ABSTARCT
Capsule: Nest success rate of Hawfinches Coccothraustes coccothraustes within our study areas averaged 36% across five seasons, a level unlikely to be driving population declines and considerably higher than was suggested by recent estimates from the long-term Nest Record Scheme.
Aims: To investigate potential habitat correlates of nest success and identify nest predators from an intensive study on Hawfinches. To compare nest success and habitat of these nests with those found by Nest Record Scheme (NRS) recorders and test whether there is evidence of a decline in nest success over time from the NRS data.
Methods: Females trapped at feed sites were fitted with radio-tags and tracked back to nest sites providing a sample of nests for subsequent monitoring. Habitat measures potentially influencing survival were collected at nest sites and modelled against nest outcome. Nest success was compared with that from the long-term Nest Record Scheme. Nest cameras were deployed to identify predators.
Results: Nest success varied among years, but the mean value was not substantially lower than other Hawfinch studies or from other species with similar nesting strategies. Apparent recent declines in success suggested by Nest Record Scheme data were not evident in our study areas. Only avian species were recorded predating nests and a number of partial predations were recorded. No correlation was found between overall success for study nests and the habitat or temporal measurements collected.
Conclusions: Within our study areas, average Hawfinch nest success and productivity appeared to be sufficient to maintain local population stability though our sample size was modest and further work would be beneficial. General nest recording may have inherent biases that lead to an over-estimation of nest failure compared to those found by more structured study. Drivers of recent UK Hawfinch declines may be operating outside of the nesting season and/or in landscapes outside primary woodland.
The Hawfinch Coccothraustes coccothraustes is one of a suite of UK birds, closely associated with woodland habitats, which have suffered major declines in recent decades. Based on a composite index of the trends of 37 widespread species, woodland birds in the UK have declined by 23% since 1970, and for a subset of woodland specialists (based on 25 species) the decline is more severe at 43% (Defra Citation2017). Hawfinches are not included in these indices as they are too scarce to be monitored by the national, annual monitoring schemes so their decline has been best documented through the breeding bird atlas surveys of 1968–72 (Sharrock Citation1976), 1988–91 (Gibbons et al. Citation1993) and 2008–11 (Balmer et al. Citation2013). These data show a 76% reduction in the number of occupied 10-km squares between the first and last periods, with the majority of loss occurring between the second and last atlases. The most recent population estimate suggests there are just 500–1000 breeding pairs largely confined to a small number of discreet geographical areas of England and Wales (Clements Citation2013), although recently revised calculations from one of the core areas mean this figure may need revising upwards a little (Lewis Citation2018). Local breeding extinctions have been noted across much of central and eastern England, with only 4% of 10-km squares in Britain now occupied (Balmer et al. Citation2013), though winter records remain more widely distributed.
Although often separated by considerable distances, the remaining Hawfinch strongholds in Britain have a distinct western bias and consist of highly wooded landscapes characterized by mature and diverse tree assemblages. Persistence of breeding populations between the atlas surveys of 1988–91 and 2008–11 was found to be more likely in areas where the proportion of broad-leaved and mixed woodland cover was greatest at both landscape and local scales (Kirby et al. Citation2015).
In contrast to the British population, the latest assessment from the Pan European Common Bird Monitoring Scheme (PECBMS) shows an overall ‘moderate increase’ of Hawfinch, with both the long-term (1980–2014) and short-term (2005–14) trends increasing (PECBMS Citation2017). However, within this overall assessment, there is considerable regional variation, with moderate declines in central and eastern Europe, moderate increases in western Europe, stable populations in northern Europe and uncertainty regarding the trend in the southern region. Reasons for the severity of the British decline and the apparent diversion from the European trend are unknown, though a number of possible factors have been implicated in wider woodland bird population declines, including: climate change, reduction in invertebrate food supplies, changes to adjacent land use, habitat modification by deer and increased predation pressure from avian and mammalian predators (Fuller et al. Citation2005). No studies have yet linked these or other changes specifically to Hawfinch declines, though Smart et al. (Citation2007) found correlative evidence suggesting declines were greater in woods with high counts of Grey Squirrel Sciurus carolinensis dreys, but this was based on a very small sample.
British Hawfinches are thought to be predominantly single-brooded, though our study showed double brooding can occur (W. B. Kirby unpubl. data). Subsequent re-nesting after failure of initial attempts appears to be common. The majority of first egg dates occur in the period late-April to late-May. Nestling Hawfinches are fed by both parents on a mixture of invertebrates and seeds, with the proportion of seeds increasing with chick age (Mountfort Citation1957, Newton Citation1967).
Our study set out to intensively study nest survival rates, fledgling productivity and causes of nest failure, and to compare these with historical data collected by volunteers for the British Trust for Ornithology's Nest Record Scheme between 1943 and 2013. The study also collected habitat data to investigate potential correlates of nesting success. Understanding the components of nest success may help identify factors contributing to recent population declines, an essential first step in designing remedial conservation actions.
Methods
Study areas
Two separate areas were used during the course of this study, centred on pre-existing ringing studies in woodlands with regular and recent Hawfinch breeding activity. The first study area (2013–16) occupied a section of the Wye river valley between the towns of Monmouth and Chepstow on the English/Welsh border. The second study area (2015–17) was close to the town of Dolgellau, Gwynedd in north Wales. The two study areas are broadly similar in terms of habitat, comprising steep-sided valleys with a high cover of species-rich, mature deciduous woodland, both natural and plantation. Pastoral farmland, rural habitation, roads and conifer plantations are other major components of both local landscapes. The study areas had no pre-defined boundaries, these being dependent on the movements of the radio-tracked birds.
Locating nest sites
Nests were located by tracking female Hawfinches fitted with miniature radio transmitters. Birds were attracted to the ground at sites baited with black sunflower seeds where they were caught using mist nests or whoosh nets. In 2013 radio-tags (Biotrack Pip Ag376, 0.76 g) were tail-mounted to the central two tail feathers (Calladine & Morrison Citation2010) but due to short tag retention times, this method was altered in future years to use a lighter-weight tag (Biotrack PicoPip Ag376, 0.65 g) glued to the birds back (Tyler et.al. Citation1996). Between mid-April and early July, tags were fitted to females that had a well-developed brood patch.
Following tag attachment, daily searches (by vehicle and on foot, dependent on access) were undertaken for all birds with extant tags within a five-kilometre buffer zone surrounding the tagging sites. Searches of suitable habitat outside the buffer zones were undertaken to look for missing birds as time permitted. When a signal was detected attempts were made to locate the bird to ascertain its position and behaviour. Although it was not always possible to actually observe the tagged bird, we could tell from the radio signal whether they were stationary for extended periods (indicating incubation or chick brooding) or moving (suggesting they were not actively nesting or had fledged chicks). Further observation allowed us to pinpoint nests, confirm feeding of fledged chicks and identify individuals not actively nesting. Searches for missing frequencies continued until either the bird (or dropped tag) was located or the tag's battery life was exceeded. Success rate in locating nest sites was around 50% with 69 nests located from 143 tags deployed. A range of factors impacted our ability to find nests, including early tag loss or failure, movement of birds outside our search area, and tagging of some birds that had already completed the nesting phase.
Nest monitoring
Nests were monitored at 2–3-day intervals from the ground using binoculars and telescopes. The height and position of nests meant that it was rarely possible to view into the nest cup, so the stage of the nesting attempt generally had to be interpreted from adult behaviour, although large chicks could sometimes be seen stretching above the rim of the nest. Fledging could normally be confirmed as Hawfinch chicks leave the nest before they can fly strongly and adults continue feeding them in the vicinity of the nest for several days before moving them further away. Remote, digital, motion activated nest cameras were erected at a sample of nests in both study areas, primarily to identify nest predators, though incidentally enhancing the effectiveness of our monitoring for these nests. Cameras were positioned 0.5–1 m from the nest and cabled to a recording unit positioned on the ground to enable easy access to change batteries and memory cards with minimal further nest disturbance (Bolton et al. Citation2007). Camera footage was reviewed where available to identify nest predators and record supplementary information not available from standard nest monitoring from the ground.
Once nesting attempts were completed, we recorded a range of measurements at the nest site including: tree species, nest height, tree height, trunk circumference, distance to nearest glade (open area greater than 100 m2), distance to woodland edge and whether the nest was concealed within Ivy Hedera helix or other creepers.
Nest Record Scheme data
Data from Hawfinch nests across Britain for the period 1943–2013 were obtained from the British Trust for Ornithology's (BTO) Nest Record Scheme (NRS). The NRS is an unstructured, volunteer-based scheme for recording nest details and outcomes for any UK breeding bird species (Crick et al. Citation2003). These nest records are not derived from predetermined search effort and the number and frequency of subsequent monitoring visits is variable. A total of 251 Hawfinch nest record histories were obtained from the BTO archive, though many contained observations from a single visit only. Although some useful information could still be obtained from these single visit records (e.g. nest height, tree species), without a known outcome, we were not able to use them in models investigating nest success. We therefore selected only those nest records that had an observed outcome and at least two visits, providing exposure days and the means to back-calculate first egg dates. This resulted in 133 NRS records being retained, though a further six did not have an estimated height, a predictor variable within the starting model, leaving a minimum of 127 nests for analysis (or 133 in iterations without the NEST HEIGHT variable – see below). Descriptive statistics used a variable number of nests, dependent on what could be discerned from the details recorded.
Statistical analysis
Patterns of nest success from the intensive study areas
Overall daily nest survival was calculated for each year and each study area using the Mayfield method (Mayfield Citation1975), where daily probability of success is calculated using the number of days the nest was monitored and the final outcome as successful or not. As nests were not visited every day, the mid-point between the penultimate and final visits was used to determine the presumed outcome date. Separate analyses were not possible for egg and chick stages as we were seldom able to accurately identify hatching date, hence the analysis relates to the whole nesting period. To obtain overall nest survival estimates, daily survival rates were raised to the power of 28 (4 days laying and 12 days each for egg and chick stages). These figures were also used to back-calculate first egg dates where nests were found post-laying.
The effect of a number of habitat and nest characteristics on overall nest success were investigated using generalized linear models (GLM), fitted using the PROC GENMOD procedure of SAS version 9.4 (SAS Institute, Cary, NC). The response variable, daily whole nest mortality, used a binomial trials model in which nest outcome (1 = failure, 0 = success) was the numerator, and the number of exposure days the denominator (Johnson Citation1979). Predictor variables considered were YEAR, STUDY AREA, FIRST EGG DATE, NEST HEIGHT, PROPORTIONAL HEIGHT, TREE CIRCUMFERENCE, OPEN AREA DISTANCE, NEST EXPOSURE, and the interaction term for YEAR*STUDY AREA. These variables were used to identify whether there were seasonal variations in nest success between and within years (YEAR, FIRST EGG DATE), between study areas (STUDY AREA), exposure of the nest to weather or predators (NEST HEIGHT, PROPORTIONAL HEIGHT, OPEN AREA DISTANCE, NEST EXPOSURE) and woodland maturity (TREE CIRCUMFERENCE). Definitions of the variables are provided in . All variables were included in the full model and stepwise backward deletion of non-significant terms (P > 0.05) was used for model selection, to identify whether any were related to nest failure rates.
Table 1. Predictor variables and definitions used in GLMs for Hawfinch nests in our intensive study areas and the Nest Record Scheme data.
Temporal patterns in nest success and nest characteristics affecting nesting success from the Nest Record Scheme data
Effects of nest characteristics and identification of temporal trends in nest success using NRS data were analysed using the same GLM structure as above but with a different set of predictor variables available from nest record cards. Nest record cards record the habitat surrounding the nest in much less detail than our study, only allowing us to investigate FIRST EGG DATE, NEST HEIGHT and differences over time, represented by the YEAR variable. All variables and definitions are provided in .
Results
Nest success in the intensive study areas
A total of 69 nests were monitored in the two study areas between 2013 and 2017. Annual nest survival rate varied between years in both study areas, ranging from 0.10 to 0.68, giving an average nest survival rate of 0.36 across all years and both study areas (). However, the apparent differences were not statistically significant (STUDY AREA: = 0.24, P = 0.62, YEAR:
= 2.14, P = 0.71, YEAR*STUDY AREA:
= 4.66, P = 0.59), probably due to the small sample sizes in any one year.
Table 2. Number of Hawfinch nests monitored per year in our two study areas showing outcome and cumulative exposure days, along with results from two European studies for comparison.
Hawfinch nest success also showed no relationship with any of the six potential predictor variables, following stepwise backwards deletion of non-significant terms (P > 0.05) within the model. There was, therefore, no apparent evidence that date of nesting (FIRST EGG DATE: coefficient = 0.0101, se = 0.0132, = 0.88, P = 0.35), nest height (NEST HEIGHT, coefficient = −0.0215, se = 0.0486,
= 0.2, P = 0.66), nest tree girth (TREE CIRCUMFERENCE: coefficient = −2.395, se = 1.439,
= 0.29, P = 0.59), position in the nest tree (PROPORTIONAL HEIGHT: coefficient = 0.0018, se = 0.0033,
= 2.6, P = 0.11), distance to open area (OPEN AREA DISTANCE: coefficient = 0.0041, se = 0.0146,
= 0.08, P = 0.78) or whether the nest was concealed within a creeper (NEST EXPOSURE: coefficient = 0.397, se = 0.421,
= 0.9, P = 0.34) influenced nest failure rates in our two study areas.
Causes of nest failure
Over the five breeding seasons, nest cameras were sited at 19 active nests (15 in Dolgellau and 4 in Wye Valley). In four cases the nest failed due to the disturbance caused in siting the cameras, and in a further two cases the camera failed to record an outcome. From the 13 nests that successfully recorded to the end of the nesting attempt, 7 fledged chicks, 2 failed due to weather and 1 failed after the female ejected chicks that had been colour ringed (nests that were adversely affected by our research actions have been excluded from all calculations). The remaining three nests failed due to predation, consisting of: (1) partial predation of eggs by Jay Garrulus glandarius then abandonment, (2) complete predation of chicks by Great Spotted Woodpecker Dendrocopos major and (3) three separate partial predations of chicks by Goshawk Accipiter gentilis (twice) and Jay. Partial predations of chicks by Jays were also recorded at a further three nests, two of which proceeded to fledge partial broods and one had an unknown outcome due to subsequent camera failure. No mammalian predators were recorded at, or close to, any nests.
Nest Record Scheme data
Overall Mayfield-adjusted nest success calculated from the NRS records was 20.7%, well below that of 36% obtained from our study, though this was highly influenced by the number of failures in the later period (1996 onwards). The predictor variables YEAR and NEST HEIGHT are highly correlated (Pearson correlation = 0.43, P > 0.0001), with average nest height increasing over time (). Both were included as predictors in separate backwards deletion models of nest success. This resulted in a similar outcome, with FIRST EGG DATE non-significant (P > 0.05) in either. The minimum adequate model retained either YEAR or HEIGHT (, ) so we could not be sure which variable was driving the observed decline in nest success. However, the decline in nest success with increasing height observed in the NRS data does not appear to fit with the findings from our study, where no such relationship was apparent (). Examination of the data set showed a high number of nest failures between 1996 and 2013 (33 failures and only 1 success, which seems biologically unlikely and may be an artefact of the data set).
Figure 1. Scatterplot of raw data from Hawfinch Nest Record Scheme, with fitted regression line showing the relationship of increasing nest height over time (Pearson correlation = 0.43 P < 0.0001).
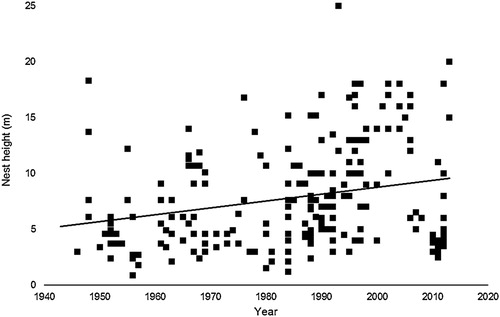
Figure 2. Predicted probability of overall nest success (line) with observed data from 1943 to 2013 nest records grouped by decade (a) and height category (b) with failures on the lower histogram and successes above.
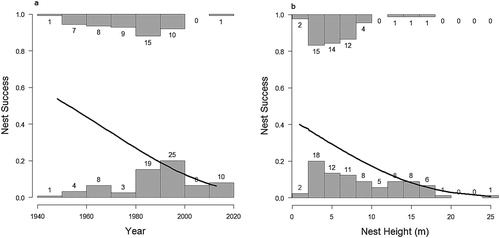
Figure 3. Observed Mayfield-adjusted overall nest success rates with increasing nest height, comparing the intensive study (squares) with Nest Record Scheme data (diamonds). Data labels show number of nests contributing to each category, error bars show 1 standard error.
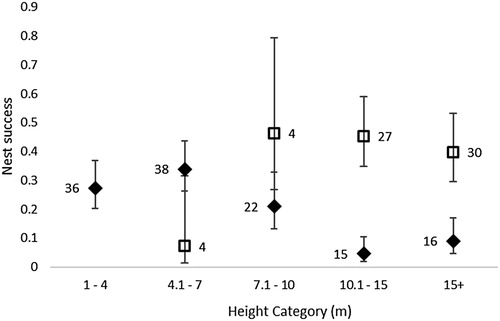
Table 3. Models describing variance in Hawfinch nest success from Nest Record Scheme data. Separate models are presented for Height and Year as the two variables are confounded. The minimum adequate models (MAM) are derived by stepwise backwards deletion of non-significant terms (P > 0.05).
Discussion
Poor reproductive success is frequently observed in declining bird populations and long-term nest monitoring has been instrumental in showing the wide range of factors that can influence this (e.g. Newton Citation2004, González-Braojos et al. Citation2017, Imlay et al. Citation2018). However, for studies to be applicable to wider populations the nests monitored need to represent the full range of natural situations. The method of nest finding in our study was designed to reduce a number of potential biases inherent in cold searching (visually searching suitable habitat), as commonly used by Nest Record Scheme recorders (Crick & Baillie Citation1996). NRS data may be biased towards early season nests, as recorders are keener at the start of the season and put in more nest finding effort. Nests may also become more difficult to find later in the season as concealing vegetation becomes denser, a factor likely to apply particularly to the Hawfinch, where later nests (and parent bird activity) are readily hidden by the developing canopy. There may also be a bias towards easier to find nests (e.g. lower or closer to woodland tracks and edges) which could have higher failure rates if they are also easier for predators to find. A further factor detected in the Hawfinch nest record histories that may have led to an over-estimation of failures, is the number that have no confirmed outcome. If, for instance, only two visits are made to a nesting area, firstly to find the nests and then to check them at a subsequent date (perhaps to ring chicks), all failed nests will be recorded with a definite outcome whereas those still active remain unknown (they may subsequently fail or fledge). If a further visit is not made, or made much later, these potentially successful nests cannot be used in the calculation of nest success rates as they have an unknown eventual outcome. We suspect this latter bias may be exerting a strong influence on the later period NRS data.
These potential biases have largely been removed from our intensive study as the nests were found by following tagged females, caught away from the nesting area. We rarely failed to find nests where nesting was suggested by the activity of the birds we were able to track. There may still be some bias towards earlier nests as we had a limited supply of tags and more were fitted early in the season (April 21, May 82, June 35, July 5). Tag battery life was six weeks, though many failed or were lost prior to this. The fact that all of our tagged birds were caught at artificial feeding sites does add a different potential bias, as supplementary food can affect reproductive success (Peach et al. Citation2014). Within the tagged bird sample there was, however, a broad spectrum of feed site use, as recorded by regular observation of individually identifiable colour rings and regular trapping throughout the season. Nests were also widely dispersed within the study areas ranging from a few hundred metres to 17 km away from feed sites.
A wide range of factors can influence nest survival and although, ultimately, predation accounts for the majority of nest failures (estimated as an average of 80% of failures across a wide range of species and habitats; Martin Citation1993), predation likelihood itself is dependent on a range of precursory factors. Local food availability is critical to nest survival, and a lack of food for nestlings can lead to direct starvation but may have other, indirect effects. Increased foraging time for adults having to fly further or search longer for food will mean less time spent at the nest which, in turn, can lead to increased energy use by chicks to keep warm in the absence of brooding and ultimately reduced growth rates and fitness. Increased absence from the nest also leaves the nestlings more vulnerable to predation as adults are less available to defend the nest or distract predators. Hungry nestlings are likely to be more vocal due to increased begging for food and thus attract the attention of potential predators (Evans Citation2004), though Hawfinch adults are capable of vigorous nest defence and we regularly observed them chasing away corvids. The high frequency of partial predation events captured on camera is likely due to adults interrupting predation events. The main nest predator for Hawfinches in this study appears to be the Jay, as was also found in the Netherlands (Bijlsma Citation1998) and Poland (Tomiałojć Citation2012). Jays similarly accounted for 8 of 11 predation events recorded at woodland Spotted Flycatcher Muscicapa striata nests using identical cameras to those deployed in this study (Stevens et al. Citation2008).
Despite the frequency with which nests are predated, there is little evidence that this has a causative role in population level declines for songbirds. Large-scale correlative studies using Common Bird Census and Breeding Bird Survey data found very few instances where increased predator numbers coincided with declines in songbird populations. In fact, in many cases populations of songbirds increased alongside increases in predator numbers, suggesting that habitat loss and/or degradation is a more likely driver of population change (Thomson et al. Citation1998, Newson et al. Citation2010). Specific to Hawfinches, no differences were found in predator encounters in survey areas where breeding Hawfinches had persisted or been lost between atlas periods (Kirby et al. Citation2015).
Perhaps the most important factor implicated in nest failures, aside from predation, is weather during the nesting period. Prolonged cold and/or wet periods may cause incubating birds to desert and can lead to widespread mortality of chicks (Newton Citation1964) as adults are not able to leave the nest to forage without leaving the chicks vulnerable to chilling. During this study poor weather events caused nest failures on several occasions, including one two-day period of persistent rain, low temperatures and strong winds on 31 May and 1 June 2015 when two of three nests under observation failed. One of the three had a nest camera, which clearly showed the brooding female desert and the nestlings die of exposure, though the female continued to return to the dead chicks intermittently over the following day. The overall low success rates of the Dolgellau nests in 2015 () could be due to an extremely wet May which recorded 111 mm of rain, the third highest total in 86 years and more than double the 86-year average (as measured at Valley weather station, Anglesey, approximately 70 km northwest of the study area (data.gov.uk)). Recent UK climate observations show a trend towards wetter summers and an increase in the number and intensity of extreme summer precipitation events, both of which could be detrimental to nesting birds (Monteith et al. Citation2016).
Failure of individual nests is not necessarily detrimental to the overall population for species that rear one brood per season. If a pair can re-lay after failure it may make no difference whether it is the first, second or a subsequent nest that produces chicks providing they have equivalent survival rates (though there may be some cost to adult reproductive fitness). Immediate post-fledging survival will be determined by weather, food availability and other factors that vary temporally over a season and between years, so it will not always be the case that early-fledging broods have the highest chance of reaching independence. However, we know from this study that it is possible (at least occasionally) for Hawfinches to re-nest after a successful attempt and this could potentially make a big difference to overall per-pair productivity. More research is necessary to establish whether multiple broods are a regular occurrence and, if so, what affects their likelihood. This is an important missing component in our current understanding of Hawfinch demography. Immediate post fledging survival and differences between first-year and adult survival are also unknown for Hawfinches. Continued efforts to ring and re-sight juveniles after fledging will enable estimates of first-year survival over time. To estimate immediate post-fledging survival requires more focused research, probably involving the radio-tracking of fledglings.
The overall nest success rate of 36% () that we detected sits between that of 27% obtained from a stable population in Poland (Tomiałojć Citation2012) and 39–59% estimated from a rapidly increasing population in the Netherlands (Bijlsma Citation1998). For other species with similar nesting strategies, woodland-nesting Spotted Flycatchers in England had an equivalent rate of 24.2% (Stevens et al. Citation2007) and Chaffinch Fringilla coelebs 33.1% (Stoate & Szczur Citation2001). A simple demographic model, using inputs from our study populations supplemented by values from similar species in the literature, suggests this should be sufficient to maintain the population at the mid-range of our estimates (online ). However, the work undertaken here was confined to two remaining strongholds where local populations are currently thought to be stable and whether they are representative of other regions of Britain where declines have been more pronounced and are ongoing is not known. Attempts to set up a study population in Kent, where there is evidence of continued decline, failed due to low numbers of birds.
The loss of breeding Hawfinches from many parts of Britain, particularly the midlands and southeastern England as documented through the national atlas projects, therefore remains largely unexplained, though possible causes are discussed by Kirby et al. (Citation2015). Within the NRS data, the correlation between increasing nest height and year () supports the hypothesis that nests are increasingly confined to woodland. Further examination of the NRS data shows that the median pre-1980 nest height was 5.5 m and post-1980 had increased to 7.6 m (the equivalent measure from our study was 15 m). There is also a pronounced change in the species composition of nesting trees, with Oak Quercus sp. and Beech Fagus sp. increasing from 7.4% to 20.1% and 1.2% to 21.2%, respectively, while Hawthorn Crataegus sp. (a traditional hedgerow species) decreased from 25.9% to 1.9%.
That breeding Hawfinches are now primarily confined to a small number of highly wooded core areas suggests that formerly suitable sites are now unable to maintain viable populations, possibly due to a deteriorating wider landscape. Biodiversity loss associated with agricultural intensification is widely documented (e.g. Donald et al. Citation2001, Stoate et al. Citation2001, Green et al. Citation2005). Specific changes that could affect Hawfinch populations include: loss of traditional orchards (Robertson & Wedge Citation2008); destruction of mature hedgerows and in-field trees (Barr & Parr Citation1994, Rackham Citation2001), and wide-scale depletion of invertebrates (Benton et al. Citation2002, Hallman et al. Citation2017) and natural seed sources (Robinson & Sutherland Citation2002). Recent outbreaks of tree disease may have further depleted potential Hawfinch food sources, in particular, the loss of 20 million elms Ulmus sp. (a spring-seeding species) to Dutch elm disease across England during the 1970s (Gibbs et al. Citation1994) coincided with the start of the Hawfinch decline. Other regular seed sources including cherry Prunus sp., Yew Taxus baccata and Wych Elm Ulmus glabra are not highly valued by commercial forestry and are frequently selected out of managed woods. Berry production on Hawthorn (another favoured food source) in hedgerows is likely to have declined with the increasing frequency of cutting associated with the mechanization of farming. Hedges within former English agri-environment scheme rules had 83% lower berry production than when left unmanaged (Staley et al. Citation2012), whilst annually cut hedges (the most frequent treatment outside of agri-environment schemes) produce only 2% of the berry weight by area compared with long-term uncut hedges (Croxton & Sparks Citation2002). Large increases in some avian predator populations have been most pronounced on farmland (Gregory & Marchant Citation1996) and while this study did not find unexpectedly high levels of nest failure in woodland, the case may be different in other habitats.
Although this study centred on two, potentially stable, core woodland-based populations, recovering Hawfinch numbers more generally is likely to require conservation efforts both within primary woodland habitats and across the wider countryside. There remain a number of gaps in our understanding of basic Hawfinch ecology and further research to establish post-fledging and first-year survival would improve confidence in our demographic modelling. As there is little within the results of this study to suggest current nest success rates are insufficient to maintain populations, further research concentrating on different stages of the life cycle would be a sensible next step. We suggest a focus on winter diet and food availability in the pre-breeding period as perhaps the next most important aspect to investigate as this ‘hungry gap’ has been identified as a factor in the decline of other seed-eating species (Siriwardena et al. Citation2008).
Acknowledgements
We extend our thanks to Natural Resources Wales, Forestry Commission England and the many other land managers who allowed access within our study areas. Assistance with collection of data in the field was ably provided by Vivien Hartwell, Judit Mateos, Rob Hughes, Molly Foulds, Mike Duckham and Annie Seddon. Adrian Thomas provided extensive logistical help and assisted with bird capture in the Wye Valley while climbers Matt Thomas and Steve Roberts erected the nest cameras there. At RSPB, Richard Gregory provided advice and continued support throughout the project and Will Peach advised on our demographic model. Nigel Butcher, Andrew Asque and Colin Gooch helped with equipment and technical issues. The British Trust for Ornithology kindly provided access to Nest Record Scheme and Retrapping Adults for Survival data and we thank all the volunteers who helped collect this. Our original manuscript was improved by the helpful comments provided by reviewers.
Additional information
Funding
References
- Baillie, S.R. & McCulloch, N. 1993. Modelling the survival rates of passerines ringed during the breeding season from national ringing and recovery data. In Lebreton, J.D. & North, P.M. (eds) Marked Individuals in the Study of Bird Population. Birkhäuser Verlag, Basel.
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. 2013. Bird Atlas 2007–11: The breeding and wintering birds of Britain and Ireland. BTO Books, Thetford.
- Barr, C.J. & Parr, T.W. 1994. Hedgerows: linking ecological research and countryside policy. In Watt, T.A. & Buckley, G.P. (eds) Hedgerow Management and Nature Conservation, 119–136. Wye College Press, Ashford, UK.
- Benton, T.G., Bryant, D.M., Cole, L. & Crick, H.Q.P. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 39: 673–687. doi: 10.1046/j.1365-2664.2002.00745.x
- Bijlsma, R.G. 1998. Breeding biology and population trend of hawfinches Coccothraustes coccothraustes in Flevoland. Limosa 71: 137–148.
- Bolton, M., Butcher, N., Sharpe F., Stevens, D.K. & Fisher, G. (2007) Remote monitoring of nests using digital camera technology. J. Field Ornithol. 78: 213–220. doi: 10.1111/j.1557-9263.2007.00104.x
- Bradbury, R.B., Kyrkos, A., Morris, A.J., Clark, S.C., Perkins, A.J. & Wilson, J.D. 2000. Habitat associations and breeding success of yellowhammers on lowland farmland. J. Appl. Ecol. 37: 789–805. doi: 10.1046/j.1365-2664.2000.00552.x
- Calladine, J. & Morrison, N. 2010.The ranging behaviour and habitat selection by three Hawfinches Coccothraustes coccothraustes in late winter in Scotland. Ornis Fennica 87: 119–123.
- Clements, R. 2013. A UK population estimate for the Hawfinch. Br. Birds 106: 43–44.
- Cox, W.A., Thompson, F.R., Cox, A.S. & Faaborg, J. 2014. Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J. Wildlife Manage. 78: 183–193. doi: 10.1002/jwmg.670
- Crick H.Q.P. & Baillie S.R. 1996. A review of the BTO’s Nest Record Scheme. Its value to the Joint Nature Conservation Committee and Country Agencies, and its methodology. BTO Res. Rep. no. 156. Thetford.
- Crick H., Dudley, C. & Glue, D. 2003. The Nest Record Scheme Handbook, Rev. edn. BTO, Thetford.
- Croxton, P.J. & Sparks, T.H. 2002. A farm-scale evaluation of the influence of hedgerow cutting frequency on hawthorn (Crataegus monogyna) berry yields. Agr. Ecosyst. Environ. 93: 437–439. doi: 10.1016/S0167-8809(02)00106-8
- Data.gov.uk. Historical monthly data for meteorological stations. https://data.gov.uk/dataset/historic-monthly-meteorological-station-data/resource/c6876eb0-8b51-4a8e-96f6-cf034d575d79.
- DEFRA. 2017. Wild bird populations in the UK, 1970 to 2016. https://www.gov.uk/government/statistics/wild-bird-populations-in-the-uk.
- Donald, P.F., Green, R.E. & Heath, M.F. 2001. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. B 268: 25–29. doi: 10.1098/rspb.2000.1325
- Evans, K.L. 2004. The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146: 1–13. doi: 10.1111/j.1474-919X.2004.00231.x
- Fuller, R.J., Noble, D.G., Smith, K.W. & Vanhinsbergh, D. 2005. Recent declines in populations of woodland birds in Britain: a review of possible causes. Br. Birds 98: 116–143.
- Gibbons, D.W., Reid, J.B. & Chapman, R.A. 1993. The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991. T.&A.D. Poyser, London.
- Gibbs, J., Brasier, C. & Webber, J. 1994. Dutch Elm Disease in Britain. Forestry Authority Research Information Note 252. The Forestry Authority, Wrecclesham.
- Green, R.E., Cornell, S.J., Scharlemann, J.P.W. & Balmford, A. 2005. Farming and the fate of wild nature. Science 307: 550–555. doi: 10.1126/science.1106049
- Gregory, R.D. & Marchant, J.H. 1996. Population trends of Jays, Magpies, Jackdaws and Carrion Crows in the United Kingdom. Bird Study 43: 28–37. doi: 10.1080/00063659609460993
- González-Braojos, S., Sanz, J.J. & Moreno, J. 2017. Decline of a montane Mediterranean pied flycatcher Ficedula hypoleuca population in relation to climate. J. Avian Biol. 48: 1383–1393. doi: 10.1111/jav.01405
- Hallmann, C.A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., Stenmans, W., Mueller, A., Sumser, H., Hoerren, T., Goulson, D. & de Kroon, H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12: e0185809. DOI:10.1371/journal.pone.0185809.
- Imlay, T.L., Mills Flemming, J., Saldanha, S., Wheelwright, N.T. & Leonard, M.L. 2018. Breeding phenology and performance for four swallows over 57 years: relationships with temperature and precipitation. Ecosphere 9: e02166. DOI:10.1002/ecs2.2166.
- Johnson, D.H. 1979. Estimating nest success: the Mayfield method and an alternative. Auk 96: 651–661.
- Kirby, W.B., Bellamy, P.E., Stanbury, A.J., Bladon, A.J., Grice, P.V. & Gillings, S. 2015. Breeding season habitat associations and population declines of British Hawfinches Coccothraustes coccothraustes. Bird Study 62: 348–357. doi: 10.1080/00063657.2015.1046368
- Lewis, J. 2018. The Hawfinch population in the forest of Dean/Wye Valley. Br. Birds 111: 168–169.
- Martin, T.E. 1993. Nest predation and nest sites: new perspectives on old patterns. Bioscience 43: 523–532. doi: 10.2307/1311947
- Mayfield, H. 1975. Suggestions for calculating nest success. Wilson Bull. 87: 456–466.
- Monteith, D., Henrys, P., Banin, L., Smith, R., Morecroft, M., Scott, T., Andrews, C., Beaumonte, D., Benham, S., Bowmaker, V., Corbett, S., Dick, J., Dodd, B., Dodd, N., McKenna, C., McMillan, S., Pallett, D., Pereira, M.G., Poskitt, J., Rennie, S., Rose, R., Schäfer, S., Lorna Sherrin, L., Tang, S., Turner, A. & Watson, H. 2016. Trends and variability in weather and atmospheric deposition at UK environmental change network sites (1993–2012). Ecol. Indic. 68: 21–35. doi: 10.1016/j.ecolind.2016.01.061
- Mountfort, G. 1957. The Hawfinch. Collins, London.
- Newson, S.E., Rexstad, E.A., Baillie, S.R., Buckland, S.T. & Aebischer, N.J. 2010. Population change of avian predators and grey squirrels in England: is there evidence for an impact on avian prey populations? J. Appl. Ecol. 47: 244–252. doi: 10.1111/j.1365-2664.2010.01771.x
- Newton, I. 1964. The breeding biology of the Chaffinch. Bird Study 11: 47–68. doi: 10.1080/00063656409476059
- Newton, I. 1967. The adaptive radiation and feeding ecology of some British finches. Ibis 109: 33–96. doi: 10.1111/j.1474-919X.1967.tb00005.x
- Newton, I. 2004.The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146: 579–600. doi: 10.1111/j.1474-919X.2004.00375.x
- Peach, W.J., Sheehan, D.K. & Kirby, W.B. 2014. Supplementary feeding of mealworms enhances reproductive success in garden nesting House Sparrows Passer domesticus. Bird Study 61: 378–385. doi: 10.1080/00063657.2014.918577
- PECBMS 2017. Population trends of common European breeding birds 2016. CSO, Prague.
- Rackham, O. 2001. Trees and Woodland in the British Landscape, Revised edn. Phoenix Press, London.
- Robertson, H. & Wedge, C. 2008. Traditional orchards and the UK biodiversity action plan. Landscp Arch. Ecol. 7: 108–117.
- Robinson, R.A. & Sutherland, W.J. 2002. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 39: 157–176. doi: 10.1046/j.1365-2664.2002.00695.x
- Sharrock, J.T.R. 1976. The Atlas of Breeding Birds in Britain and Ireland. T. & A.D. Poyser, Berkhamsted.
- Siriwardena, G.M., Baillie, S.R. & Wilson, J.D. 1998. Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study 45: 276–292. doi: 10.1080/00063659809461099
- Siriwardena, G.M., Calbrade, N.A. & Vickery, J.A. 2008. Farmland birds and late winter food: does seed supply fail to meet demand? Ibis 150: 585–595. doi: 10.1111/j.1474-919X.2008.00828.x
- Smart, J., Taylor, E., Amar, A., Smith, K., S Bierman, S., Carpenter, J., Grice, P., Currie, F., Smithers, R., Fuller, R. & Hewson, C. 2007. Habitat associations of woodland birds: implications for woodland management for declining species. RSPB Res. Rep. no.26. RSPB, Sandy.
- Staley, J.T., Sparks, T.H., Croxton, P.J., Baldock, K.C.R., Heard, M.S., Hulmes, S., Hulmes, L., Peyton, J., Amy, S.R. & Pywell, R.F. 2012. Long-term effects of hedgerow management policies on resource provision for wildlife. Biol. Conserv. 145: 24–29. doi: 10.1016/j.biocon.2011.09.006
- Stevens, D.K., Anderson, G.Q.A., Grice, P.V. & Norris, K. 2007. Breeding success of Spotted Flycatchers Muscicapa striata in southern England – is woodland a good habitat for this species? Ibis 149: 214–223. doi: 10.1111/j.1474-919X.2007.00746.x
- Stevens, D.K., Anderson, G.Q.A., Grice, P.V., Norris, K. & Butcher, N. 2008. Predators of Spotted Flycatcher Muscicapa striata nests in southern England as determined by digital nest-cameras. Bird Study 55: 179–187. doi: 10.1080/00063650809461520
- Stoate, C. & Szczur, J. 2001. Could game management have a role in the conservation of farmland passerines? A case study from a Leicestershire farm. Bird Study 48: 279–292. doi: 10.1080/00063650109461228
- Stoate, C., Boatman, N.D., Borralho, R.J.C., Rio Carvalho, C., de Snoo, G.R. & Eden, P. 2001. Ecological impacts of arable intensification in Europe. J. Environ. Manage. 63: 337–365. doi: 10.1006/jema.2001.0473
- Thomson, D.L., Green, R.E., Gregory, R.D. & Baillie, S.R. 1998. The widespread declines of songbirds in rural Britain do not correlate with the spread of their avian predators. Proc. R. Soc. Lond. B 265: 2057–2062. doi: 10.1098/rspb.1998.0540
- Tomiałojć, L. 2012. Reproduction and population dynamics of Hawfinches Coccothraustes coccothraustes in the primeval forest of Bialowieia National Park (NE Poland). Acta Ornithol. 47: 63–78. doi: 10.3161/000164512X653935
- Tyler, G.A., Green, R.E., Stowe, T.J. & Newton, A.V. 1996. Sex differences in the behaviour and measurements of Corncrakes Crex crex in Scotland. Ring. Migr. 17: 19–26. doi: 10.1080/03078698.1996.9674115
Appendix: Hawfinch Demographic Model
In order to assess whether the observed values of nest success could be linked to population declines we used a simple deterministic model to give an estimation of population change based on our average nest survival and productivity rates.
Where: Nt and Nt+1 is the population available for breeding in one year and the next. Sn, Sa, Sf and S1 are the survival rates of a nest (from egg laying to fledging), an adult (from one breeding season to the next), a fledgling (fledging to independence) and first-year bird (independence to second summer), respectively. Y1 and Y2 are young produced from pairs at first nesting attempt and re-nesting of failed pairs. P is the number of young produced per successful nesting attempt. RN is the proportion of pairs that nest again after failing at first nesting.
Table A1 shows the effect of varying the demographic rates of individual parameters (whilst holding all others at mean/mid-levels) on the number of birds potentially entering the second breeding season. The model suggests that with all parameters at the middle of their range our populations should be stable or slightly increasing.
Assumptions and caveats
Although we used actual Hawfinch data where possible (Nest survival rate, number fledged per successful nest and adult survival) other required variables were not available and we had to substitute data from other studies and other species into the model (post-fledging survival to 20 days, survival from independence to first breeding). We calculated confidence intervals as far as possible from the summary data available in the referenced articles as we did not have access to the original datasets. For re-nesting rate after initial failure, we used an upper, middle and lower range we felt was biologically realistic and fitted our spread of first egg dates (mid-April – mid-June).
