ABSTRACT
Capsule: Across Britain, breeding Eurasian Curlew Numenius arquata are less numerous and have shown greater population declines in areas with more arable farming, woodland cover and higher generalist predator abundance.
Aims: We present the first national-scale analysis of the potential drivers of Curlew population change in Britain, which is needed to guide conservation action for this globally near-threatened, declining species.
Methods: Breeding Bird Survey data and environmental predictors were used to model variation in Curlew abundance in 1995–99 and 2007–11, and population change between these periods.
Results: Arable farming and woodland cover were negatively associated with Curlew abundance and population declines. Curlew abundance was positively associated with extent of protected area coverage and gamebird numbers. Abundance and population change were positively associated with cooler temperatures and higher summer rainfall, but negatively associated with numbers of generalist predators.
Conclusions: We found support for the negative effects of intensive agriculture, forestry, increases in generalist predator populations and climate warming on Curlew abundance and population change. Effective site protection and measures to reduce generalist predator abundance may be important conservation measures, together with improving breeding habitat quality in the wider countryside.
Wader (shorebird) populations are declining worldwide (Butchart et al. Citation2010, IUCN Citation2016), with causes likely to include loss and degradation of breeding, stopover and wintering habitats due to a range of factors including agricultural intensification, coastal development, fisheries and climate change (International Wader Study Group Citation2003, Thomas et al. Citation2006, Wauchope et al. Citation2016, Pearce-Higgins et al. Citation2017, Studds et al. Citation2017). Throughout temperate Europe, breeding waders often occupy human-modified landscapes and in many countries, including the UK (Eaton et al. Citation2015), populations are rapidly declining due to a combination of changing land use practices and predation (Wilson et al. Citation2004, MacDonald & Bolton Citation2008), with future climate change likely to result in additional pressure (Pearce-Higgins Citation2010, Renwick et al. Citation2012, Pearce-Higgins et al. Citation2017). A recent addition to the UK red list is the globally near-threatened Eurasian Curlew Numenius arquata (hereafter Curlew), which has declined widely across Europe over the last 30 years (BirdLife International Citation2015). Curlew breed in a range of agricultural, semi-natural and natural open habitats across boreal and temperate regions of western, central and northern Europe and eastwards to the Russian steppes, and winter predominantly on estuaries and coastal grasslands throughout Western Europe (Cramp & Simmons Citation1983). In the UK, the species breeds predominantly on unenclosed upland heath, bog and grassland (‘moorland’) and enclosed marginal upland grassland habitats. Smaller numbers breed in lowland wet meadows and heath. The UK breeding population comprises an important proportion of both the European (28%) and global (19–27%) populations (BirdLife International Citation2015, Citation2017, Brown et al. Citation2015a, Wetlands International Citation2016), but is in rapid decline: 48% of the breeding population has been lost between 1995 and 2014 (Harris et al. Citation2016), and in Britain the species has experienced a range contraction of 17% since approximately 1990 (Balmer et al. Citation2013). Declines have been greatest in Scotland, Wales and Northern Ireland (Johnstone et al. Citation2007, Balmer et al. Citation2013, Colhoun et al. Citation2015, Harris et al. Citation2016), while in neighbouring Ireland, declines have led to a 78% range contraction since the 1980s, and as few as 200 breeding pairs may remain in the country (Kelly et al. Citation2016).
As a result of the species’ global conservation status and the importance of the UK breeding population and its long-term national decline and range contraction, the Curlew has been proposed as the UK’s highest avian conservation priority (Brown et al. Citation2015a). As a precursor to implementing effective conservation action there is an urgent need to better understand the causes of large-scale declines, which may also inform the conservation of other declining wader and upland-breeding species in the UK and more widely across Europe (Balmer et al. Citation2013, BirdLife International Citation2015).
Adult Curlew annual survival rates are high in the absence of hunting and harvesting of intertidal prey, ranging from 82% to 95% (Berg Citation1994, Valkama & Currie Citation1999, Taylor & Dodd Citation2013), pointing to low reproductive success as the likely demographic driver of breeding population declines throughout Europe (Grant et al. Citation1999, Roodbergen et al. Citation2012, Brown et al. Citation2015a). Local and regional studies have identified a range of potential hypotheses for declines (); primarily the loss and degradation of natural and semi-natural breeding habitats through changing land use, particularly agricultural intensification and afforestation. These changes have likely also contributed to increasing numbers of generalist predators in the wider landscape, as well as increasing the vulnerability of nests and chicks to predation (Douglas et al. Citation2014, Kentie et al. Citation2015).
Table 1. Hypotheses on the potential drivers of Curlew abundance and population change and support for each based on the results from three analyses: (1) correlates of Curlew abundance in 1995–99; (2) correlates of Curlew abundance in 2007–11; (3) correlates of Curlew population change between 1995–99 and 2007–11. Hypotheses were supported if all results aligned with predictions, were partially supported if some results aligned with predictions, and were not supported if results did not align with predictions. All hypotheses were proposed a priori, apart from declining quality of heath and bog habitats which arose from our results.
Review of existing evidence for population change
Changing agricultural land use practices are likely to influence Curlew population declines through habitat degradation (Baines Citation1988, Wilson et al. Citation2004), potentially reducing the quality of foraging habitats for both adults and chicks and/or making nests and chicks more vulnerable to predation, for example through habitat simplification (Whittingham & Evans Citation2004). Curlew abundance is greater in areas with more heterogeneous sward height (Pearce-Higgins & Grant Citation2006). This potentially accounts for documented short-term responses to rapid changes in grazing pressure or moorland cutting, which is likely to modify sward structure (Grant & Pearce-Higgins Citation2012, Fisher & Walker Citation2015, Douglas et al. Citation2017). Historic increases in sheep grazing in the uplands led to large-scale changes in moorland vegetation (Anderson & Yalden Citation1981, Fuller & Gough Citation1999), but there is little clear evidence this strongly affected breeding Curlew populations (Amar et al. Citation2011, Douglas et al. Citation2014, Citation2017).
Curlew habitat quality is likely to be influenced by conversion of enclosed farmland from more heterogeneous semi-natural grassland to agriculturally improved grassland through soil drainage, fertilization and re-seeding. These practices can result in a species-poor and structurally uniform sward (Baines Citation1988, Vickery et al. Citation2001), though fertilization and liming may increase the availability of some soil invertebrates (McCallum et al. Citation2015). Enclosed grassland can provide nesting sites (O’Brien Citation2004), but also foraging sites for birds breeding on nearby unenclosed moorland (Robson Citation1998), particularly if fields have high densities of soil invertebrates (Berg Citation1992, Valkama et al. Citation1998, McCallum et al. Citation2015). Curlew nests tend to be associated with intermediate to tall swards (10–45 cm) with a high frequency of tussocks (Valkama et al. Citation1998, Citation1999, Durant et al. Citation2008), and with improved grassland that includes rush cover (Baines Citation1988, Dallimer et al. Citation2010). Carrion Crow Corvus corone abundance is greater in improved grassland (Dallimer et al. Citation2010), which could result in higher predation rates in these habitats. While predation is likely to be the main cause of nest failure in agricultural grassland, farm operations and trampling by livestock may also result in nest destruction (Berg Citation1992, Grant et al. Citation1999).
Predation is very likely to be an important driver of low productivity (Berg Citation1992, Grant et al. Citation1999, Valkama & Currie Citation1999), with generalist predators such as foxes, corvids and mustelids identified as likely sources of nest predation and chick mortality (Robson Citation1998, Grant et al. Citation1999). While Red Fox Vulpes vulpes populations in the UK have been predominantly stable (Battersby & Tracking Mammals Partnership Citation2005, Noble et al. Citation2012, but see evidence for urban fox increases in Scott et al. Citation2014, and long-term declines in Harris et al. Citation2016), populations of Carrion Crow (+19%) and Raven Corvus corax (+45%) have increased over the last two decades (Amar et al. Citation2010, Harris et al. Citation2016). Curlew breeding on unenclosed moorland in areas with adjacent woodland, particularly commercial conifer plantations, are less abundant and have shown greater population declines and reduced breeding success (Pearce-Higgins et al. Citation2009a, Douglas et al. Citation2014). This may be a product of edge effects, whereby generalist predator activity may be greater on open ground adjacent to woodlands (Valkama et al. Citation1999, Douglas et al. Citation2014), though some have not found evidence for this (Amar et al. Citation2011). Aspects of moorland management to benefit gamebirds, particularly Red Grouse Lagopus lagopus scotica, have been positively associated with Curlew breeding densities, population change and breeding success (Tharme et al. Citation2001, Pearce-Higgins & Grant Citation2006, Baines et al. Citation2008, Fletcher et al. Citation2010, Douglas et al. Citation2014), though others have found no evidence of this (Amar et al. Citation2011). Lethal predator control can increase breeding success and breeding abundance of Curlew on managed grouse moors (Fletcher et al. Citation2010), and can potentially serve to offset the negative impacts of increasing woodland extent (Douglas et al. Citation2014). However, some studies have found no or only weak relationships between predator abundance and Curlew abundance or population change, suggesting that predation impacts might be spatially variable (van der Wal & Palmer Citation2008, Amar et al. Citation2010, Citation2011). In addition to predator control, rotational burning of heather moorland for grouse management may also create variation in sward height that is associated with increased Curlew breeding abundance (Pearce-Higgins & Grant Citation2006), and newly burnt areas may be used for nesting (Robson Citation1998).
Breeding Curlew are also likely to be sensitive to ongoing and future climate change, with declines predicted in the UK population by 2080 as a result of drier conditions (Renwick et al. Citation2012), and evidence that populations have shown a significant upward shift in elevation through time (Massimino et al. Citation2015). The effects of climate change may reduce the availability of invertebrate food resources for both adults and chicks, especially on peatland (Holden et al. Citation2007, Pearce-Higgins Citation2010, Carroll et al. Citation2011), but the extent to which observed population change is related to temperature and precipitation is unknown.
Here, we present the first large-scale assessment of correlates of change in British Curlew populations, which is urgently needed in order to quantify the extent to which different pressures are contributing to declines (Pearce-Higgins et al. Citation2017). Our aim is to quantify associations between Curlew abundance and recent population change, hypothesized environmental correlates including habitat, predator abundance and climate, and additional predictors which may be important for Curlew, including the extent of protected area coverage, soil type and topography. Identifying these broad-scale relationships will provide an evidence-based framework from which to develop policy-level solutions and specific conservation measures which will help reverse the decline of this species.
Methods
Curlew abundance
We used national-scale Breeding Bird Survey (BBS) data to investigate the potential drivers of variation in Curlew abundance and population change across Great Britain between 1995 and 2011 at a 1 km resolution. Specifically, we compared abundance and population change between the earliest (1995–99) and latest (2007–11) five-year periods of the BBS which matched with corresponding availability of environmental data. We did not include Curlew data from Northern Ireland as some of our predictor variables covered Great Britain only. The BBS scheme is an annual (1994–present) citizen science monitoring scheme in which observers undertake two (early April to mid-May; mid-May to late June) visits to a regionally stratified random sample of an average of 2783 one-kilometre squares (Harris et al. Citation2016). Observers record the birds seen or heard along two 1 km line transects, each comprising five 200 m transect sections. Birds are recorded in three distance bands (0–25, 25–100 and 100+ m), while flying birds are separately recorded. Observers also use a three-point ordinal system to record four weather variables: cloud cover, rain, wind and visibility.
We used Curlew count data from the two bounded distance bands to estimate the detectability of breeding Curlew (as per Johnston et al. Citation2014). Detectability was modelled using the best model structure (∼visit + visibility) for Curlew from Renwick et al. (Citation2012). Including detectability as an offset in the models relating Curlew abundance to environmental covariates (see below) allowed counts to be converted into absolute abundance estimates of Curlew density per 1 km2.
To reduce the chance of inflating counts of breeding abundance by including pre-breeding or foraging flocks of particular wader species, including Curlew, standard BBS analytical methodology includes the removal of all records with 10 or more birds per 200 m transect section (Field & Gregory Citation1999). Large counts were rare in the remaining dataset, with <0.005% of the records comprising >5 individuals per transect section. We used data from the early April to mid-May visit period when birds are more likely to be establishing territories. To obtain a single count and detectability estimate for each 1 km square in each of the 1995–99 and 2007–11 periods, we averaged annual Curlew counts and detectability across the relevant years.
Environmental correlates
The following environmental factors were quantified for the squares and periods studied:
Habitat
We used UK Land Cover Map 2007 (Morton et al. Citation2011) to calculate the absolute proportional coverage per 1 km square of the four aggregate habitat types most relevant to Curlew: arable, improved grassland, semi-natural grassland and mountain/heath/bog (Table S1, online supplementary material). We did not model associations between breeding Curlew abundance and habitats where they are unlikely to occur (e.g. urban). Following Douglas et al. (Citation2014) and Wilson et al. (Citation2014), we also derived a predictor variable describing the absolute proportional coverage of woodland (broadleaf and coniferous) in the 8 squares adjacent to the focal 1 km square (online Table S1). We were unable to quantify habitat change between 1995–99 and 2007–11 as Land Cover Map 2007 is not directly comparable to earlier land cover maps of the UK (Morton et al. Citation2011).
Habitat serves as a proxy by which to assess the impacts of agricultural intensification on Curlew populations. We predict that if intensification is an important driver of population change, then densities should be lower and/or population declines greater in intensively managed arable and improved grassland habitats, while the opposite may be true in more semi-natural habitats (e.g. semi-natural grassland, heath and bog). However, invertebrate food resources may also vary between these habitats (Pearce-Higgins & Yalden Citation2003), and could be more abundant in nutrient-rich improved grassland (Berg Citation1992, Valkama et al. Citation1998). If invertebrate abundance is limiting populations, we predict higher Curlew densities and more positive population trends in improved grassland, contrary to the prediction above. We also predict that Curlew abundance and population change will be negatively associated with greater woodland cover, as a result of a long-term afforestation programme in marginal upland areas of the UK.
Site protection
We determined the proportion of each 1 km square that was included within a protected area to test the extent to which site protection has influenced Curlew populations (online Table S1). Protected area data were obtained from the World Database on Protected Areas (IUCN & UNEP-WCMC Citation2014). We predict that site protection will have a generally beneficial impact on Curlew, even in cases where the species is not included as a designated feature, as protection may include habitat management that creates or maintains generally more favourable habitat or creates barriers to more intensive development and land use.
Topography
We used the CGIAR-SRTM 90 m raster elevation data (Jarvis et al. Citation2008, available at http://srtm.csi.cgiar.org) to calculate mean elevation (m) per 1 km square (online Table S1), an elevation2 variable to allow for known associations with intermediate altitudes and mean slope (degrees) per 1 km square (online Table S1). We predict that Curlew will be associated with intermediate altitudes, characteristic of their more upland distribution. We also predict that similar to other breeding waders, Curlew will be associated with flatter ground, potentially due to associations with wet habitats in flat areas (Pearce-Higgins & Grant Citation2006, Wilson et al. Citation2014) and an improved ability to perceive nest predators (Whittingham et al. Citation2002).
We used the European soil database to calculate the mean percentage of organic carbon content in topsoil per 1 km square (Jones et al. Citation2003, available as a raster at http://eusoils.jrc.ec.europa.eu/themes/european-data). The resolution of the database is at a 1 km scale, so values were obtained for each 1 km square by extracting the raster value for the midpoint of each square on the British National Grid using extract in the raster package in R (R Core Team Citation2015, Hijmans et al. Citation2016). Breeding wader distributions are associated with soil conditions (McCallum et al. Citation2015), likely as a consequence of important invertebrate food resources being associated with particular conditions, though this can vary by invertebrate family (Coulson Citation1988, Edwards & Bohlen Citation1996). We had no a priori prediction of potential associations between Curlew and soil organic carbon, as there is little known about the associations between the different soil invertebrate groups selected by young and adult Curlew and soil type.
Climate
We assessed climatic associations by including relevant summer and winter variables which could influence Curlew food resources or breeding habitat conditions: mean monthly minimum winter (Dec–Feb) temperature, mean monthly maximum summer (Jun–Aug) temperature and mean total winter and summer rainfall as predictors (online Table S1); however, we recognize that elevational shifts may also be indicative of a climate change effect (Chen et al. Citation2011, Massimino et al. Citation2015). Spatial climate data were obtained from the UK Met Office’s 5 km gridded data (available at http://www.metoffice.gov.uk/climatechange/science/monitoring/ukcp09/download/index.html). Annual climate data were averaged across each of the 1995–99 and 2007–11 periods for each 1 km square. We predict that Curlew abundance and population change will be negatively associated with warmer temperatures and drier conditions.
Game management
Curlew breed widely in areas of Britain that are subject to Red Grouse management (Tharme et al. Citation2001, Amar et al. Citation2011, Douglas et al. Citation2014), often at higher densities than in areas not managed for grouse (Tharme et al. Citation2001, Baines et al. Citation2008). We used a recently published map of the extent of moorland strip burning for Red Grouse per 1 km square (Douglas et al. Citation2015) as a direct measure of the extent of burning on moorland (online Table S1) and as an index of the presumed overall intensity of grouse moor management. Squares that were outside the area mapped were assigned a value of 0% of heather moorland burned on the assumption that an initial mapping exercise by Anderson et al. (Citation2009) broadly captured areas subject to moorland burning for grouse. We predict that Curlew abundance and population change will be positively associated with the extent of strip burning, through its role in creating variation in vegetation height and as a proxy for grouse moor management and consequently, predator control.
In addition to using the extent of burning as an index of the intensity of game management, we also used BBS counts (as for Curlew above) to identify areas with high numbers of gamebirds, specifically Red Grouse and Pheasant Phasianus colchicus (online Table S1). Annual count data for each species were averaged across each of the 1995–99 and 2007–11 periods for each 1 km square, and these averaged counts were included as predictors in the models of Curlew abundance and population change. Red Grouse and Pheasant counts were included as separate covariates in the model because of management differences between them. We predicted that Curlew abundance and population change would be positively associated with Red Grouse and Pheasant numbers, as a proxy for aspects of game management such as predator control.
Predator populations
In addition to using game management intensity as an index of predator control, we used BBS data to obtain indices of potential Curlew nest predators, specifically crow abundance and Red Fox occurrence (Robson Citation1998, Grant et al. Citation1999). We averaged combined annual BBS counts of Carrion and Hooded Crows Corvus cornix per 1 km square for each of the 1995–99 and 2007–11 periods (online Table S1). BBS surveyors also record mammals, and we used these data to estimate the probability of Red Fox occurrence. Foxes have a low detection rate, recorded on an average of 281 ± 53 BBS squares per year, so we modelled the probability of a fox occurring at the 10 km square level (online Table S1). Using a generalized linear mixed model with a binomial error structure and a logit link function, we modelled fox occurrence per 1 km square as the response variable, with 10 km square and year as covariates and 1 km square as a random effect, and then used the model to predict occurrence per 10 km square. Due to insufficient data, we were unable to model change in fox occurrence between time periods, so we took the average probability of fox occurrence across both time periods and used the same values for both 1995–99 and 2007–11. We predict that Curlew abundance and population change will be negatively associated with both crow abundance and fox occurrence.
Statistical analysis
We used generalized additive models (GAM) to investigate associations between environmental covariates and Curlew abundance and population change in three separate analyses (online Table S1). For all analyses, we modelled Curlew counts using a log link function with a negative binomial error structure in R using the gam function in the mgcv package (R Core Team Citation2015, Wood Citation2016), and accounted for spatial variability in Curlew abundance by including normalized easting and northing as a spatial smooth with value k = 20.
Models of Curlew abundance in 1995–99 and 2007–11
In Analyses 1 and 2, we modelled Curlew breeding abundance separately for 1995–99 and 2007–11. In each model, we included the natural log of average Curlew detectability per 1 km square across the relevant period as an offset, allowing counts to be converted into absolute abundance estimates of Curlew density per 1 km2. Observations were weighted by the inverse of the sampling effort within each 1 km square’s survey region to account for spatial variation in survey coverage (coverage varies between regions from <1% to 50%); thus, squares in regions with low survey coverage (e.g. northern Scotland) are given more weight to reduce bias in modelled abundance estimates towards better surveyed regions (e.g. southern England).
Models of Curlew population change between 1995–99 and 2007–11
In Analysis 3, we modelled the change in abundance between 1995–99 and 2007–11 by using the count in 2007–11 as the response, and the natural log of the 1995–99 count as an offset. We assumed detectability remained constant at the square level so changes in count reflect changes in density. Observations were again weighted by the inverse of regional sampling effort. For those covariates for which we had period-specific data (climate, gamebird and crow abundance), we included both the early and the late period variables as separate covariates, as well as their interaction, which means that changes in these variables can be included alongside their period-specific values, without making any assumptions about the form of any relationship between Curlew population change and predictor variables. We centred these covariates using the scale function to make interpretation of interactions between early and late measures of a variable easier and to reduce multicollinearity between interaction terms and their component main effects (Aiken et al. Citation1991). For this analysis, we used only those BBS squares (n = 241) which were surveyed in both periods and where Curlew were present in 1995–99, excluding the 22% of squares (n = 67) that were colonized as we could not take the natural log of zero. This is appropriate given our focus on identifying correlates of population declines. However, recognizing that colonized squares contain important information, we additionally used t-tests (α = 0.05) to compare mean values of predictor variables between squares colonized between 1995–99 and 2007–11 and squares which were not colonized.
Modelling approach
Given the large number of predictor variables we considered, we used a three-step modelling approach for analyses. In Step 1, we modelled univariate relationships between count and predictor variables, and retained significant (P < 0.05) predictors for inclusion in subsequent steps (online Table S1). In Step 2, we grouped variables into relevant categories (habitat, topography, climate, game management + predator populations) and using separate multiple regression models for each category, simplified through backwards deletion to a minimum adequate model (MAM; online Table S2). To address multicollinearity between predictors in the same category, we first removed covariates with high variance inflation factors (VIFs ≥ 3) and/or high (≥0.70) pairwise correlations (Dormann et al. Citation2013; see online Table S3 & S4 for correlation matrices). For highly correlated variables, we retained the predictor that had the strongest relationship with the response variable in Step 1. In Step 3, we combined the significant variables from the MAMs in Step 2 into a single model, first removing those with high VIFs and pairwise correlations in relation to other model covariates, as above. The one exception was elevation, which was strongly correlated with a number of other predictor variables as a linear term, but was retained in conjunction with its quadratic term due to the significant non-linear relationship between Curlew abundance and elevation. Finally, we simplified by backwards deletion to produce the final model.
Results
Model of Curlew abundance in 1995–99
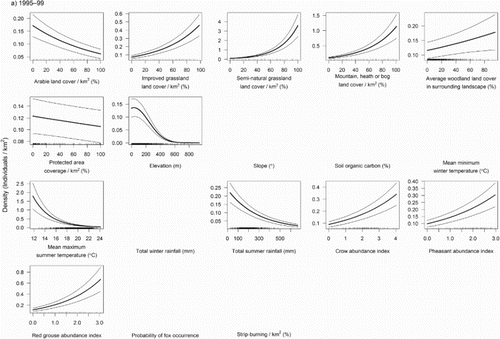
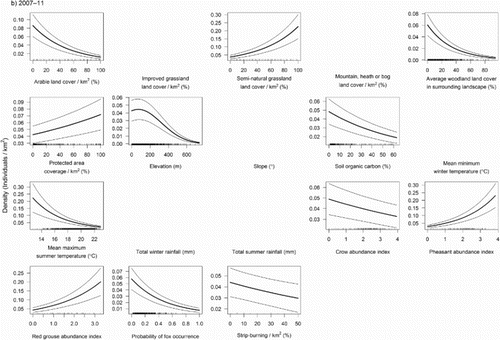
The MAM of Curlew abundance from 1995–99 included 13 predictor variables and explained 58.3% of the deviance in abundance (Analysis 1; ). Habitat variables had some of the statistically strongest associations with Curlew abundance, with positive relationships between density and the extent of semi-natural grassland, heath and bog, and improved grassland ((a)). Curlew abundance was negatively associated with arable habitat, but was weakly positively associated with an increasing proportion of woodland in the landscape. The amount of protected area coverage in a 1 km square was weakly negatively associated with Curlew density. Curlew abundance was highest at low to intermediate elevations, and was negatively related to warmer summer temperatures and high summer rainfall. Gamebird and crow abundance had strong associations with Curlew abundance; Curlew density was positively associated with both grouse and pheasant abundance, and also with crow abundance.
Figure 1. Relationships between Curlew density (individuals/km2) in (a) 1995–99 and (b) 2007–11 and environmental predictors. Thick lines show the significant predicted relationship between abundance and covariates in the final minimum adequate GAM. Thin lines show the 95% confidence intervals. Rug plots along the x-axis show the distribution of the original values of the predictor variable which were used in the model. Panels with no relationship were found to be non-significant during the stepwise modelling process.
Table 2. Final MAM parameter estimates, standard errors, z and P values from models of factors associated with abundance (in 1995–99 and 2007–11) and changes in abundance (between 1995–99 and 2007–11) of breeding Curlew in Britain. The linear elevation term was retained in the model of abundance in 1995–99 as the squared elevation term was significant in this model.
Model of Curlew abundance in 2007–11
The MAM of Curlew abundance for 2007–11 was similar (Analysis 2; ) and contained 13 predictor variables, of which 10 were also present in the 1995–99 model. This model explained 55.9% of the deviance in abundance. Habitat variables were again strongly associated with Curlew abundance. Curlew density was positively affected by the amount of semi-natural grassland, and negatively associated with arable cover ((b)). Contrary to 1995–99, lower Curlew abundance was strongly associated with a high proportion of woodland in the landscape, while protected area coverage was significantly positively related to Curlew abundance, though fairly weakly. Curlew densities peaked at a slightly higher elevation (73 m) than in 1995–99 (32 m). Curlew abundance was negatively associated with soil organic carbon content. A negative association with summer temperature was consistent between 1995–99 and 2007–11. Curlew density had strong positive associations with grouse and pheasant abundance, though was only weakly negatively associated with an increasing amount of rotational strip burning. Greater numbers of crows and probability of fox occurrence were associated with fewer Curlew, though the relationship with fox occurrence was stronger than with crow abundance.
Models of Curlew population change between 1995–99 and 2007–11
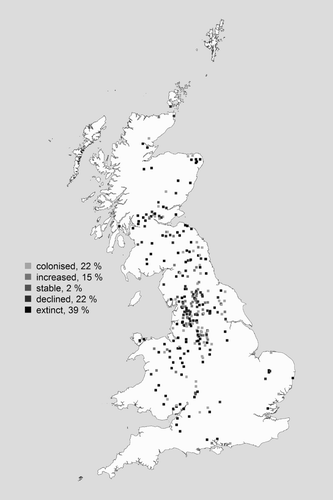
Between 1995–99 and 2007–11, Curlew declined or went extinct in 61% of 1 km squares (), but tended to colonize 1 km squares which had significantly less arable land cover and more improved grassland, semi-natural grassland and heath and bog (online Figure S1). Colonized squares were at higher elevations and had higher soil organic content, suggesting colonization of bog habitats (Figure S1). Many of these colonized areas are likely managed for gamebirds, suggested by significantly higher levels of strip burning, a higher abundance of grouse and more recently, pheasant, and lower numbers of foxes (Figure S1). Colonized squares were significantly wetter and also cooler in both summer and winter (Figure S1).
Figure 2. Patterns of Curlew population change in 1 km squares between 1995–99 and 2007–11. The 1 km squares shown (n = 308) were those surveyed in both periods and where Curlew colonized, increased, remained stable, declined or went extinct (percentage breakdown given in figure legend).
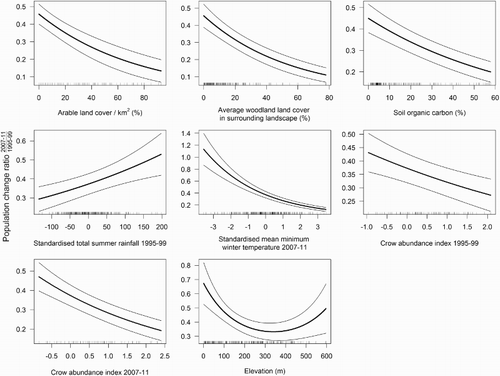
The MAM of population change on squares that retained or lost Curlew included 8 predictor variables which explained 64.1% of the model deviance (Analysis 3; ). Declines were strongly associated with increasing surrounding area of woodland habitat and high soil organic content, and were also associated with a greater extent of arable land cover (). Curlew declines were strongly associated with warmer winters in 2007–11; declines were also associated, though more weakly, with drier summers in 1995–99. Declines were greatest at intermediate to higher elevations. Higher crow abundance in both periods was associated with greater Curlew declines, and particularly strongly so in 2007–11.
Figure 3. Relationships between Curlew population change and significant environmental predictors in the final minimum adequate GAM. The 1 km squares included in the analysis (n = 241) were those surveyed in both periods and where Curlew increased, remained stable, declined or went extinct. Population change values from 1995–99 to 2007–11 are given as a ratio where a value of 1.0 = stability between the two periods. Solid lines show the significant predicted relationship between population change and covariates, while dashed lines show the 95% confidence intervals. Rug plots along the x-axis show the distribution of the original values of the predictor variable which were used in the model.
Discussion
Our study provides the most comprehensive assessment of the factors associated with patterns of British Curlew abundance and population change at a national scale. Between 1995–99 and 2007–11, we identified changes in the suite of factors explaining patterns of Curlew abundance, as well as correlates of population change over this period. Our results contribute evidence towards the different hypotheses previously suggested on the likely drivers of Curlew population declines ().
Habitat and topography
Habitat loss and degradation is one of the primary hypothesized mechanisms behind the declines of breeding Curlew through the loss of nesting and foraging sites and possibly by making nests and chicks more vulnerable to predation (Baines Citation1988, Whittingham & Evans Citation2004, Wilson et al. Citation2004, Douglas et al. Citation2014, Brown et al. Citation2015a). Habitat was indeed one of the strongest predictors of Curlew abundance in both periods, but the importance of different habitats changed over time. In both 1995–99 and 2007–11, semi-natural grassland supported the highest predicted densities of breeding Curlew of any habitat, indicating its likely importance for a significant proportion of the UK breeding population (O’Brien Citation1996). A consistent and strong negative association between arable habitat, abundance in both periods, and Curlew population trend supports the hypothesized negative impact of conversion of semi-natural habitat to enclosed farmland on breeding Curlew populations. Low breeding densities in this habitat together with greater declines in highly arable areas indicate a potential deterioration in habitat suitability and quality for nesting and foraging in agricultural landscapes, where nest destruction may also result from agricultural activities. While a relatively small proportion of the British Curlew population now occupies these habitats, loss of breeding Curlew from agricultural landscapes may have contributed to long-term declines.
While agriculturally improved grassland and mountain, heath and bog habitats were positively associated with Curlew abundance in 1995–99, our results suggest these habitats have declined in importance. This could be due to a reduction in their suitability, and/or is a result of semi-natural grassland habitats (either enclosed or unenclosed) tending to maintain breeding birds or being favoured as Curlew abundance declines. A reduced association with agriculturally improved grassland habitats in 2007–11 compared to 1995–99 may indicate potentially negative effects of intensive pastoral management (Baines Citation1988, Vickery et al. Citation2001). Although these nutrient-rich habitats have been associated with higher soil invertebrate abundance (McCallum et al. Citation2015), it appears that either there is no population-level benefit for Curlew, or alternatively, that any benefits are outweighed by other aspects of intensive grassland management.
While upland heath and, to a lesser extent, bog habitats are important breeding habitats for British Curlew, they appear to have become less important through time, as measured by negative associations between soil organic carbon and both Curlew abundance in 2007–11 and population change. In contrast to this, however, we also found that colonized squares were more likely to include a higher proportion of heath and bog habitat and have a higher soil organic carbon content. Curlew densities are generally lower in these habitats compared to semi-natural grassland, and so these apparently contradictory findings could reflect greater turnover (both extinction and colonization) in heath and bog. Alternatively, observed relationships with soil organic carbon content could also be driven by spatial variation in Curlew numbers and population change in Great Britain, with fewer birds and greater declines in north-western areas where soil organic carbon content is highest, perhaps linked to the lower productivity of these soils. Recent management of upland heath and bog habitats to re-vegetate bare peat and block drainage ditches as a climate change adaptation strategy should improve peatland habitat quality and increase invertebrate resources for breeding waders (Carroll et al. Citation2011). However, as the Diptera larvae which benefit most from drain-blocking form a lower proportion of Curlew diet than for other upland breeding waders (Pearce-Higgins Citation2010), Curlew may be a lesser beneficiary of such management.
Despite evidence that Curlew reproductive success and population change are negatively impacted by woodland around breeding sites (Valkama et al. Citation1999, Pearce-Higgins et al. Citation2009a, Douglas et al. Citation2014), Curlew abundance in 1995–99 was positively associated with woodland, albeit weakly so. More recently, however, woodland extent has had a negative impact on Curlew abundance, and population declines have been greater at sites with more woodland, patterns which are consistent with previously documented relationships. Extensive afforestation took place in the 1970s and 1980s in marginal upland habitats favoured by Curlew (Pearce-Higgins et al. Citation2007, Ratcliffe Citation2007). In 1995–99, afforested areas may not have been sufficiently mature to exclude Curlew, and only more recently have the hypothesized negative impacts of afforestation (fragmentation of open habitats and increases in generalist predators) manifested themselves. The unexpected relationship between Curlew abundance and woodland in 1995–99 might also indicate that afforestation has taken place in areas previously good for Curlew, further compounding its negative impact. This may be a result of the temporal mismatch between bird and habitat datasets, as we were limited to examining relationships with 2007 habitat data only.
Game management and predation
Previous research has suggested that grouse moor management, particularly predator control, may benefit breeding waders including Curlew (Tharme et al. Citation2001, Baines et al. Citation2008, Pearce-Higgins et al. Citation2009a, Fletcher et al. Citation2010, Douglas et al. Citation2014), and we predicted that indices of game management would therefore be positively associated with Curlew abundance. We found that red grouse and pheasant abundance, likely indices of game management, were positively associated with Curlew densities in both periods, despite management for Red Grouse and Pheasant occurring in largely different habitats from one another. Furthermore, both crow and fox abundance were negatively correlated with Curlew abundance in 2007–11, and Curlew population declines were greatest in areas with high crow abundance in both 1995–99 and 2007–11. Our results support the hypothesis that increasing predation pressure could play a role in Curlew population declines (Amar et al. Citation2010), although contrary to expectation, we found a positive association between Curlew and crow abundance in 1995–99. This relationship could arise, as for woodland cover above, from increasing crow populations in the marginal upland areas favoured by Curlew, and where Curlew have subsequently declined. Previous work has suggested that fox predation may be a particularly important driver of Curlew declines (Grant et al. Citation1999, Douglas et al. Citation2014), and our results indicate that more recently, Curlew abundance is lower in areas with a greater chance of fox occurrence. Although our findings indicate stronger evidence linking population declines to crow abundance, this is not necessarily indicative that crows are a more important predator of Curlew breeding attempts than foxes; rather, this relationship may simply reflect the finer temporal and spatial resolution of the crow data. Generally, however, our findings support the hypothesis that measures to reduce predation pressure from generalist predators such as foxes and crows are likely to be very important for Curlew.
While Red Grouse and Pheasant abundance were positively associated with Curlew abundance in both time periods, rotational strip burning was negatively associated with Curlew abundance in 2007–11, though weakly so. The amount of strip burning on British moorland has increased by 11% per year between 2001 and 2011 (Douglas et al. Citation2015). While indicative of grouse moor management and predator control, burning is also associated with a range of impacts on peatland function and habitat quality (Glaves et al. Citation2013, Brown et al. Citation2015b). Thus while low levels of strip burning could plausibly be beneficial for Curlew by creating variation in habitat structure, too much may be detrimental as burning can impact peatland hydrology and consequently soil moisture, reduce invertebrate prey, or alter vegetation structure or composition (Glaves et al. Citation2013, Brown et al. Citation2015b). Further research on the impacts of burning on Curlew would be valuable, as there is currently relatively little known about this relationship. A recent study also found that changes in Curlew abundance were positively associated with the amount of moorland cutting, which presumably relates to the creation of heterogeneity in vegetation height (Douglas et al. Citation2017).
Site protection
In human-modified landscapes such as much of Europe, protected areas have an increasingly important role in biodiversity conservation (Donald et al. Citation2007, Gray et al. Citation2016, Sanderson et al. Citation2016), either through habitat management or by creating barriers to more intensive development and land use. Significant areas of semi-natural habitats in Britain are protected as Sites of Special Scientific Interest or National Nature Reserves (national-level designations) and/or as Natura 2000 sites under the European Union’s Birds and Habitats Directives. At present, there are no Natura 2000 sites in the UK classified for breeding Curlew, although one (the North Pennine Moors SPA) has been recommended for classification (Stroud et al. Citation2001).
In 1995–99, Curlew abundance showed a weak negative association with increasing proportional coverage of protected areas. A decade later, this relationship had reversed such that Curlew densities increased with a greater extent of protected area coverage. However, the extent of protected area coverage was not a significant correlate of Curlew population change. Although this may partly reflect losses from agricultural, improved grassland habitats and retractions of Curlew to semi-natural habitats which are more likely to overlap with protected areas, the weak association with upland heath and bog habitats through time, which are even more strongly associated with protected areas, runs counter to this. Alternatively, our results are consistent with the hypothesis that protected areas provide benefits for bird populations, either directly or indirectly.
Climate change
There is increasing evidence for the impact of climate change on bird communities in the UK (Pearce-Higgins et al. Citation2015), though there is limited empirical evidence documenting effects on upland birds, despite the fact they are likely to be particularly vulnerable to climate change (Pearce-Higgins Citation2010, Carroll et al. Citation2015). Consistent negative associations between summer temperature and Curlew abundance support the findings of previous studies linking upland bird population change to summer temperatures, with negative impacts of warming on the availability and/or abundance of soil invertebrates as the likely mechanism (Pearce-Higgins Citation2010, Pearce-Higgins et al. Citation2010, Carroll et al. Citation2015). While summer temperature was not related to population change, Curlew declines were greatest in areas of high winter temperature in 2007–11. Although unlikely to be causal through direct impacts on Curlew survival (Pearce-Higgins et al. Citation2015), the effects of warming winters could be mediated through negative impacts on invertebrate prey populations (as identified recently for Lepidoptera; see Martay et al. Citation2016). Declines were also greatest in areas of low summer rainfall in 1995–99, consistent with hypothesized negative impacts of summer drought (Pearce-Higgins Citation2010). Curlew abundance in 1995–99 was negatively correlated with summer rainfall; however, this apparently contradictory pattern to the one above can potentially be explained by lower densities of breeding Curlew in the wetter, western parts of Britain compared to drier eastern areas (Pearce-Higgins & Grant Citation2006), which remains difficult to explain. In order to properly attribute observed patterns of Curlew abundance and population change to climate change, more detailed mechanistic research linking climate variables to changes in invertebrate populations and to Curlew productivity is needed if that is the mechanism involved.
Additional unmeasured factors
A number of other potential environmental pressures were not included in our analysis, primarily because the required large-scale datasets were lacking. Curlew breeding densities may be reduced in areas subject to wind farm development (Pearce-Higgins et al. Citation2009b, Citation2012), however the scale of such development is unlikely to have been sufficient to have caused significant population declines (Dobson et al. Citation2015). Similarly, there is some evidence that Curlew also appear to avoid areas of high human disturbance (Pearce-Higgins et al. Citation2006). While conditions on wintering grounds such as food availability, hunting pressure and severe weather have the potential to negatively impact survival (Taylor & Dodd Citation2013), we did not account for non-breeding season drivers of large-scale declines.
Conclusions
We assessed broad-scale patterns in Curlew abundance and population trends in Britain between 1995–99 and 2007–11, and found support or partial support for most of the hypotheses that have been suggested to explain Curlew population declines based on the findings of more localized studies (). Low reproductive success is the most likely driver of Curlew population declines (Grant et al. Citation1999, MacDonald & Bolton Citation2008, Roodbergen et al. Citation2012), with fledging rates likely to be lower than the estimate of 0.48–0.62 fledged young per breeding pair required annually to maintain a stable UK population (Grant et al. Citation1999, Brown et al. Citation2015a). We found the greatest support for the detrimental effects of arable farming, afforestation and generalist predators on both Curlew abundance and population change. These are all likely to impact reproductive success on some level, either by reducing nesting habitat suitability and quality, reducing the availability and quality of foraging habitat for chicks or increasing the vulnerability of nests and chicks to predation, either directly or indirectly.
Given the correlative nature of this study and extent of national population declines, we advocate the need for the rapid establishment of intensive studies to identify the mechanistic drivers of the patterns observed here and to test potential conservation management interventions. At these sites, variation in the extent and/or intensity of key land uses such as agriculture, forestry and grouse moor management (e.g. burning, cutting and predator control), predator abundance and invertebrate resources should be monitored and related to Curlew abundance and importantly, reproductive success. Experimental studies could be used to test potential restorative conservation management interventions, which based on our results, could include improving the habitat quality of grassland habitats and upland heath and bogs and reducing the impacts of generalist predators. Agri-environment schemes have the potential to improve habitat quality more broadly in the wider countryside, which may benefit Curlew; however this will require the deployment of evidence-based prescriptions which are spatially targeted and deployed at sufficient scale to have a meaningful benefit (but see O’Brien & Wilson Citation2011). Site protection could potentially play a key role in preventing further extensive declines, but alone, is unlikely to be a sufficient measure to reverse declines unless protection also includes management strategies likely to most benefit Curlew, including habitat restoration and reducing the negative impacts of predators. More information is also needed on the potential for habitat management to maintain and increase the abundance and availability of soil invertebrates, for example through reducing drainage (Carroll et al. Citation2011) or liming (McCallum et al. Citation2015). This would counter any negative impacts of current land management and may also serve to increase the resilience of Curlew populations to the potential negative effects of climate change.
Table S4
Download MS Excel (17.6 KB)Supplementary Material
Download MS Word (1.5 MB)Acknowledgements
Thanks to D. Massimino, A. Johnston and M. Miller for providing R code and statistical help, to D. Hodkinson and J. Wilson for helpful discussion and ideas, and to A. Hoodless and an anonymous reviewer for helpful comments which improved the manuscript. We are indebted to thousands of BBS volunteers who made this work possible.
Additional information
Funding
References
- Aiken, L.S., West, S.G. & Reno, R.R. 1991. Multiple Regression: testing and interpreting interactions. Sage, New York.
- Amar, A., Redpath, S., Sim, I. & Buchanan, G. 2010. Spatial and temporal associations between recovering populations of common raven Corvus corax and British upland wader populations. J. Appl. Ecol. 47: 253–262. doi: 10.1111/j.1365-2664.2010.01772.x
- Amar, A., Grant, M., Buchanan, G., Sim, I., Wilson, J., Pearce-Higgins, J.W. & Redpath, S. 2011. Exploring the relationships between wader declines and current land-use in the British uplands. Bird Study 58: 13–26. doi: 10.1080/00063657.2010.513412
- Anderson, P. & Yalden, D.W. 1981. Increased sheep numbers and the loss of heather moorland in the peak district, England. Biol. Conserv. 20: 195–213. doi: 10.1016/0006-3207(81)90029-X
- Anderson, B.J., Arroyo, B.E., Collingham, Y.C., Etheridge, B., Fernandez-De-Simon, J., Gillings, S., Gregory, R.D., Leckie, F.M., Sim, I.M.W., Thomas, C.D., Travis, J. & Redpath, S.M. 2009. Using distribution models to test alternative hypotheses about a species’ environmental limits and recovery prospects. Biol. Conserv. 142: 488–499. doi: 10.1016/j.biocon.2008.10.036
- Baines, D. 1988. The effects of improvement of upland, marginal grasslands on the distribution and density of breeding wading birds (charadriiformes) in northern England. Biol. Conserv. 45: 221–236. doi: 10.1016/0006-3207(88)90141-3
- Baines, D., Redpath, S., Richardson, M. & Thirgood, S. 2008. The direct and indirect effects of predation by Hen Harriers Circus cyaneus on trends in breeding birds on a Scottish grouse moor. Ibis 150: 27–36. doi: 10.1111/j.1474-919X.2008.00848.x
- Balmer, D.E., Gillings, S., Caffrey, B., Swann, R.L., Downie, I.S. & Fuller, R.J. 2013. Bird Atlas 2007-11: the breeding and wintering birds of Britain and Ireland. BTO, Thetford.
- Battersby, J. & Tracking Mammals Partnership. 2005. UK Mammals Species Status and Population Trends: first report by the Tracking Mammals Partnership. JNCC/Tracking Mammals Partnership, Peterborough.
- Berg, Å. 1992. Factors affecting nest-site choice and reproductive success of Curlews Numenius arquata on farmland. Ibis 134: 44–51. doi: 10.1111/j.1474-919X.1992.tb07228.x
- Berg, Å. 1994. Maintenance of populations and causes of population changes of curlews Numenius arquata breeding on farmland. Biol. Conserv. 67: 233–238. doi: 10.1016/0006-3207(94)90614-9
- BirdLife International. 2015. European Red List of Birds. Office for Official Publications of the European Communities, Luxembourg.
- Birdlife International. 2017. Species factsheet: Numenius arquata [online]. http://datazone.birdlife.org/species/factsheet/eurasian-curlew-numenius-arquata/refs [Accessed 10 May 2017].
- Brown, D., Wilson, J., Douglas, D., Thompson, P., Foster, S., McCulloch, N., Phillips, J., Stroud, D., Whitehead, S., Crockford, N. & Sheldon, R. 2015a. The Eurasian Curlew–the most pressing bird conservation priority in the UK. Br. Birds 108: 660–668.
- Brown, L.E., Holden, J., Palmer, S.M., Johnston, K., Ramchunder, S.J. & Grayson, R. 2015b. Effects of fire on the hydrology, biogeochemistry, and ecology of peatland river systems. Freshwater Science 34: 1406–1425. doi: 10.1086/683426
- Butchart, S.H.M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J.P.W., Almond, R.E.A., Baillie, J.E.M., Bomhard, B., Brown, C., Bruno, J., Carpenter, K.E., Carr, G.M., Chanson, J., Chenery, A.M., Csirke, J., Davidson, N.C., Dentener, F., Foster, M., Galli, A., Galloway, J.N., Genovesi, P., Gregory, R.D., Hockings, M., Kapos, V., Lamarque, J.-F., Leverington, F., Loh, J., McGeoch, M.A., McRae, L., Minasyan, A., Morcillo, M.H., Oldfield, T.E.E., Pauly, D., Quader, S., Revenga, C., Sauer, J.R., Skolnik, B., Spear, D., Stanwell-Smith, D., Stuart, S.N., Symes, A., Tierney, M., Tyrrell, T.D., Vié, J.-C. & Watson, R. 2010. Global biodiversity: indicators of recent declines. Science 328: 1164–1168. doi: 10.1126/science.1187512
- Carroll, M.J., Dennis, P., Pearce-Higgins, J.W. & Thomas, C.D. 2011. Maintaining northern peatland ecosystems in a changing climate: effects of soil moisture, drainage and drain blocking on craneflies. Glob. Change Biol. 17: 2991–3001. doi: 10.1111/j.1365-2486.2011.02416.x
- Carroll, M.J., Heinemeyer, A., Pearce-Higgins, J.W., Dennis, P., West, C., Holden, J., Wallage, Z.E. & Thomas, C.D. 2015. Hydrologically driven ecosystem processes determine the distribution and persistence of ecosystem-specialist predators under climate change. Nat. Commun. 6: 1–10. doi: 10.1038/ncomms8851
- Chen, I.-C., Hill, J.K., Ohlemüller, R., Roy, D.B. & Thomas, C.D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333: 1024–1026. doi: 10.1126/science.1206432
- Colhoun, K., Mawhinney, K. & Peach, W.J. 2015. Population estimates and changes in abundance of breeding waders in northern Ireland up to 2013. Bird Study 62: 394–403. doi: 10.1080/00063657.2015.1058746
- Coulson, J.C. 1988. The structure and importance of invertebrate communities on peatlands and moorlands, and effects of environmental and management changes. In Usher M.B. & Thompson D.B.A. (eds) Ecological Changes in the Uplands, 365–380. Wiley-Blackwell, Oxford.
- Cramp, S. & Simmons, K.E.L. 1983. The Birds of the Western Palearctic. Oxford University Press, Oxford.
- Dallimer, M., Marini, L., Skinner, A.M.J., Hanley, N., Armsworth, P.R. & Gaston, K.J. 2010. Agricultural land-use in the surrounding landscape affects moorland bird diversity. Agric. Ecosyst. Environ. 139: 578–583. doi: 10.1016/j.agee.2010.09.019
- Dobson, A.D.M., Massimino, D. & Pearce-Higgins, J.W. 2015. Modelling cumulative impacts of wind farms on birds – developing approaches and testing assumptions – Phase I, a pilot study. SWBSG Commissioned report number No. 1500.
- Donald, P.F., Sanderson, F.J., Burfield, I.J., Bierman, S.M., Gregory, R.D. & Waliczky, Z. 2007. International conservation policy delivers benefits for birds in Europe. Science 317: 810–813. doi: 10.1126/science.1146002
- Dormann, C.F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., Marquéz, J.R.G., Gruber, B., Lafourcade, B., Leitão, P.J., Münkemüller, T., McClean, C., Osborne, P.E., Reineking, B., Schröder, B., Skidmore, A.K., Zurell, D. & Lautenbach, S. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
- Douglas, D.J.T., Bellamy, P.E., Stephen, L.S., Pearce–Higgins, J.W., Wilson, J.D. & Grant, M.C. 2014. Upland land use predicts population decline in a globally near-threatened wader. J. Appl. Ecol. 51: 194–203. doi: 10.1111/1365-2664.12167
- Douglas, D.J.T., Buchanan, G.M., Thompson, P., Amar, A., Fielding, D.A., Redpath, S.M. & Wilson, J.D. 2015. Vegetation burning for game management in the UK uplands is increasing and overlaps spatially with soil carbon and protected areas. Biol. Conserv. 191: 243–250. doi: 10.1016/j.biocon.2015.06.014
- Douglas, D.J.T., Beresford, A., Selvidge, J., Garnett, S., Buchanan, G.M., Gullett, P. & Grant, M.C. 2017. Changes in upland bird abundances show associations with moorland management. Bird Study 64: 242–254. doi: 10.1080/00063657.2017.1317326
- Durant, D., Tichit, M., Kerneis, E. & Fritz, H. 2008. Management of agricultural wet grasslands for breeding waders: integrating ecological and livestock system perspectives – a review. Biodivers. Conserv. 17: 2275–2295. doi: 10.1007/s10531-007-9310-3
- Eaton, M., Aebischer, N., Brown, A., Hearn, R., Lock, L., Musgrove, A., Noble, D., Stroud, D. & Gregory, R. 2015. Birds of conservation concern 4: the population status of birds in the UK, channel Islands and Isle of man. Br. Birds 108: 708–746.
- Edwards, C.A. & Bohlen, P.J. 1996. Biology and Ecology of Earthworms, 3rd edn. Chapman & Hall, London.
- Field, R.H. & Gregory, R.D. 1999. Measuring Population Changes from the Breeding Bird Survey. Research Report No. 217. British Trust for Ornithology, Thetford.
- Fisher, G. & Walker, M. 2015. Habitat restoration for curlew Numenius arquata at the Lake Vyrnwy reserve, Wales. Conservation Evidence 12: 48–52.
- Fletcher, K., Aebischer, N.J., Baines, D., Foster, R. & Hoodless, A.N. 2010. Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J. Appl. Ecol. 47: 263–272. doi: 10.1111/j.1365-2664.2010.01793.x
- Fuller, R.J. & Gough, S.J. 1999. Changes in sheep numbers in Britain: implications for bird populations. Biol. Conserv. 91: 73–89. doi: 10.1016/S0006-3207(99)00039-7
- Glaves, D., Morecroft, M., Fitzgibbon, C., Owen, M., Phillips, S. & Leppitt, P. 2013. Natural England Review of Upland Evidence 2012 – The Effects of Managed Burning on Upland Peatland Biodiversity, Carbon and Water, Natural England Upland Evidence Review No. 004. Natural England, Peterborough.
- Grant, M.C. & Pearce-Higgins, J.W. 2012. Spatial variation and habitat relationships in moorland bird assemblages: a British perspective. In Fuller, R.J. (ed.) Birds and Habitat: Relationships in Changing Landscapes, 207–236. Cambridge University Press, Cambridge.
- Grant, M.C., Orsman, C., Easton, J., Lodge, C., Smith, M., Thompson, G., Rodwell, S. & Moore, N. 1999. Breeding success and causes of breeding failure of curlew Numenius arquata in Northern Ireland. J. Appl. Ecol. 36: 59–74. doi: 10.1046/j.1365-2664.1999.00379.x
- Gray, C.L., Hill, S.L.L., Newbold, T., Hudson, L.N., Börger, L., Contu, S., Hoskins, A.J., Ferrier, S., Purvis, A. & Scharlemann, J.P.W. 2016. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 7: 12306. doi: 10.1038/ncomms12306
- Harris, S.J., Massimino, D., Newson, S.E., Eaton, M.A., Marchant, J.H., Balmer, D.E., Noble, D.G., Gillings, S., Procter, D.A. & Pearce-Higgins, J.W. 2016. The Breeding Bird Survey 2015, BTO Research Report No. 687. British Trust for Ornithology, Thetford.
- Hijmans, R.J., van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J.A., Lamigueiro, O.P., Bevan, A., Racine, E.B. & Shortridge, A. 2016. Raster: Geographic Data Analysis and Modeling. https://cran.r-project.org/web/packages/raster.
- Holden, J., Shotbolt, L., Bonn, A., Burt, T.P., Chapman, P.J., Dougill, A.J., Fraser, E.D.G., Hubacek, K., Irvine, B., Kirkby, M.J., Reed, M.S., Prell, C., Stagl, S., Stringer, L.C., Turner, A. & Worrall, F. 2007. Environmental change in moorland landscapes. Earth Sci. Rev. 82: 75–100. doi: 10.1016/j.earscirev.2007.01.003
- International Wader Study Group. 2003. Waders are declining worldwide. Conclusions from the 2003 international wader study group conference, Cádiz, Spain. Wader Study Group Bulletin 101/102: 8–12.
- IUCN. 2016. The IUCN Red List of Threatened Species [online]. http://www.iucnredlist.org/.
- IUCN & UNEP-WCMC. 2014. The World Database on Protected Areas (WDPA) [on-line]. UNEP-WCMC, Cambridge.
- Jarvis, A., Reuter, H.I., Nelson, A. & Guevara, E. 2008. Hole-filled SRTM for the globe Version 4. Available from the CGIAR-CSI SRTM 90m Database http://srtm.csi.cgiar.org.
- Johnston, A., Newson, S.E., Risely, K., Musgrove, A.J., Massimino, D., Baillie, S.R. & Pearce-Higgins, J.W. 2014. Species traits explain variation in detectability of UK birds. Bird Study 61: 340–350. doi: 10.1080/00063657.2014.941787
- Johnstone, I., Dyda, J., & Lindley, P. 2007. The population status and hatching success of Curlews Numenius arquata in Wales in 2006. Welsh Birds 5: 78–87.
- Jones, R.J., Hiederer, R., Rusco, E., Loveland, P.J., & Montanarella, L. 2003. The Map of Organic Carbon Content in Topsoils In Europe: v 1.2 September – 2003, European Soil Bureau Research Report No. 15. Office for Official Publications of the European Communities, Luxembourg.
- Kelly, S., Donaghy, A. & O’Donoghue, B. 2016. Curlew (Numenius arquata) Surveys in the Republic of Ireland in 2015 & 2016. Presented at the International Wader Study Group Conference, Cork, Ireland.
- Kentie, R., Both, C., Hooijmeijer, J.C.E.W. & Piersma, T. 2015. Management of modern agricultural landscapes increases nest predation rates in black-tailed Godwits Limosa limosa. Ibis 157: 614–625. doi: 10.1111/ibi.12273
- MacDonald, M.A. & Bolton, M. 2008. Predation on wader nests in Europe. Ibis 150: 54–73. doi: 10.1111/j.1474-919X.2008.00869.x
- Martay, B., Monteith, D.T., Brewer, M.J., Brereton, T., Shortall, C.R. & Pearce-Higgins, J.W. 2016. An indicator highlights seasonal variation in the response of Lepidoptera communities to warming. Ecol. Indicators 68: 126–133. doi: 10.1016/j.ecolind.2016.01.057
- Massimino, D., Johnston, A. & Pearce-Higgins, J.W. 2015. The geographical range of British birds expands during 15 years of warming. Bird Study 62: 523–534. doi: 10.1080/00063657.2015.1089835
- McCallum, H.M., Park, K.J., O’brien, M.G., Gimona, A., Poggio, L. & Wilson, J.D. 2015. Soil pH and organic matter content add explanatory power to northern Lapwing Vanellus vanellus distribution models and suggest soil amendment as a conservation measure on upland farmland. Ibis 157: 677–687. doi: 10.1111/ibi.12286
- Morton, D., Rowland, C., Wood, C., Meek, L., Marston, C., Smith, G., Wadsworth, R. & Simpson, I. 2011. Final Report for LCM2007 – the new UK land cover map. Countryside Survey Technical Report No 11/07.
- Noble, D.G., Aebischer, N.J., Newson, S.E., Ewald, J.A. & Dadam, D. 2012. A comparison of trends and geographical variation in mammal abundance in the Breeding Bird Survey and the National Gamebag Census. JNCC Report 468: 1–30.
- O’Brien, M. 1996. The numbers of breeding waders in lowland Scotland. Scott. Birds 18: 231–241.
- O’Brien, M. 2004. Estimating the number of farmland breeding waders in the United Kingdom. International Wader Studies 14: 135–139.
- O’Brien, M. & Wilson, J.D. 2011. Population changes of breeding waders on farmland in relation to agri-environment management. Bird Study 58: 399–408. doi: 10.1080/00063657.2011.608117
- Pearce-Higgins, J.W. 2010. Using diet to assess the sensitivity of northern and upland birds to climate change. Clim. Res. 45: 119–130. doi: 10.3354/cr00920
- Pearce-Higgins, J.W. & Grant, M.C. 2006. Relationships between bird abundance and the composition and structure of moorland vegetation. Bird Study 53: 112–125. doi: 10.1080/00063650609461424
- Pearce-Higgins, J.W. & Yalden, D.W. 2003. Variation in the use of pasture by breeding European Golden Plovers Pluvialis apricaria in relation to prey availability. Ibis 145: 365–381. doi: 10.1046/j.1474-919X.2003.00154.x
- Pearce-Higgins, J.W., Beale, C.M., Wilson, J. & Bonn, A. 2006. Analysis of Moorland Breeding Bird Distribution and Change in the Peak District, Moors for the Future Report No. 11. Moors for the Future Partnership.
- Pearce-Higgins, J.W., Grant, M.C., Robinson, M.C. & Haysom, S.L. 2007. The role of forest maturation in causing the decline of Black Grouse Tetrao tetrix. Ibis 149: 143–155. doi: 10.1111/j.1474-919X.2006.00623.x
- Pearce-Higgins, J.W., Grant, M.C., Beale, C.M., Buchanan, G.M. & Sim, I.M.W. 2009a. International importance and drivers of change of upland bird populations. In Bonn A., Allott, T., Hubacek, K. & Stewart, J. (eds) Drivers of Environmental Change in Uplands, 209–227. Routledge, London.
- Pearce-Higgins, J.W., Stephen, L., Langston, R.H.W., Bainbridge, I.P. & Bullman, R. 2009b. The distribution of breeding birds around upland wind farms. J. Appl. Ecol. 46: 1323–1331.
- Pearce-Higgins, J.W., Dennis, P., Whittingham, M.J. & Yalden, D.W. 2010. Impacts of climate on prey abundance account for fluctuations in a population of a northern wader at the southern edge of its range. Glob. Change Biol. 16: 12–23. doi: 10.1111/j.1365-2486.2009.01883.x
- Pearce-Higgins, J.W., Stephen, L., Douse, A. & Langston, R.H.W. 2012. Greater impacts of wind farms on bird populations during construction than subsequent operation: results of a multi-site and multi-species analysis. J. Appl. Ecol. 49: 386–394. doi: 10.1111/j.1365-2664.2012.02110.x
- Pearce-Higgins, J.W., Eglington, S.M., Martay, B. & Chamberlain, D.E. 2015. Drivers of climate change impacts on bird communities. J. Anim. Ecol. 84: 943–954. doi: 10.1111/1365-2656.12364
- Pearce-Higgins, J.W., Brown, D.J., Douglas, D.J.T., Alves, J.A., Bellio, M., Bocher, P., Buchanan, G.M., Clay, R.P., Conklin, J., Crockford, N., Dann, P., Elts, J., Friis, C., Fuller, R.A., Gill, J.A., Gosbell, K., Johnson, J.A., Marquez-Ferrando, R., Masero, J.A., Melville, D.S., Millington, S., Minton, C., Mundkur, T., Nol, E., Pehlak, H., Piersma, T., Robin, F., Rogers, D.I., Ruthrauff, D.R., Senner, N.R., Shah, J.N., Sheldon, R.D., Soloviev, S.A., Tomkovich, P.S. & Verkuil, Y.I. 2017. A global threats overview for Numeniini populations: synthesising expert knowledge for a group of declining migratory birds. Bird Conserv. Int. 27: 6–34. doi: 10.1017/S0959270916000678
- Ratcliffe, D. 2007. Galloway and the Borders. Harper Collins, London.
- R Core Team. 2015. R: a language and environment for statistical computing. Version 3.2.2. R Foundation for Statistical Computing, Vienna.
- Renwick, A.R., Massimino, D., Newson, S.E., Chamberlain, D.E., Pearce-Higgins, J.W. & Johnston, A. 2012. Modelling changes in species’ abundance in response to projected climate change. Divers. Distrib. 18: 121–132. doi: 10.1111/j.1472-4642.2011.00827.x
- Robson, G. 1998. The breeding ecology of Curlew Numenius arquata on north Pennine moorland. PhD Thesis, University of Sunderland.
- Roodbergen, M., van der Werf, B. & Hötker, H. 2012. Revealing the contributions of reproduction and survival to the Europe-wide decline in meadow birds: review and meta-analysis. J. Ornithol. 153: 53–74. doi: 10.1007/s10336-011-0733-y
- Sanderson, F.J., Pople, R.G., Ieronymidou, C., Burfield, I.J., Gregory, R.D., Willis, S.G., Howard, C., Stephens, P.A., Beresford, A.E. & Donald, P.F. 2016. Assessing the performance of EU nature legislation in protecting target bird species in an era of climate change. Conserv. Lett. 9: 172–180. doi: 10.1111/conl.12196
- Scott, D.M., Berg, M.J., Tolhurst, B.A., Chauvenet, A.L.M., Smith, G.C., Neaves, K., Lochhead, J. & Baker, P.J. 2014. Changes in the distribution of red foxes (Vulpes vulpes) in urban areas in Great Britain: findings and limitations of a media-driven nationwide survey. PLoS ONE 9: e99059. doi: 10.1371/journal.pone.0099059
- Stroud, D.A., Chambers, D., Cook, S., Buxton, N., Fraser, B., Clement, P., Lewis, P., McLean, I., Baker, H., & Whitehead, S. 2001. The UK SPA Network: its scope and content. JNCC, Peterborough.
- Studds, C.E., Kendall, B.E., Murray, N.J., Wilson, H.B., Rogers, D.I., Clemens, R.S., Gosbell, K., Hassell, C.J., Jessop, R., Melville, D.S., Milton, D.A., Minton, C.D.T., Possingham, H.P., Riegen, A.C., Straw, P., Woehler, E.J. & Fuller, R.A. 2017. Rapid population decline in migratory shorebirds relying on yellow Sea tidal mudflats as stopover sites. Nat. Commun. 8: 14895. doi: 10.1038/ncomms14895
- Taylor, R.C. & Dodd, S.G. 2013. Negative impacts of hunting and suction-dredging on otherwise high and stable survival rates in Curlew Numenius arquata. Bird Study 60: 221–228. doi: 10.1080/00063657.2013.775215
- Tharme, A.P., Green, R.E., Baines, D., Bainbridge, I.P. & O’Brien, M. 2001. The effect of management for red grouse shooting on the population density of breeding birds on heather-dominated moorland. J. Appl. Ecol. 38: 439–457. doi: 10.1046/j.1365-2664.2001.00597.x
- Thomas, G.H., Lanctot, R.B. & Szekely, T. 2006. Can intrinsic factors explain population declines in North American breeding shorebirds? A comparative analysis. Anim. Conserv. 9: 252–258. doi: 10.1111/j.1469-1795.2006.00029.x
- Valkama, J. & Currie, D. 1999. Low productivity of Curlews Numenius arquata on farmland in southern Finland: causes and consequences. Ornis Fenn. 76: 65–70.
- Valkama, J., Robertson, P., & Currie, D. 1998. Habitat selection by breeding curlews (Numenius arquata) on farmland: the importance of grassland. Ann. Zool. Fenn. 35: 141–148.
- Valkama, J., Currie, D. & Korpimaki, I. 1999. Differences in the intensity of nest predation in the curlew Numenius arquata: a consequence of land use and predator densities? Ecoscience 6: 497–504. doi: 10.1080/11956860.1999.11682552
- Vickery, J.A., Tallowin, J.R., Feber, R.E., Asteraki, E.J., Atkinson, P.W., Fuller, R.J. & Brown, V.K. 2001. The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. J. Appl. Ecol. 38: 647–664. doi: 10.1046/j.1365-2664.2001.00626.x
- van der Wal, R. & Palmer, S.C.F. 2008. Is breeding of farmland wading birds depressed by a combination of predator abundance and grazing? Biol. Lett. 4: 256–258. doi: 10.1098/rsbl.2008.0012
- Wauchope, H.S., Shaw, J.D., Varpe, Ø., Lappo, E.G. Boertmann, D., Lanctot, R.B. & Fuller, R.A. 2016. Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Glob. Change Biol. 23: 1085–1994. doi: 10.1111/gcb.13404
- Wetlands International. 2016. Waterbird populatin estimates [online]. http://wpe.wetlands.org/ [Accessed 10 May 2017].
- Whittingham, M.J. & Evans, K.L. 2004. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 146: 210–220. doi: 10.1111/j.1474-919X.2004.00370.x
- Whittingham, M.J., Percival, S.M. & Brown, A.F. 2002. Nest-site selection by golden plover: why do shorebirds avoid nesting on slopes? J. Avian Biol. 33: 184–190. doi: 10.1034/j.1600-048X.2002.330210.x
- Wilson, A.M., Ausden, M. & Milsom, T.P. 2004. Changes in breeding wader populations on lowland wet grasslands in England and Wales: causes and potential solutions. Ibis 146: 32–40. doi: 10.1111/j.1474-919X.2004.00371.x
- Wilson, J.D., Anderson, R., Bailey, S., Chetcuti, J., Cowie, N.R., Hancock, M.H., Quine, C.P., Russell, N., Stephen, L. & Thompson, D.B.A. 2014. Modelling edge effects of mature forest plantations on peatland waders informs landscape-scale conservation. J. Appl. Ecol. 51: 204–213. doi: 10.1111/1365-2664.12173
- Wood, S. 2016. mgcv: Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation. https://cran.r-project.org/web/packages/mgcv.