. Sexual size dimorphism and assortative mating in the Brown Noddy. Condor 91: 868–874. doi: 10.2307/1368071
- Cramp, S. & Simmons, K.E.L. 1983. The birds of western Paleartic, Vol. III, waders to gulls. Oxford University Press, Oxford.
- Galarza, A., Hidalgo, J., Ocio, G. & Rodríguez, P. 2008. Sexual size dimorphism and determination of sex in Atlantic Yellow-legged Gulls Larus michahellis lusitanius from northern Spain. Ardeola 55: 41–47.
- Griffiths, R., Double, M.C., Orr, K. & Dawson, R.J.G. 1998. A DNA test to sex most birds. Mol. Ecol. 7: 1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x
- Hammouda, A. & Selmi, S. 2013. Morphometric sexing of Mediterranean Yellow-legged Gulls Larus michahellis michahellis breeding in the gulf of Gabès, southern Tunisia. Ostrich 84: 119–122. doi: 10.2989/00306525.2013.829131
- Harris, M.P. & Hope-Jones, P. 1969. Sexual differences in measurements of Herring and Lesser Black-backed gulls. Brit. Birds 62: 129–133.
- Helfenstein, F., Danchin, E. & Wagner, R.H. 2004. Assortative mating and sexual size dimorphism in black-legged kittiwakes. Waterbirds 27: 350–354. doi: 10.1675/1524-4695(2004)027[0350:AMASSD]2.0.CO;2
- Masello, J.F. & Quillfeldt, P. 2003. Body size, body condition and ornamental feathers of Burrowing Parrots: variation between years and sexes, assortative mating and influences on breeding success. Emu 103: 149–161. doi: 10.1071/MU02036
- Massaro, M., Davis, L.S. & Darby, J.T. 2003. Carotenoid-derived ornaments reflect parental quality in male and female yellow-eyed penguins (Megadyptes antipodes). Behav. Ecol. Sociobiol. 55: 169–175. doi: 10.1007/s00265-003-0683-3
- McGraw, K.J. 2006. Mechanics of carotenoid-based coloration. In Hill, G.E. & McGraw, K.J. (eds) Bird Coloration. Vol. I, Mechanisms and Measurements: 177–242. Harvard University Press, Cambridge.
- Morales, J., Alonso-Álvarez, C., Pérez C., Torres, R., Serafino, E. & Velando, A. 2009. Families on the spot: sexual signals influence parent-offspring interactions. Proc. R. Soc. B. Bio. 276: 2477–2483. doi: 10.1098/rspb.2008.1942
- Mougin, J.L. 2000. Pairing in the Cory’s shearwater (Calonectris diomedea) of Selvagem Grande. J. Ornithol. 141: 319–326. doi: 10.1007/BF02462241
- Olson, V.A. & Owens, I.P.F. 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 13: 510–514. doi: 10.1016/S0169-5347(98)01484-0
- Peig, A.J. & Green, A.J. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118: 1883–1891. doi: 10.1111/j.1600-0706.2009.17643.x
- Pike, T.W., Blount, J.D., Bjerkeng, B., Lindstro, M.J. & Metcalfe, N.B. 2007. Carotenoids, oxidative stress and female mating preference for longer lived males. Proc. R. Soc. B. Bio. 274: 1591–1596. doi: 10.1098/rspb.2007.0317
- Rintamäki, P.T., Lundberg, A., Alatalo, R.V. & Höglund, J. 1998. Assortative mating and female clutch investment in Black Grouse. Anim. Behav. 56: 1399–1403. doi: 10.1006/anbe.1998.0904
- Safran, R.J. & McGraw, K.J. 2004. Plumage coloration, not length or symmetry of tail-streamers, is a sexually selected trait in North American Barn Swallows. Behav. Ecol. 15: 455–461. doi: 10.1093/beheco/arh035
- SAS Institute. 1999. SAS/STAT User’s Guide. SAS Institute, Cary, NC.
- Sydeman, W.J., Penniman, J.F., Penniman, T.M., Pyle, P. & Ainley, D.G. 1991. Breeding performance in the western gull: effects of parental age, timing of breeding and year in relation to food availability. J. Anim. Ecol. 60: 135–149. doi: 10.2307/5450
- Wagner, R.H. 1999. Sexual size dimorphism and assortative mating in Razorbills (Alca torda). Auk 116: 542–544. doi: 10.2307/4089388
- Widemo, F. & Saether, S.A. 1999. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trend Ecol. Evol. 14: 26–31. doi: 10.1016/S0169-5347(98)01531-6
- Zahavi, A. & Zahavi, A. 1997. The Handicap Principle. A Missing Piece of Darwin’s Puzzle. Oxford University Press, New York.
