ABSTRACT
Capsule: Wood Warblers Phylloscopus sibilatrix showed significant selection for tree species and woodland characteristics at staging and wintering sites in sub-Saharan Africa.
Aims: To investigate home range size, habitat and tree species selection of Wood Warblers at a staging site in Burkina Faso (Koubri) and a wintering site in Ghana (Pepease).
Methods: Comparing habitat recorded at locations of radio-tagged birds and at control points, we investigated whether there was habitat and tree species selection. We also compared home range size of individual birds between the two sites.
Results: Home range size did not differ between the two sites. There was significant selection for tree species at both Koubri and Pepease: Anogeissus leiocarpus and Albizia zygia, respectively. At Koubri, there was significant avoidance of the most common tree species (Azadirachta indica, Mangifera indica (both non-native), Vitellaria paradoxa and Acacia spp.). In addition, there was a preference for taller trees and greater tree density at both sites. However, the probability of a point being used declined with increasing number of taller (>14 m) trees.
Conclusion: Fine-scale selection of woodland habitats suggests that Wood Warblers are likely to suffer the consequences of ongoing land-use change in their West African wintering grounds.
Over 2 billion Palearctic passerines and near-passerines winter in sub-Saharan Africa (Hahn et al. Citation2009), spending more time there than they do on the breeding grounds (Sheehan & Sanderson Citation2012). Yet there is a paucity of information on the ecology of most migrant birds in winter or on migration (Vickery et al. Citation2014). This is important because, across Europe, long-distance migrants have suffered greater population declines than either resident species or short-distance migrants (Sanderson et al. Citation2006, Hewson et al. Citation2007), and although considerable work has been carried out on the breeding grounds, the causes of decline remain unknown for most species (Vickery et al. Citation2014).
There is evidence linking annual survival rates and breeding population trends of a number of Afro-Palearctic migrants with conditions on the wintering grounds, in particular variation in annual rainfall and drought in the Sahel (Winstanley et al. Citation1974, Peach et al. Citation1991, Norman & Peach Citation2013). Species wintering in the arid zone just south of the Sahara, such as Common Whitethroat Sylvia communis, Sedge Warbler Acrocephalus schoenobaenus and Sand Martin Riparia riparia, underwent population declines from the late 1960s to 1980s, which were associated with long-term below-average rainfall and reduced flooding in the Sahel (Zwarts et al. Citation2009), and are thought to be mediated through effects on habitat condition and food availability (Stoate & Moreby Citation1995). In more recent years, however, populations of many of these species have stabilized, coincident with annual rainfall in the Sahel returning towards pre-drought levels (Nicholson Citation2005). In contrast, species wintering further south in West Africa, in the more humid Guinea forest zone, such as Common Nightingale Luscinia megarhynchos, Spotted Flycatcher Muscicapa striata and Wood Warbler Phylloscopus sibilatrix, are now experiencing more severe population declines than species wintering further north (Hewson & Noble Citation2009, Thaxter et al. Citation2010, Vickery et al. Citation2014). Without an obvious overriding climatic driver, the causes of these later declines are largely unknown, but could include land-use change on the wintering grounds (Adams et al. Citation2014), the effect of longer migration distances on arrival dates on the breeding grounds (Ockendon et al. Citation2012) or an interaction between these and conditions on the breeding grounds (Morrison et al. Citation2013).
Globally, as a result of an increasing human population and economic development, there has been a long-term conversion of natural to man-made agricultural landscapes, mainly at the expense of natural grasslands and forests (Klein Goldewijk Citation2001). Across sub-Saharan Africa, agricultural land cover increased by 57% from 1975 to 2000, at the expense of natural vegetation (Brink & Eva Citation2009). At a regional scale, West Africa has undergone similar land-use changes; assuming the current forest-agricultural mosaic was once dense forest, Norris et al. (Citation2010) estimated that 83% of Guinea rainforest has been converted, mainly to agricultural use. Similar increases in cropland cover at the expense of woodland and other natural vegetation has also taken place (Gray Citation1999, Braimoh Citation2004, Braimoh & Vlek Citation2005, Alo & Pontius Citation2008, Paré et al. Citation2008).
The Wood Warbler is an insectivorous Palaearctic migrant, breeding across Europe and into Siberia (Cramp Citation1988), predominantly in broadleaved forest, where they favour an open structure with limited understory vegetation (Mallord et al. Citation2012a). It overwinters in the humid forest zone in Africa, its range extending from Sierra Leone to western Kenya (Urban et al. Citation1997). Wood Warblers have declined in many European countries, and by 35% continent-wide between 1980 and 2012 (EBCC Citation2014). These declines have been most severe in the north and west of its breeding range (Burfield & van Bommel Citation2004), for example, by 66% in the UK from 1995 to 2012 (Baillie et al. Citation2014). The causes of their decline are largely unknown, although evidence suggests that the drivers are unlikely to be operating on the breeding grounds (Mallord et al. Citation2012b, Citation2016).
As with most other species wintering in the humid zone (Vickery et al. Citation2014), little is known regarding the Wood Warblers’ ecology on the non-breeding grounds. In this study, we investigated their habitat requirements at two sites in sub-Saharan Africa, firstly in the Sudan-savannah zone in Burkina Faso, and secondly, in the Guinea forest-savannah transition zone in Ghana (WWF Citation2015). Evidence suggests that these may represent a staging and wintering area, respectively, with birds moving southwards as conditions become too dry in the Sudan-savannah: Wood Warblers tend to be only recorded in northern Ghana in October/November (Dowsett-Lemaire & Dowsett Citation2014), and are absent from the forest zone in Ghana until mid-November (RSPB, unpubl. data). As a surrogate measure of habitat quality, we estimated and compared home range sizes at each site – larger home ranges would suggest poorer habitat quality (Zabel et al. Citation1995). Based on radio tracking locations, we investigated whether there was evidence for selection of tree species and habitat characteristics. We discuss our findings in the context of recent and ongoing land-use change in West Africa, and the conservation of Wood Warblers and Afro-Palearctic migrants more broadly.
Methods
Study sites
The study was conducted at two study sites, Monastére Sainte-Benoit, in Koubri, central Burkina Faso (12°13′N 01°21′W; hereafter ‘Koubri’), and at a site near Pepease on the Kwahu Plateau in Eastern Region, Ghana (06°39′N 0°42′W; hereafter ‘Pepease’). Koubri (250 ha) consists of mainly dry woodland (with some areas of taller moist forest along water courses) and low-intensity subsistence agriculture, crops including Mango Mangifera indica and Sorghum bicolor. Pepease (350 ha) consists of a mosaic of small-scale cultivation, especially of Plantain Musa paradisiaca and Palm Elaeis guineensis (for oil and wine). A variable number of trees were retained within crop fields, and these were interspersed with small patches of secondary forest (patch area, 0.01–4.8 ha). The rainy season in the Sudan-savannah zone coincides with the European summer, and is finished by September/October. In contrast, the Guinea forest and forest-savannah transition zones of Ghana experience two rainy seasons, September–November and March–July (Dowsett-Lemaire & Dowsett Citation2014).
Radio tracking and home ranges
Birds were caught using mist nets and audio lures of the call and song of the Wood Warbler. A total of 50 birds were caught at Koubri from 2012 to 2014 (n = 15, 16 and 19 in 2012, 2013 and 2014, respectively), and 33 birds were caught at Pepease from 2011/12 to 2013/14 (n = 10, 16 and 7 in 2011/12, 2012/13 and 2013/14, respectively). All birds were fitted with a numbered metal ring (Ghana Ringing Scheme, University of Ghana, Legon) and a unique combination of three coloured plastic rings (A.C. Hughes Ltd, London, UK). In addition, 26 birds at Koubri and 25 at Pepease were fitted with miniature radio transmitters (PicoPip tag, Biotrack Ltd, Wareham, Dorset, UK). The tags were dropped from two birds, and another individual was predated at Koubri, leaving n = 23 birds that were tracked for one day or more (total 315 bird days; ); at Pepease, the signal was quickly lost from five tags, leaving n = 20 tracked for one day or more (total 212 bird days; ). The tags (0.29 g) constituted 2.9–3.8% (mean, 3.2%) of the birds’ mass (mean = 9.2 g, range 7.9–10.4 g). Transmitters were attached to the two central tail feathers with Superglue, and tied with dental floss. Birds were weighed with an electronic balance (to nearest 0.1 g), and wing (maximum chord) and tarsus measurements taken (Redfern & Clark Citation2001). Controlling for wing length and site in a multiple regression, there was no difference in the mass of tagged and untagged birds (mean ± sd; tagged = 9.1 ± 0.6; untagged = 9.1 ± 0.6; F79 = 0.1, P = 0.73). Each bird was assigned an age (either first-year or adult) based on the amount of wear on the primary and tail feathers (Koubri; 39/50 (78%) first-year; 11/50 (22%) adult; Pepease: 17/33 (51%) first-year; 16/33 (49%) adult; Svensson Citation1992). Although males tend to have longer wings than females (Cramp Citation1988, RSPB unpubl. data), we did not sex birds as there is overlap in wing measurements. Birds were tracked from the day after capture (to allow birds to settle after the tagging process), using a Telonics TR-4 Receiver (Telonics Electronics Consultants, Arizona, USA) and directional Yagi 3-element antennae (Mariner Radar Ltd, Lowestoft, UK.), on a mean of 3.3 (range, 1–7) occasions daily between 06:00 and 18:00 GMT, over a period of 1–21 days (). Once the bird was located, an attempt was made to observe it, confirming its identity with the unique colour ring combination (identity confirmed for 1426/1489 sightings; 95.8%), and a precise location taken using a Global Positioning System handset. In the remainder of cases where the bird was not observed (i.e. when the bird flew off before being located), a best estimate was taken of its location but no habitat measurements were recorded under those circumstances.
Table 1. Mean number of fixes and the days over which birds were tracked at Koubri in Burkina Faso and Pepease in Ghana, with mean size of home range, measured as the 50% and 90% kernel density, and the MCP100.
Calculation of home range size was carried out in the ‘adehabitatHR’ (Calenge Citation2006) package in R (version 3.2.1; R Core Team Citation2015). We calculated home range size as the 50% (KD50; core foraging range) and 90% (KD90; home range) fixed kernel density using the reference (href) approach to estimate the smoothing factor (Worton Citation1989). Although considered unreliable (Borger et al. Citation2006), 100% minimum convex polygons (MCP100) were also calculated to look at overall use of the environment, and to compare with other studies.
Tree and habitat selection
Habitat measurements (; online supplementary information, Table S1) were recorded at each location where a radio-tagged bird was found and at control points, which were located at the intersections of a 50 m grid overlaying both study sites. Measurements were taken within a 25 m radius from the ‘Main tree’, which was either the tree in which the bird was found, or the tree at the centre of a control point. At Koubri, an attempt was made to identify all species of tree; at Pepease, where the number and diversity of trees is higher, this was not possible, and therefore we recorded whether or not a tree was Albizia zygia (noted early in the study to be used by Wood Warblers).
Table 2. Variables recorded at actual bird locations and control points. All measurements taken within a 25 m radius of the Main tree.
Tree density (‘treedens’) was obtained by taking the average distance of the five nearest trees, that is, the lower the average distance, the greater the density. Similarly, we calculated the average height (‘treeht’) and diameter at breast height (‘treedbh’; Koubri only) of the five nearest trees (, online supplementary Table S1).
We also recorded evidence of the removal of wood by local people, whether burning had occurred, either in the current or previous year, and the identity of any crops and fruits being cultivated ().
Statistical analysis
Home range sizes, (KD50, KD90 and MCP100) were compared between sites in a generalized linear model with normal error structure. As estimates of home range size can be dependent on the number of fixes taken or the number of days over which birds were tracked, especially KD90 and MCP100, only those birds for which we had more than ten fixes (Borger et al. Citation2006) were included (Koubri, n = 17; Pepease, n = 20), as well as including the number of days over which individual birds were tracked in our models. Home range areas were log-transformed to equalize their variances.
The preference for particular tree species was assessed at the broad (home range) and fine (point) scales. To assess whether certain tree species were being used above what would be expected from their availability, Jacobs Preference Indices (Jacobs Citation1974) were calculated for each species, for each bird individually. Significance of preference was based on the non-parametric Sign Test (Hayward & Kerley Citation2005), which tests whether the median value of Jacobs D for each species across all individual birds was different to zero. To avoid pseudoreplication, repeat occurrences in the same tree were excluded, giving mean (± sd; range) number of trees per bird at Koubri, n = 43 (±13.7; 9–69) and n = 20.6 (±13.1; 5–51) at Pepease. The identity of the trees at the closest n control points to actual bird locations was used as a measure of tree species availability at the home range scale (where n = number of actual bird locations; distance to control points, mean ± sd, Koubri, 118.8 m ± 5.5; Pepease, 102.7 m ± 1.9). At the fine scale, tree species availability was taken to be the proportions of each species within the five nearest trees.
As habitat measurements were taken within a 25 m radius, to ensure independence of bird locations and controls for analysis of wider habitat selection, the number of points was reduced further so that all were at least 50 m apart. Due to overlapping home ranges, there was some duplication of control points (Koubri, n = 65/97 points used more than once; Pepease, 34/120). The mean number of points (± sd; range) per bird (actual and control), was 13.8 (±5.5; 6–26; Koubri); and 8.3 (±4.4; 3–19; Pepease; mapping carried out in ArcGIS (ESRI)). To compare actual bird locations with control points, a generalized linear mixed model was fitted using the glmmML package (Broström Citation2013) in R, specifying a Binomial error distribution and logit link function. Bird identity was included as a random effect to allow for variation between birds and the fact that some control points were duplicated between birds. Model selection was based on the information-theoretic approach (Burnham & Anderson Citation2002). Given what is known of Wood Warbler ecology, it was considered that the main predictors of habitat use would be related to tree density and structure, with possible quadratic effects. Therefore, initially, the full model included only tree-related variables. Models were simplified by including only simple terms, and then testing quadratic terms singly and rejecting those that did not improve the AIC of the model. The remaining full models were:
(see for variable definition; superscript 2 denotes quadratic effect) were then evaluated using the ‘dredge’ function in R, using the MuMIn package (Barton Citation2014), where all possible models are run (210 = 1024 and 29 = 512 possible models, respectively).
For Pepease only, non-tree variables were added to the best model (lowest AIC), that is, measures of human wood removal (‘yes’, ‘no’), evidence of burning (‘yes’, ‘no’) and the main crops and fruit grown (). Crop type was reduced to Cassava and Plantain, which were the main crops at 60% of points, along with ‘other’ crops and ‘none’. Likewise, fruits were reduced to Palm (24%), ‘other’ (17%) and ‘none’ (59%).
Results
Home ranges
Although they tended to be larger at Koubri (), there was no significant difference in the size of home ranges (log-transformed) between the two sites (MCP100: F1,36 = 3.1, P = 0.09; KD50: F1,36 = 1.7, P = 0.20; KD90: F1,36 = 1.8, P = 0.19).
Tree selection
At both Koubri and Pepease, Wood Warblers showed a strong, significant preference for a single tree species at both spatial scales, albeit different at each site: Anogeissus leiocarpus at Koubri (% used vs available, 56.6 vs. 11.4 and 27.0 at home range and point scales, respectively), and A. zygia at Pepease (48.4 vs. 22.0 and 27.2; online supplementary Table S2). In contrast, at Koubri, the three most frequently encountered tree species, Azadirachta indica (2.8 vs. 21.5 and 6.6), Vitellaria paradoxa (1.8 vs. 14.5 and 2.7), and M. indica (0 vs. 7.9 and 0.8), were all avoided at one or more of the two scales; Grewia bicolor (3.0 vs. 1.4 and 9.6) was favoured at the home range scale, but avoided at the Point scale, while Acacia spp. (3.4 vs. 5.6 and 6.1) were avoided at the Point scale (online supplementary Table S2).
Habitat selection
Koubri
There were two models with ΔAIC <2 compared to the best model (online supplementary Table S4). From the significant predictors in the best model, the probability of a point being an actual bird location was twice as great when A. leiocarpus was the main tree ((a)), and increased with increasing tree cover (<10% probability of occupancy at 1–4% cover, increasing to > 70% at > 65% cover; (b)). Probability of occupancy increased with the height of the main tree (35–99% across height range, 2–30 m; (c)), tree density (97–38% probability, at increasing mean distance to nearest five trees, 1–36 m; (d)) and number of trees of height 3–7 m (57–97% probability over range 0–70 trees; (e)). There was a lower probability of occurrence with increasing number of trees of height > 14 m (75–53% over range 0–19 trees; (f)). Relationships were similar, but often stronger, when the main tree was not Anogeissus; Figure S1). The only extra (non-significant) variable in the second model (online supplementary Table S4) was the number of trees, height 7–14 m (increasing probability with increasing number of trees). The best model () correctly predicted the use/non-use of points by Wood Warblers in 80% of cases (use, 78.3% correct; non-use, 81.7% correct). The standard deviation of the random term (‘bird id’) was small (0.00002), suggesting consistency of results among individuals.
Figure 1. Significant predictors of the probability of occurrrence of Wood Warblers at Koubri in Burkina faso (regression lines from the best model in with mean values for other variables, ‘treesp’ = Anogeissus and ‘treecov’ = 3): (a) tree cover, (b) tree species, (c) height of the Main tree (in which bird was located or at centre of the control point – see methods), (d) average distance to the Main tree of the five nearest trees (i.e. inverse of tree density), and the number of trees within 25 m radius of height, (e) 3–7 m and (f) >14 m.
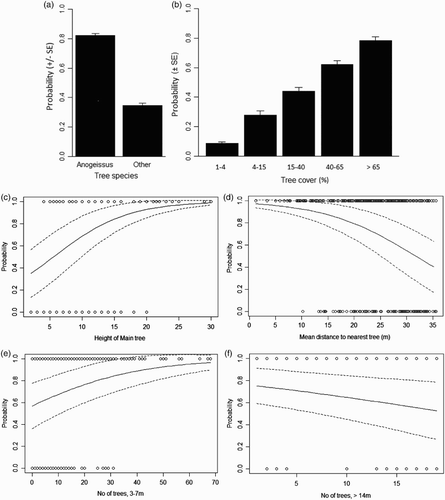
Table 3. Results of the logistic regression analysis to determine which habitat variables predicted the habitat use by Wood Warblers at a wintering site at Koubri Monastery in Burkina Faso. The first, full model results are given along with the best model (of all possible models) evaluated with AIC, along with the model-averaged parameter estimates from the top models (the six models within 4 AIC points of the top model). Note that Treesp ‘Anogeissus’ and Treecov ‘2’ are the reference categories.
Pepease
There were five models with ΔAIC <2 compared to the best model (online supplementary Table S5). From significant variables in the best model, the probability of a point being an actual bird location was twice as great in A. zygia ((a)). Probability of occupancy increased with increasing tree cover (15% at 1–4% cover to 75% at 40–65% cover, declining to 50% at > 65% cover; (b)). Probability peaked at 96% at an optimal height of the main tree of 23 m ((c)), at an optimum number of seven trees of height 3–7 m (peak probability, 91%; (d)), and at an increasing number of trees of height, 7–14 m (84–99% over the range 0–31 trees; (e)). Relationships were similar when the main tree was not Albizia; online supplementary Figure S2). The best model () correctly predicted the use/non-use of point by Wood Warblers in 77.7% of cases (use, 77.1% correct; non-use, 78.3% correct). The standard deviation of the random term (‘bird’) was small (0.00003), suggesting consistency of results between individuals.
Figure 2. Significant predictors of the probability of occurrrence of Wood Warblers at Pepease in Ghana (regression lines from the best model in with mean values for other variables, ‘treesp’ = Albizia and ‘treecov’ = 3): (a) tree cover, (b) tree species, (c) Main tree height, and the number of trees of height, (d) 3–7 m and (e) 7–14 m.
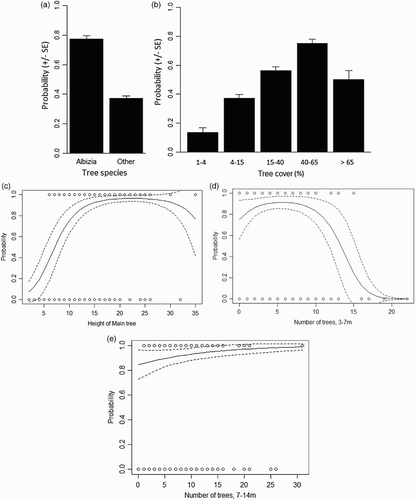
Table 4. Results of the logistic regression analysis to determine which habitat variables predicted the habitat use by Wood Warblers on their wintering grounds at Pepease in Ghana. The first, full model results are given along with the best model (of all possible models) evaluated with AIC, along with the model-averaged parameter estimates from the top models (the 21 models within 4 AIC points of the top model). Note that Treesp ‘Albizia’ and Treecov ‘2’ are the reference categories.
There was no evidence for an effect of crop ( = 0.9, P = 0.82) or fruit type (
= 0.9, P = 0.64), or evidence of burning (
= 0.8, P = 0.36) or wood removal (
= 0.03, P = 0.86) on the probability of a point being an actual bird location.
Discussion
The majority of studies on declining Afro-Palaearctic migrant passerines have been undertaken on the breeding grounds (Vickery et al. Citation2014), hence this is the first detailed study of the Wood Warbler in wintering and staging areas in sub-Saharan Africa. Our results suggest that the species has similar habitat preferences at sites in both Burkina Faso and Ghana and that, despite the differences in vegetation associated with different bioclimatic zones, home range sizes were similar at both sites. Within a well-wooded landscape, birds strongly preferred particular tree species, which differed according to the site’s location, A. leiocarpus at Koubri and A. zygia at Pepease. In addition, the most commonly occurring trees at Koubri, including two non-native species (A. indica and M. indica), were avoided. Taller trees were also favoured, particularly where they were ‘emergent’ from the surrounding canopy; the probability of a point being an actual bird location tended to increase with the number of trees, except for those over 14 m in height. The models were reasonably good at discriminating between actual bird locations and unused controls, suggesting that unlike some open-country species (Hulme & Cresswell Citation2012, Blackburn & Cresswell Citation2015), even within well-wooded landscapes, the availability of suitable habitat has the potential to be limiting for Wood Warblers.
The size of home ranges () was similar at Koubri and Pepease, despite bioclimatic differences in vegetation characteristics, suggesting habitat quality and food availability may have been similar for Wood Warblers at both sites (Zabel et al. Citation1995, Pasinelli Citation2000). It would have been interesting, however, to have continued to track birds at Koubri later in the season, as the Sudan-savannah environment became drier, to see whether home range sizes were larger under such increased stress. MCP100 were, however, non-significantly larger at Koubri, and this may be explained by the use of roosting trees up to 1 km from the core foraging range, often in non-native Eucalyptus. Although there was no significant selection shown for Eucalyptus (online supplementary Table S3), 65% (11/17) of birds roosted in these trees. Although raptors and waterbirds commonly roost in stands of non-native eucalypts, including in Africa (Smith Citation1974), we can find no other recorded instance of an Afro-Palearctic passerine migrant making an effort to do so. We speculate that the height of the trees and the slim green leaves of Eucalyptus trees may provide adequate cover and camouflage for roosting Wood Warblers. There are few estimates of home range size of Palaearctic migrants in Africa (e.g. Arbeiter & Tegetmeyer Citation2011), however, the mean MCP100 of birds at Koubri (12 ha) was identical to that reported for a close congener, the Willow Warbler Phylloscopus trochilus, in similar Sudan-savannah habitat in northern Ghana (Willemoes et al. Citationin prep). Like this species, Wood Warblers are not territorial on the wintering grounds, instead forming loose, ephemeral intra- and inter-specific feeding flocks (pers. obs.). Home range estimates derived from radio transmitters with a short battery life (≤21 days in this study) should be considered as a snapshot of birds’ activities; given the positive effect of the number of days over which birds were tracked on our home range estimates, it is likely that the area covered over an entire winter would be larger than that reported here (Arbeiter & Tegetmeyer Citation2011).
On their breeding grounds in Europe, Wood Warblers utilize a variety of wooded habitats, where structure has been shown to be more important than species composition (Wesołowski Citation1985, Bibby et al. Citation1989, Marti Citation2007, Delahaye & Vandevyvre Citation2008, Mallord et al. Citation2012a). In contrast, tree species composition may be important in the non-breeding season. Certain species of tree and shrub are important to migrants, notably Salvadora persica for its fruit during the pre-migratory fattening period in the spring (Stoate & Moreby Citation1995). We did not collect data on invertebrate abundance, so we cannot say whether this was a factor in the Wood Warblers’ tree preferences. In Ghana, although Wood Warblers have been found in a number of tree species, there is a suggestion that they favour crowns of tall feathery-foliaged trees, including A. zygia (Dowsett-Lemaire & Dowsett Citation2014). The crown of A. leiocarpus, the favoured tree at Koubri, is also generally sparse (Hennenberg et al. Citation2005), suggesting that an open canopy structure that increases the physical availability of prey is important; greater tree height, included in the best model, may also contribute to this openness. Just as important as preferences are the tree species that were actively avoided, which included the most frequently encountered species at Koubri. Two of these species, A. indica and M. indica, are non-native, the latter generally planted as a crop; studies have previously shown the benefits of native trees and shrubs for both resident and migrant species in Africa (Douglas et al. Citation2014). In the Sahel region of Nigeria, A. indica was found to be avoided by migrant Common Whitethroats, possibly due to low abundance of potential invertebrate prey (Stoate Citation1997). V. paradoxa is widely grown in the Sudan-savannah zone for its oil for use in the cosmetic and confectionary industries (Lovett & Haq Citation2000), but has been found to be rarely used by either resident or migrant birds (Zwarts et al. Citation2015). For species occurring over a wide area, tree preferences are likely to vary according to the particular region (Wilson & Cresswell Citation2006), and as a consequence are likely to be flexible, but further studies are required in other wintering and staging areas to quantify this. Even if certain tree species are (statistically) avoided by birds, if a tree is particularly abundant it may still be ecologically important. However, in our study, this appears not to be the case, with very low use of the most common species, for example, Azadirachta, 2.8% use vs. 21.5% availability (online supplementary Table S3).
Apart from selection of individual tree species, the probability of occupancy increased with increasing woodland cover, highlighting the importance of retaining trees on African farmland for the benefit of Afro-Palearctic migrants (Jones et al. Citation1996). The mean number of trees was approximately 175 and 80 per hectare at Koubri and Pepease, respectively (online supplementary Table S2). The exception was the number of tall trees (> 14 m), which showed a negative relationship at both sites, although not retained in the best models for Pepease. Although apparently counter-intuitive, as birds prefer taller trees, at Koubri, the average height of trees used by birds was only 11.7 m (online supplementary Table S2), while at Pepease, the negative relationship may be indicative of birds’ avoidance of areas of closed-canopy forests (RSPB, unpubl. data). The relationships between the various woodland characteristics are quite shallow ((c)–(f) and (c)–(e)), suggesting that habitat is suitable for Wood Warblers over a large range in ‘quality’. However, this is due of the effect of the favoured tree (Anogeissus or Albizia) on occurrence; when the relationships are modelled assigning the main tree to the non-favoured category (i.e. ‘other’), the effect of the surrounding landscape is more important (online supplementary Figures S1(a)–(d) and S2(a)–(c)).
In Burkina Faso and Ghana, as with elsewhere within the species’ wintering range (Brink & Eva Citation2009), the trend has been for the conversion of woodlands to what is perceived to be higher value cropland (Gray Citation1999, Owubah et al. Citation2001, Alo & Pontius Citation2008, Ouedraogo et al. Citation2010). This has resulted in the loss of biodiversity (Norris et al. Citation2010), and could detrimentally affect the sustainability of the farming systems themselves through the loss of ecosystem functions and services. Although there is no direct evidence that loss of non-breeding habitat is driving the ongoing decline of Wood Warblers, birds show fine-scale selection (in terms of tree height, species and density) that suggests that the species could be vulnerable to woodland loss. Therefore, it will be important to understand the socio-economic drivers of these changes and the key factors that determine ‘tree management’ on farmland. Encouraging farmers to retain trees on farmland will require a diversity of approaches including demonstrating and, where possible, enhancing the resources provided by native trees, such as medicines, firewood, charcoal, shade (Parrotta et al. Citation2009) and habitat for crop pollinators (Ricketts Citation2004). For instance, the use of A. zygia as a means of reclaiming degraded cocoa lands is a potential way of increasing suitable tree cover on farmland (Akim-Kwapong & Teklehaimanot Citation1995).
However, this runs counter to current research that suggests that the conservation of forest biodiversity in West Africa and other tropical regions is enhanced by intensifying agriculture on already modified croplands in order to reduce future deforestation (land sparing; Green et al. Citation2005, Gockowski & Sonwa Citation2011, Phalan et al. Citation2011), rather than low-intensity agriculture across the landscape (land sharing). Although we do not have the data to support either model, Palaearctic migrants, including Wood Warblers, are likely to lose out under land sparing, as they rarely occur in closed forests (Morel & Morel Citation1992), instead occurring at low densities across the wider, agricultural, landscape (Vickery et al. Citation2014), and may be a group of species that require adoption of wildlife-friendly farming practices (Wright et al. Citation2012) that integrate the needs of birds and people (Norris et al. Citation2010).
The potential problems facing Afro-Palearctic migrants as a result of land-use change in Africa has long been recognized (Moreau Citation1970). Although there is evidence that open-country migrant species may benefit from agricultural intensification (e.g. Whinchat Saxicola rubetra; Hulme & Cresswell Citation2012, Blackburn & Cresswell Citation2015), if such intensification involves the further loss of trees, this study suggests that those species reliant on a more mature wooded landscape are likely to suffer negative consequences. The challenge to conservationists will be to promote sustainable land use that not only benefits biodiversity, but also helps to alleviate the poverty of local people (Roe et al. Citation2013).
Supplementary Information
Download MS Word (122 KB)Acknowledgements
We are grateful to the support provided by the Birdlife International partner organizations in Burkina Faso and Ghana, Naturama and Ghana Wildlife Society. We thank the monks and nuns at Monastére Sainte-Benoit at Koubri, and the chiefs and local farmers from villages surrounding our Pepease study site for allowing us to work on their land. Kwame Boafu undertook fieldwork in 2014, and Paul Britten, David Fouracre, Emma Teuten and Sharolyn Parnham provided assistance with ArcGIS. We appreciate comments from Will Cresswell and an anonymous referee which improved the manuscript.
References
- Adams, W.M., Small, R.D.S. & Vickery, J.A. 2014. The impact of land use change on migrant birds in the Sahel. Biodiversity 15: 101–108. doi: 10.1080/14888386.2014.931822
- Alo, C.A. & Pontius Jr, R.G. 2008. Identifying systematic land-cover transitions using remote sensing and GIS: the fate of forests inside and outside protected areas of Southwestern Ghana. Environ. & Planning B 35: 280–295. doi: 10.1068/b32091
- Anim-Kwapong, G.J. & Teklehaimanot, Z. 1995. Reclamation of degraded cocoa lands using Albizia zygia. Land Degrad. & Rehab. 6: 109–123. doi: 10.1002/ldr.3400060205
- Arbeiter, S. & Tegetmeyer, C. 2011. Home range and habitat use by Aquatic Warblers Acrocephalus paludicola on their wintering grounds in Northwestern Senegal. Acta Ornithol. 46: 117–126. doi: 10.3161/000164511X625883
- Baillie, S.R., Marchant, J.H., Leech, D.I., Massimino, D., Sullivan, M.J.P., Eglington, S.M., Barimore, C., Dadam, D., Downie, I.S., Harris, S.J., Kew, A.J., Newson, S.E., Noble, D.G., Risely, K. & Robinson, R.A. 2014. BirdTrends 2014: Trends in Numbers, Breeding Success and Survival for UK Breeding Birds. BTO Research Report 662. BTO, Thetford. http://www.bto.org/birdtrends
- Barton, K. 2014. MuMIn: Multi-model Inference. R package version 1.10.5. http://CRAN.R-project.org/package=MuMIn
- Bibby, C.J., Bain, C.G. & Burges, D.J. 1989. Bird communities of highland birchwoods. Bird Study 36: 123–133. doi: 10.1080/00063658909477014
- Blackburn, E. & Cresswell, W. 2015. Fine-scale habitat use during the non-breeding season suggests that winter habitat does not limit breeding populations of a declining long-distance Palearctic migrant. J. Avian Biol. 46: 622–633. doi: 10.1111/jav.00738
- Borger, L., Franconi, N., de Michele, G., Gantz, A., Meschi, F., Manica, A., Lovari, S. & Coulson, T. 2006. Effects of sampling regime on the mean and variance of home range size estimates. J. Anim. Ecol. 75: 1393–1405. doi: 10.1111/j.1365-2656.2006.01164.x
- Braimoh, A.K. 2004. Seasonal migration and land-use change in Ghana. Land Degrad. & Develop. 15: 37–47. doi: 10.1002/ldr.588
- Braimoh, A.K. & Vlek, P.L.G. 2005. Land-cover change trajectories in Northern Ghana. Environ. Manage. 36: 356–373. doi: 10.1007/s00267-004-0283-7
- Brink, A.B. & Eva, H.D. 2009. Monitoring 25 years of land cover change dynamics in Africa: a sample based remote sensing approach. Appl. Geog. 29: 501–512. doi: 10.1016/j.apgeog.2008.10.004
- Broström, G. 2013. glmmML: Generalized Linear Models with Clustering. R package version 1.0. http://CRAN.R-project.org/package=glmmML
- Burfield, I. & van Bommel, F. 2004. Birds in Europe: Population Estimates, Trends and Conservation Status. Birdlife International, Cambridge.
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. Springer, New York.
- Calenge, C. 2006. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197: 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
- Cramp, S. 1988. The Birds of the Western Palearctic, Vol. 6. Oxford University Press, Oxford.
- Delahaye, L. & Vandevyvre, X. 2008. Le Pouillot siffleur (Phylloscopus sibilatrix) est-il une espèce indicatrice de al qualité des forêts feuillues ardennaises? Aves 45: 3–14.
- Douglas, D.J.T., Nalwanga, D., Katebaka, R., Atkinson, P.W., Pomeroy, D.E., Nkuutu, D. & Vickery, J.A. 2014. The importance of native trees for forest bird conservation in tropical farmland. Anim. Cons. 17: 256–264. doi: 10.1111/acv.12087
- Dowsett-Lemaire, F. & Dowsett, R.J. 2014. The Birds of Ghana. Tauraco Press, Liège.
- EBCC 2014. Trends of Common Birds in Europe, 2015. Accessed July 28, 2015. http://www.ebcc.info/index.php?ID=587.
- Gockowski, J. & Sonwa, D. 2011. Cocoa intensification scenarios and their predicted impact on CO2 emissions, biodiversity conservation, and rural livelihoods in the Guinea Rain Forest of West Africa. Environ. Manage. 48: 307–321. doi: 10.1007/s00267-010-9602-3
- Gray, L.C. 1999. Is land being degraded? A multi-scale investigation of landscape change in Southwestern Burkina Faso. Land Degrad. & Develop. 10: 329–343. doi: 10.1002/(SICI)1099-145X(199907/08)10:4<329::AID-LDR361>3.0.CO;2-I
- Green, R.E., Cornell, S.J., Scharlemann, J.P.W. & Balmford, A. 2005. Farming and the fate of wild nature. Science 307: 550–555. doi: 10.1126/science.1106049
- Hahn, S., Bauer, S. & Liechti, F. 2009. The natural link between Europe and Africa – 2.1 billion birds on migration. Oikos 118: 624–626. doi: 10.1111/j.1600-0706.2008.17309.x
- Hayward, M.W. & Kerley, G.I.H. 2005. Prey preferences of the lion (Panthera leo). J. Zool. Lond. 267: 309–322. doi: 10.1017/S0952836905007508
- Hennenberg, K.J., Goetze, D., Minden, V., Traoré, D. & Porembski, S. 2005. Size-class distribution of Anogeissus leiocarpus (Combretaceae) along forest-savannah ecotones in northern Ivory Coast. J. Trop. Ecol. 21: 273–281. doi: 10.1017/S0266467404002160
- Hewson, C.M., Amar, A., Lindsell, J.A., Thewliss, R.M., Butler, S., Smith, K. & Fuller, R.J. 2007. Recent changes in bird populations in British broadleaved woodland. Ibis. 149 (Suppl. 2): 14–28. doi: 10.1111/j.1474-919X.2007.00745.x
- Hewson, C.M. & Noble, D.G. 2009. Population trends of breeding birds in British woodlands over a 32-year period: relationships with food, habitat use and migratory behaviour. Ibis 151: 464–486. doi: 10.1111/j.1474-919X.2009.00937.x
- Hulme, M.F. & Cresswell, W. 2012. Density and behaviour of Whinchats Saxicola rubetra on African farmland suggest that winter habitat conditions do not limit European breeding populations. Ibis 154: 680–692. doi: 10.1111/j.1474-919X.2012.01258.x
- Jacobs, J. 1974. Quantitative measurement of food selection. Oecologia 14: 413–417. doi: 10.1007/BF00384581
- Jones, P., Vickery, J., Holt, S. & Cresswell, W. 1996. A preliminary assessment of some factors influencing the density and distribution of Palearctic passerine migrants wintering in the Sahel zone of West Africa. Bird Study 43: 73–84. doi: 10.1080/00063659609460997
- Klein Goldewijk, K. 2001. Estimating global land use change over the past 300 years: the HYDE database. Global Biogeochem. Cycles 15: 417–433. doi: 10.1029/1999GB001232
- Lovett, P.N. & Haq, N. 2000. Evidence for anthropic selection of the Sheanut tree (Vitellaria paradoxa). Agrofor. Syst. 48: 273–288. doi: 10.1023/A:1006379217851
- Mallord, J.W., Charman, E.C., Cristinacce, A. & Orsman, C.J. 2012a. Habitat associations of Wood Warblers Phylloscopus sibilatrix breeding in Welsh oakwoods. Bird Study 59: 403–415. doi: 10.1080/00063657.2012.727780
- Mallord, J.W., Orsman, C.J., Cristinacce, A., Butcher, N., Stowe, T.J. & Charman, E.C. 2012b. Mortality of Wood Wabler Phylloscopus sibilatrix nests in Welsh oakwoods: predation rates and the identification of nest predators using miniature nest cameras. Bird Study 59: 286–295. doi: 10.1080/00063657.2012.669359
- Mallord, J.W., Smith, K.W., Bellamy, P.E., Charman, E.C. & Gregory, R.D. 2016. Are changes in breeding habitat responsible for population declines of long-distance migrant birds? Bird Study. doi:10.1080/00063657.2016.1182467
- Marti, J. 2007. Zur Habitatwahl des Waldlaubsängers Phylloscopus sibilatrix im Kanton Glarus. Der Ornithol. Beobach. 104: 45–52.
- Moreau, R.E. 1970. Changes in Africa as a wintering area for Palearctic birds. Bird Study 17: 95–103. doi: 10.1080/00063657009476262
- Morel, G.J. & Morel, M-Y. 1992. Habitat use by Palearctic migrant passerine birds in West Africa. Ibis 134 (Suppl. 1): 83–88.
- Morrison, C.A., Robinson, R.A., Clark, J.A., Risely, K. & Gill, J.A. 2013. Recent population declines in Afro-Palearctic migratory birds: the influence of breeding and non-breeding seasons. Divers. Distrib. 19: 1051–1058. doi: 10.1111/ddi.12084
- Nicholson, S. 2005. On the question of the ‘recovery’ of the rains in the West African Sahel. J. Arid Environ. 63: 615–641. doi: 10.1016/j.jaridenv.2005.03.004
- Norman, D. & Peach, W.J. 2013. Density-dependent survival and recruitment in a long-distance Palearctic migrant, the Sand Martin Riparia riparia. Ibis 155: 284–296. doi: 10.1111/ibi.12036
- Norris, K., Asase, A., Collen, B., Gockowski, J., Mason, J., Phalan, B. & Wade, A. 2010. Biodiversity in a forest-agriculture mosaic – the changing face of West African rainforests. Biol. Cons. 143: 2341–2350. doi: 10.1016/j.biocon.2009.12.032
- Ockendon, N., Hewson, C.M. Johnston, A. & Atkinson, P.W. 2012. Declines in British-breeding populations of Afro-Palearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study 59: 111–125. doi: 10.1080/00063657.2011.645798
- Ouedraogo, I., Tigabu, M., Savadogo, P., Compaoré, H., Odén, P.C. & Ouadba, J.M. 2010. Land over change and its relation with population dynamics in Burkina Faso, West Africa. Land Degrad. & Develop. 21: 453–462.
- Owubah, C.E., Le Master, D.C., Bowker, J.M. & Lee, J.G. 2001. Forest tenure systems and sustainable forest management: the case of Ghana. Forest Ecol. & Manage. 149: 253–264. doi: 10.1016/S0378-1127(00)00557-0
- Paré, S., Söderberg, U., Sandewall, M. & Ouadba, J.M. 2008. Land use analysis from spatial and field data capture in southern Burkina Faso, West Africa. Agric, Ecosyst. & Environ. 127: 277–285. doi: 10.1016/j.agee.2008.04.009
- Parrotta, J.A., Oteng-Yeboah, A. & Cobbinah, J. (eds) 2009. Traditional Forest-Related Knowledge and Sustainable Forest Management in Africa. Proceedings of Conference, Accra, October 2008, IUFRO, Vienna.
- Pasinelli, G. 2000. Oaks (Quercus sp.) and only oaks? Relations between habitat structure and home range size of the middle spotted woodpecker (Dendrocopos medius). Biol. Cons. 93: 227–235. doi: 10.1016/S0006-3207(99)00137-8
- Peach, W., Bailie, S. & Underhill, L. 1991. Survival of British Sedge Warblers Acrocephalus schoenobaenus in relation to West African rainfall. Ibis 133: 300–305. doi: 10.1111/j.1474-919X.1991.tb04573.x
- Phalan, B., Onial, M., Balmford, A. & Green, R.E. 2011. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333: 1289–1291. doi: 10.1126/science.1208742
- R Core Team. 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
- Redfern, C.P.F. & Clark, J.A. 2001. Ringers’ Manual. British Trust for Ornithology, Thetford.
- Ricketts, T.H. 2004. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Cons. Biol. 18: 1262–1271. doi: 10.1111/j.1523-1739.2004.00227.x
- Roe, D., Elliott, J., Sandbrook, C. & Walpole, M. (eds). 2013. Biodiversity Conservation and Poverty Alleviation: Exploiting the Evidence for a Link. John Wiley & Sons, Ltd, Chichester.
- Sanderson, F.J., Donald, P.F., Pain, D.J., Burfield, I.J. & van Bommel, F.P.J. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Cons. 131: 93–105. doi: 10.1016/j.biocon.2006.02.008
- Sheehan, D.K. & Sanderson, F.J. 2012. Seeing the bigger picture: how anthropogenic landscape modification in Africa affects declining migratory birds and the need for trans-continental research and conservation. Ibis 154: 659–662. doi: 10.1111/j.1474-919X.2012.01275.x
- Smith, K.D. 1974. The utilization of gum trees by birds in Africa. Ibis 116: 155–164. doi: 10.1111/j.1474-919X.1974.tb00235.x
- Stoate, C. 1997. Abundance of Whitethroats Sylvia communis and potential invertebrate prey, in two Sahelian sylvi-agricultural habitats. Malimbus 19: 7–11.
- Stoate, C. & Moreby, S.J. 1995. Premigratory diet of trans-Saharan migrant passerines in the western Sahel. Bird Study 42: 101–106. doi: 10.1080/00063659509477156
- Svensson, L. 1992. Identification Guide to European Passerines, 4th edn. Privately published, Stockholm.
- Thaxter, C.B., Joys, A.C. Gregory, R.D., Baillie, S.R. & Noble, D.G. 2010. Hypotheses to explain patterns of population change among breeding bird species in England. Biol. Cons. 143: 2006–2019. doi: 10.1016/j.biocon.2010.05.004
- Urban, E.K., Hilary Fry, C.H. & Keith, S. 1997. The Birds of Africa, Volume 5: Thrushes to Puffback Flycatchers. Princeton University Press, Princeton, NJ.
- Vickery, J.A., Ewing, S.R., Smith, K.W., Pain, D.J., Bairlein, F., Škorpilova, J. & Gregory, R.D. 2014. The decline of Afro-Palearctic migrants and an assessment of potential causes. Ibis 156: 1–22. doi: 10.1111/ibi.12118
- Wesołowski, T. 1985. The breeding ecology of the Wood Warbler Phylloscopus sibilatrix in primaeval forest. Ornis Scand. 16: 49–60. doi: 10.2307/3676575
- Willemoes, M., Tøttrup, A.P., Lerche-Jørgensen, M., Jacobsen, E.M., Reeve, A.H. & Thorup, K. in prep. Response to non-breeding habitat changes in long-distance migratory songbirds varies among species.
- Wilson, J.M. & Cresswell, W. 2006. How robust are Palearctic migrants to habitat loss and degradation in the Sahel? Ibis 148: 789–800. doi: 10.1111/j.1474-919X.2006.00581.x
- Winstanley, D., Spencer, R. & Williamson, K. 1974. Where have all the Whitethroats gone? Bird Study 2: 1–14.
- Worton, B.J. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70: 164–168. doi: 10.2307/1938423
- Wright, H.L., Lake, I.R. & Dolman, P.M. 2012. Agriculture – a key element for conservation in the developing world. Conserv. Lett. 5: 11–19. doi: 10.1111/j.1755-263X.2011.00208.x
- WWF. 2015. Terrestrial ecoregions. Accessed October 19, 2015. http://www.worldwildlife.org/biome-categories/terrestrial-ecoregions
- Zabel, C.J., McKelvey, K. & Ward Jr, J.P. 1995. Influence of primary prey on home-range size and habitat-use patterns of northern spotted owls (Strix occidentalis caurina). Can. J. Zool. 73: 433–439. doi: 10.1139/z95-049
- Zwarts, L., Bijlsma, R.G., van der Kamp, J., Wymenga, E., Zwarts, J. & Visser, D. 2009. Living on the Edge: Wetlands and Birds in a Changing Sahel. KNNV Publishing, Zeist, Pays-Bas.
- Zwarts, L., Bijlsma, R.G., van der Kamp, J., Sikkema, M. & Wymenga, E. 2015. Moreau’s paradox reversed, or why insectivorous birds reach high densities in savanna trees. Ardea 103: 123–144. doi: 10.5253/arde.v103i2.a2
