ABSTRACT
Capsule: Barn Swallows Hirundo rustica were more likely to forage along arable field margins that were enhanced with wildflowers or legumes than control grass margins.
Aims: To determine if foraging Barn Swallows displayed preferences for specific arable field boundary habitats (grass margins versus floristically enhanced margins) that were managed as part of an agri-environment scheme. We also aim to determine how Barn Swallow food abundance related to these habitats.
Methods: Two foraging activity surveys took place on all grass and floristically enhanced margins (n = 56) present within the 600 m foraging range of seven Barn Swallow colonies during June and July 2016. Margin habitat use was measured by recording the presence/absence of foraging individuals during surveys, the total number of individuals and by calculating an index of foraging activity. Habitat information relating to adjacent boundary type, transect crop type and neighbouring crop type were also recorded.
Results: Foraging Barn Swallows were significantly more likely to be recorded when survey transects included a floristically enhanced margin, but there was no significant impact of floristically enhanced margins on the total number of individuals recorded or on the index of foraging activity. Foraging activity was higher along grass verges and hedgerows when compared to treelines and was positively related to length weighted Diptera abundance (a measure of food biomass).
Conclusion: Our results suggest that there may be a role for floristically enhanced margins in the conservation of Barn Swallows on arable farmland. More research, however, is needed to determine whether invertebrate-rich agri-environment scheme habitats can influence colony size or improve the breeding success of this species.
Agricultural intensification has been linked to European farmland bird declines (Donald et al. Citation2001) as well as the declines of a range of other taxa that occupy farmland, including invertebrates (New Citation2005). Declining invertebrate populations have contributed to the population declines of a variety of bird species that depend on them as a food resource, including Grey Partridge Perdix perdix (Potts Citation2012), Yellowhammer Emberiza citrinella and Corn Bunting Emberiza calandra (Brickle et al. Citation2000, Hart et al. Citation2006). Across Europe, agri-environment schemes (AES) have been introduced which, in part, aim to halt biodiversity declines by reducing the negative impact of intensive agricultural practices (Ovenden et al. Citation2008). In the UK, specific farmland bird habitats have been developed that aim to boost productivity through increasing summer invertebrate food supplies and providing additional nesting habitat.
Previous studies on interactions between agricultural management, invertebrate abundance and bird foraging preferences have focused on partial granivores which forage for invertebrates on the ground or within the vegetation when feeding chicks (e.g. Tree Sparrow Passer montanus, Yellowhammer, Cirl Bunting Emberiza cirlus and Corn Bunting; Peach et al. Citation2001, Setchfield et al. Citation2012, McHugh et al. Citation2016, McHugh et al. Citation2017). Studies examining the relationship between purely insectivorous species and AES have tended to focus on breeding waders, with few studies measuring the invertebrate provisions of AES habitats (Schmidt et al. Citation2017). Because research has been heavily biased towards ground-feeding birds, a knowledge gap exists relating to aerial invertebrates and their predators, such as the hirundines and swifts. Where hirundines have been studied in the context of agricultural management, research has tended to focus on grassland-dominated rather than arable habitats (Evans et al. Citation2003a) or compared bird abundance and activity in arable versus grass fields (Evans et al. Citation2007, Henderson et al. Citation2007) and have shown that arable fields tend to provide low-quality foraging habitat for Barn Swallows Hirundo rustica (Evans et al. Citation2007). Thus far there has been no research into their use of AES arable field margins.
This study targeted foraging Barn Swallows, one of the most widely distributed breeding species in Britain, occurring in most lowland landscapes (Balmer et al. Citation2013). Over 80 aerial invertebrate families have been recorded in their diet, but in the UK they have been shown to preferentially hunt large Diptera, including Muscidae (Turner Citation2006). Across Europe, BirdLife International (Citation2015, Citation2018) has estimated that they have undergone moderate declines of approximately 25% in three generations. In the UK, the population is stable at a national level, but local declines have been observed (Evans et al. Citation2003a, Robinson et al. Citation2003), which have been attributed the loss of suitable nest sites (Evans et al. Citation2003b), in addition to hedgerow loss, field enlargement and lower food availability within arable dominated landscapes (Henderson et al. Citation2007). As with other farmland bird species, farmland management through AES may have important implications for this species on arable land. For Barn Swallows, it has been suggested that field margins and hedgerows managed for birds and invertebrates under AES may be beneficial, providing access from nest sites is appropriate (Vickery et al. Citation2004, Henderson et al. Citation2007).
In this study, we compare the likelihood of foraging Barn Swallows being present, as well as the maximum number of Barn Swallows observed and the duration of foraging events, between two AES field margin types (grass margins versus floristically enhanced margins). Grass margins are typically sown with five tussock-forming grass species and they are commonly deployed on arable land in the UK (Natural England Citation2014). They can be used for a wide variety of purposes such as preventing soil erosion and run-off to watercourses, protecting archaeological features or forming links between wildlife habitats (Natural England Citation2014). In contrast, floristically enhanced margins are more floristically and structurally diverse, with mixes containing up to 21 wildflower species (Carvell et al. Citation2007). Because of this increased diversity they contain a greater abundance and diversity of invertebrates (Woodcock et al. Citation2013, Holland et al. Citation2014) and their implementation under AES is recommended to increase spring-summer invertebrate food resources for breeding farmland birds (Natural England Citation2014). Additionally, we compare the results of our Barn Swallow models with invertebrate abundance data and discuss whether AES has the potential to improve arable farmland quality for Barn Swallows.
Methods
Data collection
Foraging surveys were conducted on five farms in Hampshire and Dorset, southern England and represent five Barn Swallow colonies (). In May, a preliminary visit to each farm was made to ensure breeding Barn Swallows were present and to estimate the number of breeding pairs. Each nest was observed for 15 minutes to determine nest activity, between two and eight pairs were recorded on each farm. All active nests were recorded in farm buildings and colonies were a minimum of 2.3 km and maximum of 39.6 km from neighbouring Barn Swallow colonies included in the study. Barn Swallows are central place foragers during the breeding season, meaning that foraging surveys needed to take place within 600 m of Barn Swallow nest sites; this is the maximum distance they are known to forage under normal weather conditions (Bryant & Turner Citation1982). The foraging habitats present within 600 m of the colonies were determined through farm records and were verified with in-field observations. Habitats comprised woodland, grassland, cereal (Wheat Tritcum aestivum and Barley Hordeum vulgare) and Oilseed Rape Brassica napus. All farms were under an AES and AES habitats present included grass margins, wildflower margins, pollen and nectar margins and wild bird seed mixture plots.
Table 1. Grid references for the Barn Swallow colonies under investigation, the number of breeding pairs known to be present at each site and the number of grass and enhanced margins surveyed on each site.
Two foraging surveys were conducted in 2016, one in June and a second in July along field boundary belt transects. Surveys were conducted over this period because Loske (Citation1992) observed that Barn Swallows preferentially forage along field edges at this time of year. To be included in the study the area within 600 m of each colony needed to contain both enhanced and grass margins. However, enhanced margin habitats tended to only be present on farms involved in Higher Level Stewardship and because of this the study farms had high margin coverage across their land. As a result there were insufficient instances of fields without AES margins to use as no-margin controls.
Belt transects were 70 m long by 35 m wide and included a six-meter-wide grass or enhanced margin (wildflower or pollen and nectar) which ran the whole length of the transect in addition to a cereal crop or oilseed rape. The 70 m transect was located centrally along the field edge and all Barn Swallows seen foraging over the transect were counted. All six-meter margins within 600 m of Barn Swallow colonies which were adjacent to either a cereal crop or oilseed rape were surveyed, resulting in 52 belt transect surveys (grass margins, n = 37; enhanced wildflower margin, n = 15). Field boundary type along 70 m transects was recorded as either grass verges (most of which included a fence, n = 16), hedgerows (n = 19) or treelines (n = 17). Land use in the field neighbouring the transect field was also recorded as either arable (n = 40) or grassland (n = 12).
Surveys were 20 minutes in duration and took place between 06:00 and 11:00 hours when Beaufort wind speed was between zero and four, with light or no rain. When hunting insects, Barn Swallows undertake a characteristic foraging flight which entails abrupt and frequent changes in their flight path. Individuals were excluded from analysis if they were engaged in other activities, such as alarm calling or mobbing predators. The highest number of simultaneously foraging Barn Swallows observed within a one-minute period was used to approximate the total number of individuals using the transect and the total of each maximum count per minute was used to calculate an index of foraging activity.
In addition, aerial invertebrates were collected using lightweight kite nets. These data were used to model the effect of habitat on invertebrate abundance and were included as a covariate in the Barn Swallow models. Aerial invertebrate samples were taken at a height of 0.5 to 1.5 m above the vegetation as recommended by Evans et al. (Citation2007) and each sample consisted of 30 sweeps. Transect belts were sampled at four locations, two at the interior, within margins, and two at the exterior edge of belts within the crop. Invertebrates were frozen on the day of sampling and preserved in alcohol before calculating total Muscidae abundance (one of the larger prey items preferred by Barn Swallows) and total Diptera abundance per sample. An index of biomass was calculated by measuring the length of each recorded Diptera, to the nearest millimetre, and the sum of Diptera x Diptera length included as a term in the model (length weighted Diptera abundance). The overall length measurements were taken from the frons to the tip of the abdomen. Appendages extending beyond these points (wing covers, ovipositors, etc.) were disregarded.
The potential confounding variable percentage cloud cover was recorded as a proxy for air pressure. Cloud cover causes the air beneath the clouds to cool and sink creating low pressure which should cause insects to descend nearer to the ground, this may in turn impact our foraging observations if Barn Swallow are more likely to forage for invertebrates near the ground when cloud cover is high.
Data analysis
Before undertaking statistical analysis, the data were explored following the method outlined by Zuur et al. (Citation2010). Data exploration and statistical analysis were implemented in Rv3.2.0 (R Core Team Citation2017). Co-linearity was investigated using Pearson’s correlation coefficients with multi-panel scatter plots. Low correlation was evident between the variables under investigation (Pearson’s R ranged from −0.09 to 0.27). Dot plots were used, in addition to histograms, to determine if it was necessary to transform explanatory variables, resulting in the log + 1 transformation of the cloud cover data and length weighted Diptera.
Two surveys were excluded from the analysis which had been conducted on an enhanced and a control margin at the same time by two observers. No Barn Swallows were observed on the control margin, but the number of foraging Barn Swallows recorded on the enhanced margin was far greater than any other survey (34 individuals compared to our second highest count of 11, which was also on an enhanced margin). This interesting observation may present a potential line of future enquiry, but within the confines of the study presented here, it is inconsistent with the wider methodology.
Statistical models were based on generalized linear mixed effects models (GLMM) and were built using the package lme4 (Bates et al. Citation2015) with Barn Swallow presence, total number of individuals and index of foraging activity being used as response variables. For each response variable we aimed to identify: (1) the effect of habitat on Barn Swallows and (2) the effect of habitat on Barn Swallows given the effect of the best Dipteran predictor. In order to identify the best Dipteran predictor four models were compared for each Barn Swallow response variable: (1) null model, (2) Diptera abundance, (3) length weighted Diptera and (4) Muscidae abundance. An information theory approach was taken to identify the best Dipteran predictor for each response variable and models were compared based on Akaike Information Criteria (AIC) weights (Burnham & Anderson Citation2002).
GLMM models investigating the effect of habitat were hierarchically structured with transect crop type (cereal or oilseed rape), margin type (enhanced or grass) and boundary type (verge, hedgerow or treeline) included as the fixed effects of primary interest. Visit (June or July) was also included as a fixed effect to determine whether foraging behaviours differed over the breeding period. Neighbouring crop (arable or grassland) and percentage cloud cover were included in models as confounding factors. Colony was incorporated in all models as a random effect to account for differential colony size between farms. The Barn Swallow presence models used a binomial distribution and logit link to account for Barn Swallows either being present or absent over the survey period. GLMMs for the total number of individuals and foraging activity index models were first built using a Poisson error distribution and log link, however the final models used a negative binomial error distribution and log link to account for overdispersion present in the Poisson models using the package glmmADMB (Fournier et al. Citation2012, Skaug et al. Citation2016). Where appropriate habitat models were rebuilt to identify the effect of habitat on Barn Swallow given the best Dipteran predictor.
Muscidae abundance, Diptera abundance and length weighted Diptera abundance were also modelled in relation to the sampled habitat type (grass margin, enhanced margin, oilseed rape or cereal), cloud cover, boundary type and visit. Neighbouring crop and percentage cloud cover were again included in models as confounding factors. Length weighted Diptera abundance was log + 1 transformed for inclusion in a linear mixed effect model, whilst Diptera abundance and Muscidae abundance were modelled using negative binomial GLMMs. Both models included the random effects colony and transect, where transect was nested within colony, to account for pseudoreplication between study sites and between transects.
Results
Use of belt transects by foraging Barn Swallows
Over the two survey periods 112 surveys were conducted. Barn Swallows were recorded as present on 35.7% of belt transects. Where foraging Barn Swallows were present the total number of individuals ranged from 1 to 34 with a mean (± se) of 1.17 ± 0.34 recorded across all surveys. The mean index of foraging activity was 6.33 ± 3.90.
The total number of individual Barn Swallows recorded during a survey was significantly negatively correlated to percentage cloud cover (). After controlling for the confounding variables, GLMMs indicated that the probability of observing Barn Swallows along belt transects which included an enhanced margin was up to 12.56% higher than those that included grass margins ( & , ). However, we found no significant difference in the total number of individuals recorded or the index of foraging activity between control grass margins and enhanced margins ( & ). Foraging activity was estimated to significantly decrease along transects where treelines were present when compared to grass verges, with no significant difference between hedgerows and grass verges ( & ). No model showed significant effects of visit, transect crop type or neighbouring crop type.
Figure 1. Estimated probability of observing a foraging Barn Swallow during surveys. Probabilities and standard errors were back transformed from the generalized linear mixed effects model estimates and account for the other variables modelled.
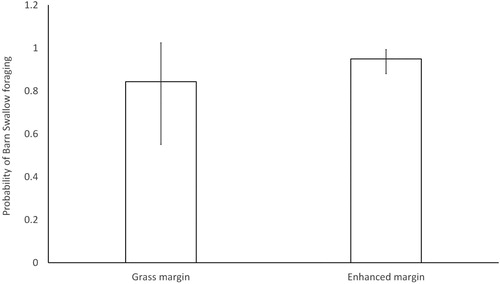
Table 2. Results of generalized linear mixed effects models of associations between field edge habitats and Barn Swallow presence (binomial model), total number of individuals (negative binomial model) and foraging activity (negative binomial model). All models included the random effect colony and control for the effects of cloud cover and visit.
The best Dipteran predictor for Barn Swallow presence and foraging activity was identified as Diptera abundance, which had the highest Akaike weights of 0.46 and 0.52, respectively (). The total number of individual Barn Swallows recorded was best explained by the null model (Akaike weight = 0.41), the effect of food abundance on this response variable was therefore not investigated any further (). Models investigating the effect of habitat and Diptera abundance revealed a significant positive relationship between Barn Swallow foraging activity and Diptera abundance (). Foraging activity was again negatively associated with the presence of treelines and Barn Swallow were more likely to be present along enhanced margin habitats, with Diptera abundance having no effect on their distribution ().
Table 3. Comparison of alternative Dipteran models with details of the degrees of freedom (DF), AIC values, differences in AIC values and Akaike weights for all models.
Table 4. Results of generalized linear mixed effects models of associations between field edge habitats, Diptera abundance and Barn Swallow presence (binomial model) and foraging activity (negative binomial model). All models included the random effect colony and control for the effects of cloud cover and visit.
Aerial invertebrates
448 aerial invertebrate samples were taken from 56 belt transects. We recorded a mean ± sd of 3.37 ± 15.13 Diptera in cereal crops, 7.29 ± 15.41 in enhanced margins, 5.05 ± 9.10 in grass margins and 45.5 ± 15.52 in oilseed rape. Diptera abundance and length weighted Diptera abundance were significantly higher in oilseed rape, grass margins and enhanced margins when compared to cereal crop ( & ). Muscidae abundance, however, did not differ significantly between the sampled habitats (). In addition, Diptera abundance declined with increasing cloud cover and increased where treelines were present ().
Table 5. Results of generalized linear mixed effects models of associations with Diptera abundance (negative binomial model) and Muscidae abundance (negative binomial model) Colony is included in the model as a random effect, with transect ID nested within colony.
Discussion
Margin and crop habitats
Our study identifies a significant relationship between Barn Swallow presence and floristically enhanced margins. This expands upon comparative studies of foraging by Evans et al. (Citation2007) which found that Barn Swallows spent a higher proportion of their time hunting along hedgerows. When we modelled foraging activity and the estimated number of individuals the same trend was apparent although not statistically significant. Previous studies have shown that enhanced margins can improve the breeding success or breeding densities of other farmland birds (Buner et al. Citation2005, Aebischer & Ewald Citation2010, McHugh et al. Citation2016, McHugh et al. Citation2017) and that farmland which includes enhanced margin habitats support more Common Linnet Linaria cannabina, Reed Bunting Emberiza schoeniclus and Goldfinch Carduelis carduelis (Redhead et al. Citation2018). The foraging activity of several bird species were increase when enhanced margins were available (Clarke et al. Citation1997). This may also be the case for Barn Swallows since these habitats can provide chick-food invertebrates over a longer period than grass margins owing to their increased plant diversity and extended flowering period (Vickery et al. Citation2002, Citation2009), although no previous studies have considered aerial foragers.
Across all the habitats we sampled, Diptera abundance was greatest in oilseed rape. Despite this, our comparison of Barn Swallow foraging over oilseed rape and wheat found no significant difference between the two crops. Foraging preferences may be influenced not only by prey abundance but also habitat structure. A study focussing on the diets of aerial insectivores demonstrated that Barn Swallows tend to consume more agricultural pests of oilseed rape whilst the crop is being harvested (Orlowski et al. Citation2014). In the period directly before harvest, the agricultural pests of this crop clearly decreased in the diet of Barn Swallows and increased during harvest as they are displaced from the crop making them accessible (Cramp Citation1998, Turner Citation2006). This crop and its invertebrate population may therefore become more important to foraging Barn Swallows later in the season as the UK oilseed rape harvest usually takes place during July/August.
Measures of Diptera
We identified a positive relationship between the Barn Swallow index of foraging activity and Diptera abundance. This may in part explain the observed relationship with enhanced margins since Diptera abundance was second highest in this habitat. Dietary studies suggest that although Barn Swallows can feed on a wide variety of aerial invertebrates, they may be more selective when rearing their young (Turner Citation1980, Citation1982, Henderson et al. Citation2007). For example, in earlier broods more than 70% of the diet can be composed of larger flies such as Tabanidae, Syrphidae and Muscidae (Turner Citation2006) whilst other prey species, including Scathophagidae, are infrequently selected in relation to their availability (Waugh Citation1978, Turner Citation2006). Egger (Citation2000) found that insects caught in good weather, were larger (averaging 7.4 mm) than in bad weather (4.9 mm). Barn Swallows may therefore be selecting this habitat because of specific prey species associated with it and the relative abundance of these food items. Demonstrating this using our invertebrate data was hindered by the fact that Tabanidae and Syrphidae were recorded too infrequently to model (they were present in only 2.5% and 10.2% of samples, respectively). We found that there was no difference in the abundance of Muscidae between habitats, and that this taxon alone was a poor predictor of Barn Swallow presence and activity.
The low abundance of large flies recorded may be explained by the invertebrate sampling method used since Barn Swallows are known to exploit dense aggregations of aerial invertebrates that, because of their very patchy distribution, may not necessarily be detected by kite netting. In particular, the sampling method may have under represented the abundance of Syphridae on the study transects, since our samples were taken at a height of 0.5 m to 1.5 m above the vegetation and Syphridae will spend more time on or close to nectaring flowers. Previous studies have shown that floristically enhanced margins hold a significantly higher abundance and diversity of Syrphidae compared to grass margins and cereal crops (Haenke et al. Citation2009). Despite invertebrate sampling taking place directly after Barn Swallow surveys, the sampling method used may not accurately represent the foraging behaviour of Barn Swallows during surveys, due to the narrow height range sampled. If this study were to be repeated, we would recommend that aerial invertebrates be sampled at a variety of different heights and that estimations of Barn Swallow foraging heights also be made.
Boundary types
Our study found a positive relationship between Diptera abundance and the presence of treelines in arable fields, but this did not result in increased Barn Swallow foraging activity. Increased Diptera abundance may stem from the greater volume of habitat provided by the margin’s increased vegetative cover or could be explained by the increased shelter provided which is particularly important to small, wind sensitive invertebrates (Evans et al. Citation2003a).
The fact that Barn Swallow foraging activity decreased along treelines when compared to grass verges, in line with the findings of Evans et al. (Citation2003a), is likely to be a product of the species’ foraging technique which is more efficient in open habitats. Norberg (Citation1990) found that in bad weather Barn Swallow energy requirements increase, resulting in individuals increasing the amount of time they spend gliding, which is slower but more energy efficient than flapping. This slower flight is thought to be used when they are feeding on swarms of insects (Turner Citation2006), such as those that aggregate along field boundaries in bad weather. When temperatures are high, on the other hand, Barn Swallows increase their flight speed and become more manoeuvrable, allowing them to catch larger strong-flying insects such as Brachyceran flies, the distribution of which is less dependent on shelter along field boundaries (Turner Citation1980).
Conclusions
The foraging activity of Barn Swallows was best predicted by food availability and the absence of treelines; this relationship is most likely linked to the high mobility of Barn Swallows and their airborne prey. In contrast, the likelihood of Barn Swallows using the local landscape was best predicted by the presence of AES floristically enhanced margins even given the effect of Diptera abundance, which suggests some real impact of agricultural habitat suitability for this species. This finding adds to the growing body of evidence that AES management can influence bird distribution on farmland and benefit wildlife (McHugh et al. Citation2016, McHugh et al. Citation2017, Walker et al. Citation2018). Arable farmland conservation strategies for foraging Barn Swallows could include planting wildflower strips adjacent to grass verges and hedgerows, but more research is needed to determine whether invertebrate-rich AES habitats can influence colony size or improve the breeding success of this species. Additionally, research focused on the relative importance of habitat versus landscape-scale management may also benefit this species, since this area of their ecology is poorly understood (Henderson et al. Citation2007).
Table 6. Result of linear mixed effects model of associations with log transformed length weighted Diptera abundance. Colony is included in the model as a random effect, with transect ID nested within colony.
Current AES policy does not consider how arable land can be managed to benefit aerial insectivores, but our results suggest that there may be a role for AES in the conservation of Barn Swallows. The proposed restructuring of UK AES offers opportunities to incorporate new targets for aerial insectivores which could allow the new scheme to widen their beneficial impacts, whilst also providing improved value for money.
Acknowledgements
We are grateful to the landowners who provided us with access to their land over the study. We thank two anonymous reviewers and C.J. Heward for comments on an earlier version of the manuscript.
Additional information
Funding
References
- Aebischer, N.J. & Ewald, J.A. 2010. Grey Partridge Perdix perdix in the UK: recovery status, set-aside and shooting. Ibis 152: 530–542. doi: 10.1111/j.1474-919X.2010.01037.x
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.L. 2013. Bird Atlas 2007–11: the breeding and wintering birds of Britain and Ireland. BTO Books, Thetford.
- Bates, D., Machler, M., Bolker, B. & Walker, S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. DOI: 10.18637/jss.v067.i01.
- BirdLife International. 2015. European Red List of Birds. Office for Official Publications of the European Communities, Luxembourg.
- BirdLife International. 2018. Species factsheet: Hirundo rustica. http://www.birdlife.org.
- Brickle, N.W., Harper, D.G.C., Aebischer, N.J. & Cockayne, S.H. 2000. Effects of agricultural intensification on the breeding success of Corn Buntings Miliaria calandra. J. Appl. Ecol. 37: 742–755. doi: 10.1046/j.1365-2664.2000.00542.x
- Buner, F., Jenny, M., Zbinden, N., Naef-Daenzer, B. 2005. Ecologically enhanced areas – a key habitat for re-intriduced Grey Partridges Perdix perdix. Biol. Conserv. 124: 373–381. doi: 10.1016/j.biocon.2005.01.043
- Bryant, D.M. & Turner, A.K. 1982. Central place foraging by swallows (Hirundinidae): the question of load size. Anim. Beh. 30: 845–856. doi: 10.1016/S0003-3472(82)80158-9
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Multimodel Inference: a practical information-theoretic approach, 2nd edn. Springer, New York.
- Carvell, C., Meek, W.R., Pywell, R.F., Goulson, D. & Nowakowski, M. 2007. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J. Appl. Ecol. 44: 29–40. doi: 10.1111/j.1365-2664.2006.01249.x
- Clarke, J.H., Jones, N.E. & Hill, D.A. 1997. The management of set-aside within a farm and its impact on birds. Weeds. The 1997 Brighton Crop Protection Conference, British Crop Protection Council; pp. 1170–1184.
- Cramp, S. 1998. The Complete Birds of the Western Palaearctic on CD-ROM. Oxford University Press, Oxford.
- Donald, P.F., Green, R.E. & Heath, M.F. 2001. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R Soc. Lond. B. Biol. Sci. 268: 25–29. doi: 10.1098/rspb.2000.1325
- Egger, B. 2000. Foraging performance of Barn Swallows Hirundo rustica in relation to food supplies and consequences for nestling growth and survival. Diploma Thesis, University of Bern and Swill Ornithological Institute.
- Evans, K.L., Bradbury, R.B. & Wilson, J.D. 2003a. Selection of hedgerows by swallows Hirundo rustics foraging on farmland: the influence of local habitat and weather. Bird Study 50: 8–14. doi: 10.1080/00063650309461284
- Evans K.L., Wilson J.D. & Bradbury R.B. 2003b. Swallow Hirundo rustica population trends in England: data from repeated historical surveys. Bird Study 50: 178–181. doi: 10.1080/00063650309461310
- Evans, K.L., Wilson, J.D. & Bradbury, R.B. 2007. Effects of crop type and aerial invertebrate abundance on foraging Barn Swallows Hirundo rustica. Agric. Ecosyst. Environ. 122: 267–273. doi: 10.1016/j.agee.2007.01.015
- Fournier, D.A, Skaug, H.J, Ancheta, J., Ianelli, J., Magnusson, A., Maunder, M., Nielsen, A. & Sibert, J. 2012. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 27: 233–249. doi: 10.1080/10556788.2011.597854
- Haenke, S., Scheid, B., Schaefer, M., Tscharntke, T. & Thies, C. 2009. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J. Appl. Ecol. 46: 1106–1114. doi: 10.1111/j.1365-2664.2009.01685.x
- Hart, J.D., Milsom, T.P., Fisher, G., Wilkins, V., Moreby, S.J., Murray, A.W.A. & Robertson, P.A. 2006. The relationship between yellowhammer breeding performance, arthropod abundance and insecticide applications on arable farmland. J. Animal Ecol. 43: 81–91. doi: 10.1111/j.1365-2664.2005.01103.x
- Henderson, I., Holt, C. & Vickery, J. 2007. National and regional patterns of habitat association with foraging Barn Swallows Hirundo rustica in the UK. Bird Study 54: 371–377. doi: 10.1080/00063650709461497
- Holland, J.M., Storkey, J., Lutman, P.J.W., Birkett, T.C., Simper, J. & Aebischer, N.J. 2014. Utilisation of agri-environment scheme habitats to enhance invertebrate ecosystem service providers. Agric. Ecosyst. Environ. 183: 103–109. doi: 10.1016/j.agee.2013.10.025
- Loske, K.H. 1992. Nestlingsnahrung der Rauchschwalbe (Hirundo rustica) in Mittelwestfalen. Vogelwarte 35: 186–201.
- McHugh, N.M., Goodwin, C.E., Hughes, S., Leather, S.R. & Holland, J.M. 2016. Agri-environment scheme habitat preferences of Yellowhammer Emberiza citrinella on English farmland. Acta Ornithologica 51: 199–209. doi: 10.3161/00016454AO2016.51.2.006
- McHugh, N.M., Prior, M., Grice, P.V., Leather, S.R. & Holland, J.M. 2017. Agri-environmental measures and the breeding ecology of a declining farmland bird. Biol. Conserv 212: 230–239. doi: 10.1016/j.biocon.2017.06.023
- New, T.R. 2005. Invertebrate Conservation and Agricultural Ecosystems. Cambridge University Press, Cambridge.
- Natural England. (2014) Delivering the HLS package for farmland birds: advisory note for land management teams. http://www.naturalengland.org.uk/Images/farmland-bird-guidance_tcm6-30540.pdf.
- Norberg, U.M. 1990. Vertebrate Flight: Mechanics, Physiology, Morphology, Ecology and Evolution. Springer-Verlag, Heidelberg.
- Orłowski, G., Karg, J., Karg, G. & Ballard, G. 2014. Functional invertebrate prey groups reflect dietary responses to phenology and farming activity and pest control services in three sympatric species of aerially foraging insectivorous birds. PloS one 9: e114906. doi: 10.1371/journal.pone.0114906
- Ovenden, G.N., Swash, A.R.H. & Smallshire, D. 2008. Agri-environment schemes and their contribution to the conservation of biodiversity in England. J. Appl. Ecol. 35: 955–960. doi: 10.1111/j.1365-2664.1998.tb00014.x
- Peach, W.J., Lovett, L.J., Wotton, S.R. & Jeffs, C. 2001. Countryside stewardship delivers Cirl Buntings (Emberiza cirlus) in Devon, UK. Biol. Conserv. 101: 361–373. doi: 10.1016/S0006-3207(01)00083-0
- Potts, G.R. 2012. Partridges: Countryside Barometer. Collins, London.
- R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
- Redhead, J.W., Hinsley, S.A., Beckmann, B.C., Broughton, R.K. & Pywell, R.F. 2018. Effects of agri-environmental habitat provision on winter and breeding season abundance of farmland birds. Agric. Ecosyst. Environ. 251: 114–123. doi: 10.1016/j.agee.2017.09.027
- Robinson, R.A., Crick, H.Q.P. & Peach, W.J. 2003. Population trends of Barn Swallows Hirundo rustica breeding in Britain. Bird Study 50: 1–7. doi: 10.1080/00063650309461283
- Schmidt, J.U., Eilers, A., Schimkat, M., Krause-Heiber, J., Timm, A., Siegel, S., Nachtigall, W. & Kleber, A. 2017. Factors influencing the success of within-field AES fallow plots as key sites for the Northern Lapwing Vanellus vanellus in an industrialised agricultural landscape of Central Europe. J. Nat. Conserv. 35: 66–76. doi: 10.1016/j.jnc.2016.12.001
- Setchfield, R.P., Mucklow, C., Davey, A., Bradter, U. & Anderson, G.Q.A. 2012 An agri-environment option boosts productivity of Corn Buntings Emberiza calandra in the UK. Ibis 154: 235–247. doi: 10.1111/j.1474-919X.2011.01207.x
- Skaug H, Fournier D, Bolker B, Magnusson A & Nielsen A. 2016. Generalized Linear Mixed Models using ‘AD Model Builder’. R package version 0.8.3.3.
- Turner, A.K. 1980. The use of time and energy by aerial feeding birds. Ph.D. Thesis, University of Stirling.
- Turner, A.K. 1982. Optimal foraging by Swallows (Hirundo rustica): prey size selection. Anim. Behav. 30: 862–872. doi: 10.1016/S0003-3472(82)80160-7
- Turner, A.K. 2006. The Barn Swallow. Poyser, London.
- Vickery, J.A., Bradbury, R.B., Henderson, I.G., Eaton, M.A. & Grice, P.V. 2004. The role of agri-environment schemes and farm management practices in reversing the decline of farmland birds in England. Biol. Conserv. 119: 19–39. doi: 10.1016/j.biocon.2003.06.004
- Vickery, J., Carter, N. & Fuller, R.J. 2002. The potential value of managed cereal field margins as foraging habitats for farmland birds in the UK. Agric. Ecosyst. Environ. 89: 41–52. doi: 10.1016/S0167-8809(01)00317-6
- Vickery, J.A., Feber, R.E. & Fuller, R.J. 2009. Arable field margins managed for biodiversity conservation: a review of food resource provision for farmland birds. Agric. Ecosyst. Environ 133: 1–13. doi: 10.1016/j.agee.2009.05.012
- Walker, L.K., Morris, A.J., Cristinacce, A., Dadam, D., Grice, P.V. & Peach, W.J. 2018. Effects of higher-tier agri-environment scheme on the abundance of priority farmland birds. Anim. Conserv. DOI: 10.1111/acv.12386.
- Waugh, D.R. 1978. Predation strategies in aerial feeding birds. Ph.D. Thesis, Stirling University.
- Woodcock, B.A., Savage, J., Bullock, J.M., Nowakowski, M., Orr, R., Tallowin, J.R.B. & Pywell, R.F. 2013. Enhancing beetle and spider communities in agricultural grasslands: the roles of seed addition and habitat management. Agric. Ecosyst. Environ 167: 79–85. doi: 10.1016/j.agee.2013.01.009
- Zuur, A.F., Ieno, E.N. & Elphick, C.S. 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol 1: 3–14. doi: 10.1111/j.2041-210X.2009.00001.x
