ABSTRACT
Capsule: We assess biometric variation in the threatened Dupont’s Lark along a wide fraction of its distribution area analysing the largest data set for this bird species available to date, comprising a 28-year period and including birds captured in Spain, Morocco and Tunisia.
Aims: To analyse Dupont’s Lark morphology evaluating five potential sources of divergence: (I) sexual dimorphism, (II) macro-regional differences, (III) climate, (IV) isolation and (V) intraspecific competition.
Methods: Multivariate analysis was used to summarize biometric data. Sexual dimorphism and macro-regional divergence were assessed by generalized linear mixed models. Climate, isolation and intraspecific competition effects on phenotype were explored by means of model averaging.
Results: Sexes differed in wing shape suggesting a sexual selection pressure on males for aerial displays. Males showed longer bills after controlling for body size. We found an increasing Spain < Morocco < Tunisia trend in body size and bill length and volume. Tunisian populations showed more pointed wings than Moroccan and Iberian ones. Maximum temperature increased bill size as predicted by Allen’s rule. We found support for Bergmann’s rule in relation to temperature in the breeding season and water availability. Intraspecific competition was associated with more rounded wing shapes suggesting a pressure related to breeding display performance. Isolation reduced wingtip pointedness.
Conclusion: We found convincing results for wing morphology variation in relation to intrasexual competition, as males seem to be subjected to a sexual selection pressure for aerial display and this adaptation strengthens when intraspecific competition increases. We also found solid support for Allen’s rule, with results suggesting that bill size plays an important role in the bird’s thermoregulation, which does not occur in the case of tarsus. Although Bergmann’s rule is not supported in relation to minimum annual temperature, we found a relationship with thermal conditions in the breeding season, as well as water availability.
Environmental and ecological heterogeneity along a species’ distribution range generates different selective pressures with organisms developing different adaptive responses (Grant et al. Citation1976). These pressures can result in observable phenotypic divergence when populations are subject to some degree of geographic isolation, which can be evident even in short time periods (Boag & Grant Citation1981). Mutation, genetic drift and flow, and natural and sexual selection are the main mechanisms that explain evolutionary change and therefore local adaptation (Slatkin Citation1987, Coyne Citation2011), although phenotypic plasticity may also be responsible for morphological or behavioural adaptations to local conditions (Price Citation2006). The engine that feeds local adaptation is environmental variation resulting in a change in the frequency distribution of given phenotypes within a population (Kingsolver et al. Citation2001).
Phenotypic and genetic differences between populations are usually linked to isolation. Limited geographic connectivity results in a classic isolation by distance, due to limitations in dispersal as distance between populations increases (Wright Citation1943). Among other forces of divergence, sexual selection is considered a positive one, as it can trigger rapid evolution of phenotypic traits (West-Eberhard Citation1983, Panhuis et al. Citation2001), such as those related to sexual display, mate recognition and choice (Seddon et al. Citation2013). In birds, bill size and shape appear to be related to acoustic communication due to its effect on singing rate and intensity (Westneat et al. Citation1993, Nowicki Citation1987), which is in turn influenced by breeding behaviour and subjected to sexual selection. Wing shape can also be a sexually selected trait in species with song flights or aerial displays in their breeding behaviour (Able Citation2004).
In addition to sexual selection, local conditions produce characteristic morphological adaptations. Biometric variation of endothermic organisms is related to environmental conditions due to their thermoregulation needs. Following Bergmann’s rule (Citation1847; see revision by Rensch Citation1938 for races within a species), a larger body size is expected in colder habitats to reduce surface-to-volume ratio, and thus, to improve heat maintenance. Allen’s rule predicts longer appendages in warmer than in colder areas for the same species, due to their function as heat-loss body parts (Allen Citation1877). In the case of birds, bare body parts (bill and tarsus) are particularly useful as thermoregulatory elements, contrary to feathered ones, due to plumage insulation capacity (Evans & Heiser Citation2004, Gill Citation2007).
Local conditions can also promote morphological adaptations within a species, according to the niche they exploit (Bicudo et al. Citation2010). For example, limb length is influenced by locomotion and habitat structure, as it can support greater movement speed from increasing stride length (Alexander Citation1977, Zeffer & Norberg Citation2003). In birds, a more insectivorous diet can favour longer tarsi, as it enables more efficient prey-chasing (de Juana et al. Citation2004). Bill size and shape are closely associated with food type and feeding behaviour (Remsen Citation2003). Wing shape is related to flight capacity and can be influenced by movement patterns, habitat structure and breeding behaviour (Voelker Citation2001). Narrow and pointed wings allow for a higher speed with lower energy consumption during long flights than broad, rounded wings, which optimize acceleration and short-distance speed as well as manoeuvrability in dense habitats (Tellería et al. Citation2001, Pérez-Tris et al. Citation2003).
Here we explore biometric divergence along a biogeographical gradient in an extremely endangered steppe bird with a wide, but fragmented distribution: the Dupont’s Lark Chersophilus duponti (Vieillot 1820). Dupont’s Lark is a small (17–18 cm, 32–47 g; de Juana & Suárez Citation2016) and scarce passerine of the Alaudidae family, restricted to the Mediterranean region. It is strongly selective in its habitat, occurring exclusively in flat, treeless, natural steppe-land systems (Garza & Suárez Citation1990, Garza et al. Citation2005, Seoane et al. Citation2006) and diet composition, being eminently insectivorous (Cramp & Simons Citation1978, Suárez Citation2010, de Juana & Suárez Citation2016). Such strong specialization, along with its sedentary behaviour and wide range distribution, offers a good opportunity to test ecogeographical rules. Some support for Bergmann’s rule in Dupont’s Lark has recently been found (Vögeli et al. Citation2017), as wing and tarsus length, surrogates of body size, increased at high elevations where minimum temperatures are lower. However, no differences in wing morphology or differences between sexes have been detected, including any potential effect of intraspecific competition (Vögeli et al. Citation2017).
Males compete for the best territories and defend them using aerial song displays (Suárez Citation2010, de Juana & Suárez Citation2016). As broader wings reduce wing loading and increase hovering capacity (Able Citation2004), they can constitute a sexually selected trait in species exhibiting such breeding display behaviour (Voelker Citation2001). Because these displays are associated with singing (Suárez Citation2010, de Juana & Suárez Citation2016), bill morphology is another trait potentially subject to sexual selection pressure. Along with the different notes that the bird emits when it sings, forming the phrases of its repertoire, the configuration of the vocal tract is modified in coordination with the syrinx to maintain a suitable filtering function for the different frequencies. This results in very precise open-close bill movements during the song, which encounters severe constraints when bill size increases (Podos Citation2001). Thus, smaller bills should be expected in denser populations, where intraspecific competition is assumed to be stronger.
In addition, we explore morphological divergence between sexes. In Dupont’s Lark, males and females differ in body size (Cramp & Simmons Citation1978, de Juana & Suárez Citation2016, Vögeli et al. Citation2007). However, little is known about the relative differences in biometric traits, controlling for the effect of body size, which could reveal underlying ecological divergence processes. The different breeding behaviours between sexes could select for broader wings (as a sexually selected trait for flight displays, Suárez Citation2010, de Juana & Suárez Citation2016) and smaller bills (for a better singing performance, Podos Citation2001) in males than in females.
Finally, we evaluate the morphological variability at a macro-regional scale, comparing the three unconnected regions: Spain, Morocco and Tunisia. The long-term lack of connectivity in these global populations (García et al. Citation2008a) suggests that the different biometric traits may have undergone a divergence process.
Methods
Bird captures
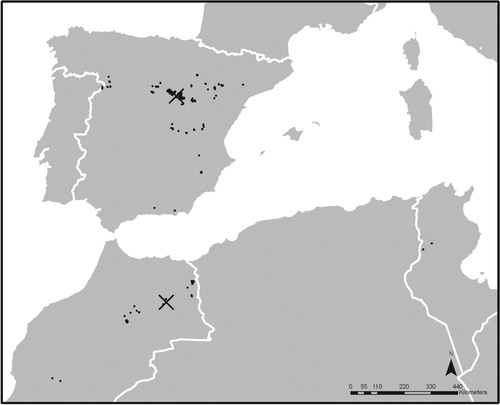
Dupont’s Lark populations in Spain, Morocco and Tunisia were sampled from 1986 to 2013 (). Spain accounts for the largest number of captured birds, with 614 adult individuals trapped during the breeding period (February–July). In Morocco, 8 expeditions were carried out from 2004 to 2010, both in breeding and non-breeding seasons, collecting 139 individuals of which 121 were included in the sample, after removing juvenile-plumaged birds and those with incomplete biometric measurements. One survey in Tunisia (January 2007) collected a smaller, but valuable, sample of 13 individuals that constitutes the only existing capture data for this species thus far (see online Supplemental Table S1). In all cases, birds were captured with baited spring net traps, using a male singing playback (Kroodsma Citation1989, Kroodsma et al. Citation2001). Due to the species’ elusive and terrestrial behaviour, this is the most effective trapping method, although a strong bias towards trapping territorial males (Table S1) must be assumed (Suárez Citation2010). Birds were marked with a numbered metal ring and released at the place of capture after processing and geolocation.
Figure 1. Geographic origins of Dupont’s Lark samples in Spain, Morocco and Tunisia during the period 1986–2013. Black dots indicate capture sites, black crosses mark Spanish and Moroccan population centroids based on geographic coordinates of capture points. For a global distribution map of the species, see de Juana and Suárez (Citation2016). For a more detailed distribution in Spain see Suárez (Citation2010) and, in Morocco, García et al. (Citation2008b).
Biometry
Juveniles were identified by their diagnostic plumage before moulting (EURING age 3, following Svensson Citation1992) and all birds were sexed by means of biometry according to Vögeli et al. (Citation2007). Among other measurements, we recorded bill length (distance from the tip of the bill to its insertion in the skull, to the nearest 0.1 mm), bill depth (height at the level of the nostril, to the nearest 0.1 mm), bill width (at the level of the nostril, to the nearest 0.1 mm), tarsus length (tarsometatarsus, to the nearest 0.1 mm), wing length (maximum flattened chord to the nearest 0.5 mm) and primary distances (lengths from the distal extreme of the folded wing to the tip of each primary, in descending order, excluding P10, to the nearest 0.5 mm).
Statistics
We tested for morphological differences associated with season by analysis of variance (anova) over the main biometric traits (wing, bill and tarsus lengths) on the Moroccan subsample, the only region for which we had data for different seasons.
Biometric data were summarized into synthetic variables of ecological sense (). We obtained a body size index from a principle components analysis (PCA) on tarsus and wing lengths (online Supplemental Appendix A1). To test flight capacity hypotheses, we described wing morphology by means of a PCA with primary distances (Senar et al. Citation1994, Tellería & Carbonell Citation1999, Tellería et al. Citation2001, Pérez-Tris et al. Citation2003; online Appendix A2). Bill volume was estimated by the cone equation (online Appendix A3). We also used tarsus and bill length as direct measures. Juveniles were removed from the analysis to ensure that any birds with incomplete skeletal development were excluded (Jenni & Winkler Citation1994).
Table 1. Response variables for Dupont’s Lark morphological analysis and their ecological meaning.
We used RStudio software version 1.1.383 with R version 3.4.3 in all the analyses (R Development Core Team Citation2008).
I. Sexual dimorphism
We analysed sexual dimorphism using the Spanish data only, as we lacked a sufficient sample size for birds from Morocco and Tunisia (Table S1). Taking into account the unequal sample size and the variability in measures introduced by different ringers, we performed a generalized linear mixed effects model (GLMM) followed by a type III sum of squares (SS) anova. The model included the ringer as a random effect factor (n = 9 different ringers) and sex as a fixed effect. We controlled the covariate effect of body size on the different biometric traits (response variables). For this purpose, we included body size index as a covariate in the models of bill volume, bill length and wing morphology. In the case of tarsus length, we used wing length as a body size estimator, because the body size index was built with this response variable itself (). GLMMs models were carried out with the nlme R package (Pinheiro et al. Citation2015). Once each model was performed, an anova table was obtained by means of car R package (Fox & Weisberg Citation2011), selecting type III SS to control unbalanced design.
Table 2. Predictors used in model construction to assess Dupont’s Lark biometry, and their ecological meaning. An X indicates that the predictor was included in the model for each response variable.
II. Macro-regional differences
The same method was used to assess the species’ morphological divergence among the three unconnected large-scale regions: Spain, Morocco and Tunisia. We carried out GLMMs controlling for the ringer and body size effects, followed by a type III SS anova and a post hoc Dunnett’s Tukey–Kramer test (Dunnett Citation1980), accounting for the unbalanced design. Tunisian individuals were removed from posterior analysis (climate, isolation and intraspecific competition) due to their low sample size.
III. Climate
For Spanish and Moroccan male-only observations, climatic variables were obtained to test the hypotheses related to ecogeography. For each bird’s location, we extracted its climate data from the WorldClim database (Hijmans et al. Citation2010) by means of GIS software, including the mean value of the series 1950–2000 for the following variables: temperature annual range, mean temperature of the coldest month, mean temperature of the hottest month, mean temperature in April and cumulative annual rainfall ().
IV. Isolation
To test our hypotheses related to isolation, we included the Euclidean distance from the capture location to its global population’s centroid (Spain or Morocco), which has previously been used in similar approaches (Vögeli et al. Citation2010, Méndez et al. Citation2014). Additionally, we included the ratio between distance to the centroid and population size to take into account that, for the same distance, an individual belonging to a small population is expected to be more isolated than that of a larger one.
To calculate the centroid, we averaged the geographic coordinates of all our capture points, as our sampling effort was positively correlated with population size (following García et al. Citation2008b and Suárez Citation2010: r = 0.19, P = 0.05 in the whole sample; r = 0.76, P < 0.001 in Spain-only sample; r = 0.33, P = 0.67 in Morocco-only sample).
V. Intraspecific competition
Population density was used as a surrogate variable for the intensity of male–male interactions. We estimated density from the number of territories (population size) and occupancy area of each population available for Spain (Suárez Citation2010) and Morocco (García et al. Citation2008b). Using population size and density as the only main explanatory factors could mislead the meaning of density as a competition estimator, as small nuclei with a low number of males could offer the same high density as large, very populated nuclei, where intraspecific competition should be expected to be more intense. Hence, we introduced both the pure effects and the interaction (simple multiplication) of population density and population size as intraspecific competition estimators.
The effect of climate, isolation and intraspecific competition on each morphological trait was assessed by GLMM, with the ringer effect as a random factor. All predictors were standardized by the decostand function in the vegan package in R, using method standardize, which scales mean to zero and units the variance (Oksanen et al. Citation2011). We analysed Spanish and Moroccan populations separately, as they constitute independent genetic and morphological units (García et al. Citation2008a, Méndez et al. Citation2011). For each morphological (response) variable, a global model was built including the following predictors ().
In the model for body size, we tested Bergmann’s rule including not only the minimum temperature, but also the temperature annual range and the averaged April temperature (see Olalla-Tárrega et al. Citation2009), to evaluate the effect of seasonality and thermal stress during the breeding season, respectively. We also introduced annual rainfall as an indicator of water availability (see Vogeli et al. Citation2017). We tested the effect of isolation by introducing into the model the distance to the centroid and its ratio with population size. Intraspecific competition was then analysed through population density, population size and the interaction.
For bill length and volume, as well as for tarsus length, we evaluated Allen’s rule by means of annual maximum temperature. The same above-mentioned predictors of isolation and intraspecific competition were included. The covariate effect of body size on these traits was also controlled. We used directly the body size index in bill length and volume models, but not for tarsus length, as the index was constructed with this measure itself; similarly, we used wing length as an estimator of body size in the tarsus model.
With respect to wing morphology, we addressed the effect of isolation and intraspecific competition by building three different models (one for each component of the PCA) with the same detailed variables. We also introduced the body size index to control its covariate effect on wing morphology, as larger birds need larger wings to maintain wing loading.
For each biometric trait, the complete set of models was extracted by the dredge function in MuMIn library in R (Bartoń Citation2012), with the plausible models selected by means of Akaike’s information criterion (AIC), considering as the confidence set of models those included in the 95% cumulated model weights (Burnham & Anderson Citation2002). We dismissed the use of corrected AIC, as the number of observations was always at least 40 times that of predictors in the most parameterized model on the set (n/K > 40; Burnham & Anderson Citation2002). We applied model averaging over the set of models, obtaining for each predictor the weighted value of its estimator, the unconditional standard error based on Burnham and Anderson’s revised formula (Citation2004) and its z and P values, identifying those significant effects.
Results
No morphological differences associated with season were found for the Moroccan sample, the only one with this kind of variation, after testing by anova (wing length, P = 0.94; bill length, P = 0.57; tarsus length, P = 0.12).
The PCA for the evaluation of variability patterns in wing morphology (online Appendix A2) identified three highly explanatory components (eigenvalues > 1), which accounted for 78.7% of explained variance. Principle component 1 (PC1; 38.8%) was mainly associated with the proximal primary region, incrementing P1 to P4 lengths and resulting in a proportionally wider wing. PC2 (23.1%) was related to the distal extreme of the wing, leading to high negative scores for P8 and P9 distances, and generating a more pointed wingtip. PC3 (16.8%) gave information about the mid-primary region, reducing P5 to P7 and producing a more pointed overall wing (see online Figure S1 for a graphic evaluation of the effect of each component on wing morphology).
For body size, we obtained only one component (eigenvalue > 1; 65.1% of explained variance), which identified a covariation pattern of wing and tarsus lengths, constituting a useful body size index (online Appendix A1).
I. Sexual dimorphism
Dupont’s Lark males showed a significantly larger body size and proportionally longer bill than females (). No significant dimorphism was detected in bill volume or relative tarsus length, although males showed higher values in both traits (). Wing shape diverged between sexes with males showing lower values in all three morphological indices, thus presenting shorter P1–P4 feathers (PC1), shorter P8–P9 feathers (PC2) and longer P5–P7 feathers (PC3), resulting in a more accented oar-shaped (rounded) wing morphology (online Figure S2). Due to the significant sexual dimorphism and the strong bias towards males in the whole sample, females were removed from subsequent analyses and wing shape and body size estimations were recalculated with new PCAs using the male-only sample (online Tables S2 and S3).
Table 3. Sexual dimorphism in Dupont’s Lark, mean and standard deviation of each morphological trait. P-value by means of anova type III SS after a GLMM.
II. Macro-regional differences
The three large-scale unconnected regions showed different phenotypes according to body size, bill length and volume, and wing morphology in PC2 index, but they did not differ in relative tarsus lengths (). The values of all the size-type variables increased in the same direction: Spain < Morocco < Tunisia (followed also by tarsus length, although with a non-significant effect). According to wing shape, Tunisian males showed the highest PC2 scores, resulting in a more pointed wingtip (online Figure S3).
Table 4. Morphological differences in male Dupont’s Larks among the three unconnected regions: Spain, Morocco and Tunisia. Mean and standard deviation of each morphological trait. P-value by means of anova type III SS conducted after a GLMM. Post hoc Dunnett’s modified Tukey–Kramer pairwise multiple comparison test was carried out to assess significant differences, indicated with superscript letters, meaning that regions sharing the same letter do not differ in that morphological trait. In the case of Wing morph. PC1, we obtained a significant value in the anova test but no significance in the post hoc comparison, as the latter is more conservative (Cardinal & Aitken Citation2006).
III. Climate
No effect of minimum annual temperature on body size was found, but colder breeding seasons and lower water availability were significantly associated with a larger body size in Morocco ().
Table 5. Model averaging results for each morphological response variable in male Dupont’s Larks: variables included in the averaged model, weighted estimator, unconditional standard error following Burnham and Anderson’s revised formula (Citation2004), relative importance, z and P values.
Regarding Allen’s rule, the maximum temperature showed no effect on tarsus length, but bill length (in Morocco) and volume (in Spain) were affected as predicted. In Spain, bill volume increased in the hottest regions and in Morocco bill length showed a positive relationship with maximum temperature, supporting Allen’s rule in both regions ().
IV. Isolation
The distance to the centroid and its ratio with population size had no effect on body size, bill length, bill volume or tarsus length, but it did on wing morphology. The models revealed a negative effect of the distance on PC2 in the Spanish populations (), indicating less pointed wingtips (shorter P8 and P9) in the more isolated populations (online Figure S1).
V. Intraspecific competition
Population size (in Spain) and its interaction with population density (in Morocco) had a negative effect on body size, indicating smaller Dupont’s Larks in larger and denser populations, respectively. In Spain, bill length and volume were reduced with the interaction between population size and density, and tarsus length was also smaller in denser populations. On the contrary, in Morocco population size positively affected bill volume (). Wing morphology was not affected by intraspecific competition in Morocco, but was in Spain, where males from larger and denser populations presented more rounded wings (shorter P8 and P9, PC1; longer P5–P7, PC3) (, online Figure S1).
Discussion
I. Sexual dimorphism
As a general rule in the Alaudidae family, males are larger than females (de Juana et al. Citation2004), a pattern shared by Dupont’s Lark (Suárez Citation2010, Vögeli et al. Citation2007, de Juana & Suárez Citation2016). Our results are consistent with this and, moreover, show that males have proportionally longer bills and different wing morphology.
We predicted a relatively smaller bill in males, as a sexually selected trait for better song performance (Podos Citation2001). This is not supported by our results, as males had proportionally longer bills than females. This difference could be a result of a divergence in feeding behaviour between sexes, as feeding habitat may influence bill shape: a longer bill increases accessibility to prey (Leisler & Winkler Citation2015) and widens the variety of potential prey sizes (Herrera Citation1978). However, more information about species and sex-specific diet and food availability is needed to test such a prediction.
In relation to wing morphology, the interpretation of the three morphological indices as a whole reveals a more rounded wing in males: narrower proximal primary region (P1 to P4), longer mid-primaries (P5 to P7) and shorter wingtips (P8 and P9). This divergence is consistent with the differential breeding behaviour between sexes, with males performing aerial displays as a crucial aspect of their territorial behaviour (Suárez Citation2010, de Juana & Suárez Citation2016). Moreover, it suggests that wing morphology is potentially sexually selected in the species, as occurs in the Skylark, a closely related species (Møller Citation1991). Thus, male–male competition and female choice for longer or more frequent displays could be shaping males’ wings for this more beneficial morphology.
II. Macro-regional differences
Among the subspecies duponti, African Dupont’s Larks are larger than Spanish ones, and the race margaritae is in turn larger than the former (Suárez Citation2010). Our results coincide with this description and show an ascending north-to-south and west-to-east (Spain < Morocco < Tunisia) trend in body size, bill length and volume.
In the case of wing morphology, Tunisian Dupont’s Larks have a significantly longer wingtip (P8 and P9) than Moroccan and Iberian ones, resulting in a more pointed distal extreme of the wing. Morocco presented the least pointed wings and Spain showed an intermediate level of pointedness. As more rounded morphologies optimize hovering capacity by reducing wing loading (Able Citation2004) and are more beneficial for aerial displays, this result may indicate that Moroccan populations have a greater capacity for breeding displays than Tunisian and Iberian populations.
However, divergence in wing morphology can also be attributed to differences in habitat structure or movement patterns of the different populations. Pointed shapes allow a higher speed with lower energy consumption during long flights than rounded wings, which optimize acceleration and short-distance speed, as well as manoeuvrability in dense habitats (Tellería et al. Citation2001, Pérez-Tris et al. Citation2003). In the case of Dupont’s Lark, the few available records of dispersive events, that account for maximum linear distances of 33 km (García Antón et al. Citation2015), 37 km (Bota et al. Citation2016), 80 km (García & Requena Citation2015) and 105 km (Dies et al. Citation2010), could be considered short-medium distance movements, and thus not long enough to modify wing morphology. Knowledge about movements in the African populations is lacking. A similar explanation could be considered for the effect of habitat structure as a potential force for such observed differences, due to the strong habitat specialization of the species.
III. Climate
The validity of Bergmann’s rule has remained controversial since its postulation in 1847 (see for example Blackburn et al. Citation1999, Morales-Castilla et al. Citation2012). Olalla-Tárrega et al. (Citation2009) proposed other environmental factors influencing body size, such as heat balance, water availability, primary productivity or habitat availability, highlighting the complexity of this global rule and the difficulty of separating the effect of temperature from other factors that can influence body size. In a recent study, Vögeli et al. (Citation2017) showed a decrease in Dupont’s Lark wing length and cranium size with rainfall, and an increase with aridity and maximum temperature, with birds tending to be larger at lower latitudes. They found some support for Bergmann’s rule using wing and tarsus length, as surrogates of body size, which increased at high elevations where minimum temperatures are lower. We obtained similar results, with the body size index increasing where the temperature of the breeding season and rainfall are lower. However, these relationships were only found in Morocco, where Dupont’s Larks are larger, and where breeding periods are colder and water availability decreases (see Supplemental Table S4). The absence of this pattern in Spain points to the lack of connectivity between these two regions, which have been isolated from each other for about 24,000 years (García et al. Citation2008a), and which may have caused local adaptations in body size.
Our results suggest support for Allen’s rule, as maximum temperature increased bill size in both regions as predicted. In Spain, Dupont’s Lark presented a larger bill volume when the temperature of the hottest month was higher, while bill length showed the same response in Morocco. On the contrary, tarsus length was not influenced by such thermal stress in any of the regions, supporting the prediction that the bill could be a more efficient thermoregulatory element than the tarsus in birds, because its function can be controlled by bill panting, increasing or decreasing heat loss when needed (Gill Citation2007). This same relative thermoregulatory effectiveness of the bill and tarsus has been recently shown in a multi-species study (Symonds & Tattersall Citation2010). It has also been suggested that the heat loss function of the bill is particularly important in environments where water is limiting, as in Dupont’s Lark habitat (Greenberg et al. Citation2012).
IV. Isolation
Body size, bill and tarsus were not influenced by isolation, but we found an effect of distance to the centroid on wing morphology in Spain, where PC2 is negatively influenced by this variable.
The reduction of PC2 indicates a shorter wingtip (P8 and P9 feathers) in the more isolated populations. Although PC2 only makes reference to the two outermost feathers and not to the overall wing shape (which makes it difficult to interpret as an adaptation for flight displays), this result indicates a less pointed wingtip as a population moves away from the centroid, and more pointed wings in the closer nuclei, contrary to that expected by our prediction.
V. Intraspecific competition
The increase in male–male competition was associated in Spain with shorter P8–P9 and longer P5–P7 feathers, which produces a more rounded shape of the wing, as occurred in males against females. Such wing morphology could be attributed to a better hovering capacity (Able Citation2004), as can be beneficial for aerial display performance, as mentioned above. In this sense, the Dupont’s Lark in Spain follows our prediction of the existence of sexual selection pressures over wing shape, which would be stronger in those populations with higher intraspecific competition.
Different results for Spain and Morocco could be attributed to the long-term isolation of the two regions, with differential evolutionary processes according to morphological traits. In addition, taking into consideration the significant larger body size of the Moroccan Dupont’s Larks, wing shape could be constrained to such adaptations because of a bird’s weight.
This result differs from that of Vögeli et al. (Citation2017), who failed to find an effect of intraspecific competition in Dupont’s Lark morphological traits. This different result may be attributable to the different methods used to estimate intraspecific competition. Vögeli et al. (Citation2017) used the mean distance of the nearest singing neighbours in each occupied patch as a proxy for Dupont’s Lark density. In our study, we used the interaction between population size and bird density to account for the potential confounding effect that the number of individuals in the population can have in the interpretation of density as a male–male competition estimator, expecting that, for the same bird density, the intensity of intraspecific competition will be stronger in larger populations.
Tarsus length is negatively associated with population density in Spain, pointing to local conditions not evaluated here (such as food availability or habitat structure influencing locomotion) as explanations for this result (see Vögeli et al. Citation2017).
Conclusion
We found morphological divergence in Dupont’s Lark related to sexual dimorphism, different evolutionary processes in three large-scale regions, climate, isolation and intraspecific competition. We found sexual dimorphism in wing shape, with males having a more favourable morphology for hovering than females. This type of flight-related morphology was also positively associated with intraspecific competition in Spain, suggesting that breeding flight displays are triggered by competition processes and play an important role in the ecology of the species.
Differences in drivers of bird morphology between regions suggest the existence of different competing effects in Morocco and Spain. Indeed, some morphological traits could be influenced by local conditions not evaluated in this work, such as food availability or habitat structure (Vögeli et al. Citation2017), suggesting that more research at a local scale is needed.
The strong relationship between body size and temperature of the breeding season, as well as water availability, found in the Moroccan population, supports the recent interpretations of Bergmann’s rule (Gill Citation2007, Olalla-Tárraga et al. Citation2009, Vögeli et al. Citation2017). In the case of Allen’s rule, bill size was affected by maximum temperature in both regions analysed, suggesting the operation of this ecogeographic rule in this trait and bird.
Finally, the divergence observed among the three large-scale regions and the absence of the same morphological patterns between Spain and Morocco in the models of the different traits, reinforce the idea that their long-term isolation and independent evolutionary processes may have led to inherent local adaptations and highlights the convenience of treating them as independent conservation units.
Acknowledgments
We wish to express our acknowledgement to Francisco Quico Suárez, who led the Dupont’s Lark research group until his early death in 2010. Many people collected data in the field; we especially wish to thank: A. Agirre, R. Aymí, M. Calero, E. Carriles, J. T. García, I. Hervás, J. H. Justribó, E. G. de la Morena, J. J. Oñate and J. Viñuela. We also want to thank two anonymous reviewers who notably improved the original manuscript with their comments and suggestions. All birds were captured and processed following the Wild Birds Ringing Manual (Pinilla Citation2000) and under the correspondent official licenses.
Additional information
Funding
References
- Able, K.P. 2004. Birds on the move: flight and migration. In Podulka, S., Rohrbaugh, R.W., Jr. & Bonney, R. (eds.) Handbook of Bird Biology, 5.1–5.100. Cornell Lab of Ornithology, Ithaca, NY.
- Alexander, R. 1977. Terrestrial locomotion. In Alexander, R. & Goldspink, G. (eds.). Mechanics and Energetics of Animal Locomotion. 168–203. Chapman And Hall, London.
- Allen, J.A. 1877. The influence of physical conditions in the genesis of species. Radical Rev. 1: 108–140.
- Bartoń, K. 2012. Mumin: Multi-model inference. R Package Version 1.7.7.
- Bergmann, C. 1847. Ueber Die Verhältnisse Der Wärmeökonomie Der Thiere Zu Ihrer Grösse. Gottinger Studien 3: 595–708.
- Bicudo, J.E.P.W., Buttemer, W.A. & Chappell, M.A. 2010. Ecological and Environmental Physiology of Birds. Oxford University Press, Oxford.
- Blackburn, T.M., Gaston, K.J. & Loder, N. 1999. Geographic gradients in body size: a clarification of Bergmann’s rule. Divers. Distrib. 5: 165–174. doi: 10.1046/j.1472-4642.1999.00046.x
- Boag, P.T. & Grant, P.R. 1981. Intense natural selection in a population of Darwin’s finches (Geospizinae) in the Galápagos. Science 214: 82–85. doi: 10.1126/science.214.4516.82
- Bota, G. & Guixé, D. 2016. La Alondra Ricotí En Cataluña: Evolución Histórica De Una Población En El Límite Del Area De Distribución. II Meeting of the Dupont’s Lark Experts Group, Madrid.
- Burnham, K. & Anderson, D. 2002. Model Selection and Multimodel Inference: a practical information-theoretic approach. Springer-Verlag, Berlin.
- Burnham, K. & Anderson, D. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociolog. Methods Res. 33: 261–304. doi: 10.1177/0049124104268644
- Cardinal, R.N. & Aitken, M.R.F. 2006. ANOVA for the Behavioural Sciences Researcher. Lawrence Erlbaum Associates, New York.
- Coyne, J.A. 2011. Speciation in a small space. Proc. Natl. Acad. Sci. USA 108: 12975–12976. doi: 10.1073/pnas.1110061108
- Cramp, S. & Simmons, K.E.L. 1978. Handbook of the Birds of Europe, the Middle East and North Africa. Volume 5. The Birds of the Western Palearctic: Tyrant Flycatchers to Thrushes. Oxford University Press, Oxford.
- De Juana, E. & Suárez, F. 2016. Dupont's Lark (Chersophilus Duponti). In Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A. & De Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona. http://www.hbw.com/node/57638.
- De Juana, E., Suárez, F. & Ryan, P. 2004. Larks (Alaudidae). In Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & De Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona. http://www.hbw.com/node/52302.
- Dies, B., Alcocer, T., Llorens, V., Piera, M., Marín, P. & Ruiz, P. 2010. Alondra Ricotí: una primera cita para l’Albufera. http://www.birdingalbufera.es/?q=es/node/496.
- Dunnett, C.W. 1980. Pairwise multiple comparisons in the unequal variance case. J. Am. Stat. Assoc. 75: 796–800. doi: 10.1080/01621459.1980.10477552
- Evans, H.E. & Heiser, J.B. 2004. What's inside: anatomy and physiology. In Podulka, S., Rohrbaugh, RW., Jr. & Bonney, R. (eds.). Handbook of Bird Biology, 4.1–4.162. Cornell Lab Of Ornithology, Ithaca, NY.
- Fox, J. & Weisberg, S. 2011. An R Companion to Applied Regression, 2nd edn. Sage, Thousand Oaks, CA.
- García, T. & Requena, C. 2015. Alondra Ricotí en las Salinas de Marchamalo. http://pachequerobirder.blogspot.com.es/2015/08/alondra-ricoti-en-las-salinas-de.html.
- García, J.T., Suárez, F., Garza, V., Calero-Riestra, M., Hernández, J. & Pérez-Tris, J. 2008a. Genetic and phenotypic variation among geographically isolated populations of the globally threatened Dupont’s Lark Chersophilus duponti. Mol. Phylogenet. Evol. 46: 237–251. doi: 10.1016/j.ympev.2007.06.022
- García, J.T., Suárez, F., Garza, V., Justribó, J.H., Oñate, J., Hervás, I., Calero, M. & García De La Morena, E.L. 2008b. Assessing the distribution, habitat, and population size of the threatened Dupont's Lark Chersophilus duponti in Morocco: lessons for conservation. Oryx 42: 592-599. doi: 10.1017/S0030605308000653
- García Antón, A., Garza, V. & Traba, J. 2015. Dispersión De Más De 30 Km En Un Macho De Primer Año De Alondra Ricotí (Chersophilus Duponti) En El Sistema Ibérico. I Meeting of the Dupont’s Lark Experts Group, Granada.
- Garza, V. & Suárez, F. 1990. Distribución, Población Y Selección De Hábitat De La Alondra De Dupont (Chersophilus duponti) En La Península Ibérica. Ardeola 37: 3–12.
- Garza, V., Suárez, F., Herranz, J., Traba, J., García De La Morena, E.L., Morales, M.B., González, R. & Castañeda, M. 2005. Home range, territoriality and habitat selection by the Dupont’s Lark Chersophilus duponti during the breeding and postbreeding periods. Ardeola 52: 133–146.
- Gill, F.B. 2007. Physiology. In Gill, F.B. (ed.). Ornithology, 3rd edn, 141–179. W.H. Freeman and Company, New York, NY.
- Grant, P.R., Grant, B.R., Smith, J.N.M., Abbott, I.J. & Abbott, L.K. 1976. Darwin's finches: population variation and natural selection. Proc. Natl. Acad. Sci. USA 73: 257–261. doi: 10.1073/pnas.73.1.257
- Greenberg, R., Cadena, V., Danner, R.M. & Tattersall, G. 2012. Heat loss may explain bill size differences between birds occupying different habitats. Plos ONE 7: E40933. doi: 10.1371/journal.pone.0040933
- Herrera, C.M. 1978. Individual dietary differences associated with morphological variation in Robins Erithacus rubecula. Ibis 120: 542–545. doi: 10.1111/j.1474-919X.1978.tb06825.x
- Hijmans R.J., Cameron S.E. & Parra, J.L. 2010. Worldclim: global weather stations. Museum of Vertebrate Zoology, University of California, Berkeley, in collaboration with Peter Jones and Andrew Jarvis (CIAT), and with Karen Richardson (Rainforest CRC). http://www.worldclim.org/.
- Jenni, L. & Winkler, R. 1994. Moult and Ageing of European Passerines. Academic Press, New York, NY.
- Kingsolver, J.G., Hoekstra, H.E., Hoekstra, J.M., Berrigan, D., Vignieri, S.N., Hill, C.E., Hoang, A., Gibert, P. & Beerli, P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157: 245–261. doi: 10.1086/319193
- Kroodsma, D.E. 1989. Suggested experimental designs for song playbacks. Anim. Behav. 37: 600–609. doi: 10.1016/0003-3472(89)90039-0
- Kroodsma, D.E, Byers, B.E., Goodale, E., Johnson, S. & Liu, W. 2001. Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61: 1029–1033. doi: 10.1006/anbe.2000.1676
- Leisler, B. & Winkler H.B. 2015. Evolution of island warblers: beyond bills and masses. J. Avian Biol. 46: 236–244. doi: 10.1111/jav.00509
- Méndez, M., Tella, J.L. & Godoy, J.A. 2011. Restricted gene flow and genetic drift in recently fragmented populations of an endangered steppe bird. Biol. Conserv. 144: 2615–2622. doi: 10.1016/j.biocon.2011.07.011
- Méndez, M., Vögeli, M., Tella, J.L. & Godoy, J.A. 2014. Joint effects of population size and isolation on genetic erosion in fragmented populations: finding fragmentation thresholds for management. Evol. Appl. 7: 506–518. doi: 10.1111/eva.12154
- Møller, A.P. 1991. Influence of wing and tail morphology on the duration of song flight in skylarks. Behav. Ecol. Sociobiol. 28: 309–314. doi: 10.1007/BF00164379
- Morales-Castilla, I., Rodríguez, M.A. & Hawkins, B.A. 2012. Deep phylogeny, net primary productivity, and global body size gradient in birds. Biol. J. Linn. Soc. 106: 880–892. doi: 10.1111/j.1095-8312.2012.01917.x
- Nowicki, S. 1987. Vocal tract resonances in oscine bird sound production: evidence from birdsongs in a helium atmosphere. Nature 325: 53–55. doi: 10.1038/325053a0
- Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O'hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H. & Wagner, H. 2011. Vegan: community ecology package. R Package Version 2.0-2. http://cran.r-project.org/package=vegan.
- Olalla-Tárraga, M.A., Diniz-Filho, J.A.F., Bastos, R.P. & Rodríguez, M.A. 2009 Geographic body size gradients in tropical regions: water deficit and anuran body size in the Brazilian Cerrado. Ecography 32: 581–590. doi: 10.1111/j.1600-0587.2008.05632.x
- Panhuis, T.M. Butlin, R., Zuk, M. & Tregenza, T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16: 364–371. doi: 10.1016/S0169-5347(01)02160-7
- Pérez-Tris, J., Ramírez, A. & Tellería, J.L. 2003. Are Iberian Chiffchaffs Phylloscopus (Collybita) brehmii long-distance migrants? An analysis of flight-related morphology. Bird Study 50: 146–152. doi: 10.1080/00063650309461306
- Pinheiro, J., Bates, D., Debroy, S., Sarkar, D. & R Core Team 2015. Nlme: linear and nonlinear mixed effects models. R Package Version 3.1-122. http://cran.r-project.org/package=nlme.
- Pinilla, J. 2000. Manual para el anillamiento científico de aves. SEO/BirdLife y DGCN-MIMAM, Madrid.
- Podos, J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin’s finches. Nature 409: 185–188. doi: 10.1038/35051570
- Price, T.D. 2006. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 209: 2368–2376. doi: 10.1242/jeb.02183
- Remsen, J.V. 2003. The ‘Coerebidae': a polyphyletic taxon that dramatizes historical over-emphasis on bill shape as a taxonomic character. J. Avian Biol. 34: 321–323. doi: 10.1111/j.0908-8857.2003.03313.x
- R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation For Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.r-project.org.
- Rensch, B. 1938. Some problems of geographical variation and species formation. Proc. Linn. Soc. Lond. 150: 275–285. doi: 10.1111/j.1095-8312.1938.tb00182k.x
- Seddon, N., Botero, C.A., Tobias, J.A., Dunn, P.O., Macgregor, H.E.A., Rubenstein, D.R., Uy, J.A.C., Weir, J.T., Whittingham, L.A. & Safran, R.J. 2013. Sexual selection accelerates signal evolution during speciation in birds. Proc. R. Soc. Lond. Ser. B 280, 20131065. doi: 10.1098/rspb.2013.1065
- Senar, J.C., Lleonart, J. & Metcalfe, N.B. 1994. Wing-shape variation between resident and transient wintering Siskins Carduelis spinus. J. Avian Biol. 25: 50–54. doi: 10.2307/3677293
- Seoane, J., Justribó, J.H., García, F., Retamar, J., Rabadá, C. & Atienza, J.C. 2006. Habitat-suitability modelling to assess the effects of land-use changes on Dupont’s lark Chersophilus duponti: A case study in the Layna Important Bird Area. Biol. Conserv. 128: 241–252.
- Slatkin, M. 1987. Gene flow and the geographic structure of natural populations. Science 236: 787–792. doi: 10.1126/science.3576198
- Suárez, F. 2010. La Alondra Ricotí (Chersophilus duponti). Dirección General Para La Biodiversidad. Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid.
- Svensson, L. 1992. Identification Guide to European Passerines. British Trust for Ornithology, Hertfordshire.
- Symonds, M.R & Tattersall, G.J. 2010. Geographical variation in bill size across bird species provides evidence for Allen’s rule. Am. Nat. 176: 188–197. doi: 10.1086/653666
- Tellería, J.L. & Carbonell, R. 1999. Morphometric variation of five Iberian Blackcap Sylvia atricapilla populations. J. Avian Biol. 30: 63–71. doi: 10.2307/3677244
- Tellería, J.L., Pérez-Tris, J. & Carbonell, R. 2001. Seasonal changes in abundance and flight-related morphology reveal different migration patterns in Iberian forest passerines. Ardeola 48: 27–46.
- Voelker, G. 2001. Morphological correlates of migratory distance and flight display in the avian genus Anthus. Biol. J. Linn. Soc. 73: 425–435. doi: 10.1111/j.1095-8312.2001.tb01371.x
- Vögeli, M., Serrano, D., Tella, J.L., Méndez, M. & Godoy, J.A. 2007. Sex determination of Dupont’s Lark Chersophilus duponti using molecular sexing and discriminant functions. Ardeola 54: 69–79.
- Vögeli, M., Serrano, D., Pacios, F. & Tella, J.L. 2010. The relative importance of patch habitat quality and landscape attributes on a declining steppe-bird metapopulation. Biol. Conserv. 143: 1057–1067. doi: 10.1016/j.biocon.2009.12.040
- Vögeli, M., Serrano, D., Méndez, M. & Tella, J.L. 2017. Morphological variation in the specialist DuPont’s Lark Chersophilus duponti: geographical clines vs. local ecological determinants. J. Ornithol. 158: 25–38. doi: 10.1007/s10336-016-1383-x
- West-Eberhard, M.J. 1983. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58:155–183. doi: 10.1086/413215
- Westneat, M.W., Long, J.H., Hoese, W. & Nowicki, S. 1993. Kinematics of birdsong: functional correlation of cranial movements and acoustic features in sparrows. J. Exp. Biol. 182: 147–171.
- Wright, S. 1943. Isolation by distance. Genetics 28:114–138.
- Zeffer, A. & Norberg, U.M. 2003. Leg morphology and locomotion in birds: requirements for force and speed during ankle flexion. J. Exp. Biol. 206: 1085–1097. doi: 10.1242/jeb.00208
