ABSTRACT
Capsule: Male White-throated Dippers Cinclus cinclus are more likely and quicker to respond to the playback of song than females, but both sexes are more likely to respond before the onset of breeding than after.
Aims: Territoriality and the function of song in female birds have rarely been studied outside of the tropics or Australasia. We investigated territoriality and song function in males and females of a Northern temperate species, the White-throated Dipper.
Methods: We conducted playback trials on established pairs and compared the responses of males and females according to the sex of the simulated intruder and the timing of playback relative to the onset of breeding. A response was classified as movement towards the speaker, singing or both.
Results: Males were significantly more likely and quicker to respond to playback than females, but neither sex responded differently to the playback of male and female song. Both sexes were more likely to respond to playback before breeding had begun than after.
Conclusions: Our results suggest that both males and females are territorial but that males take the dominant role in defence. Female song appears to elicit a similar response to male song and may play a role in territoriality or mate defence.
The two main functions of birdsong are mate attraction and territorial defence (Catchpole & Slater Citation1995, Catchpole Citation1996). Individuals that sing at a higher rate, using larger repertoires or with higher levels of complexity, can pair up earlier (Catchpole Citation1980), attain better quality territories (Buchanan & Catchpole Citation1997, Manica et al. Citation2014), have longer territory tenure (Hiebert et al. Citation1989, Potvin et al. Citation2013) and thus achieve greater reproductive success (McGregor et al. Citation1981, Lambrechts & Dhondt Citation1986, Potvin et al. Citation2013). The vast majority of research has focused on male song, but in recent years there has been growing interest in the song of female birds (Hall & Langmore Citation2017, Odom & Benedict Citation2018). Once thought to be rare, female song is now known to be widespread and evidence suggests that it is ancestral (Odom et al. Citation2014). It is most prevalent in non-migratory species with year-round territoriality (Price et al. Citation2008, Tobias et al. Citation2016), and is closely associated with monogamy and sexual monochromatism (Najar & Benedict Citation2015, Webb et al. Citation2016). However, studies of the function of song in females are largely restricted to duetting species (Hall & Peters Citation2008, Logue & Krupp Citation2016) and those that live in the tropics and Australasia (Slater & Mann Citation2004, Brunton & Li Citation2006, Odom & Benedict Citation2018).
Different mating systems appear to select for different functions of female song. Females in polygynous species can defend either a physical space (Beletsky Citation1982) or the parental investment they may receive from their partner (Yasukawa & Searcy Citation1982); song in these species has been shown to deter other females, thereby reducing the incidence of extra-pair copulation or polygyny (Langmore & Davies Citation1997). In monogamous species with year-round territoriality, it is thought that there is high selection pressure for joint territorial defence which may be more efficient if each partner defends against same-sex intruders (Farabaugh Citation1982, Hoelzel Citation1986, Langmore Citation1998). In some cases, a female’s defence of her partner can result in exclusive mating access or a reduction in polygyny (Hoelzel Citation1986). By contrast, mate defence by males not only increases their chance of paternity (Topp & Mennill Citation2008) but can also increase their likelihood of extra-pair mating (Hoelzel Citation1986).
Joint territorial defence has been the subject of many playback trials, with intrasexual aggression being prevalent among the responses. In studies of New Zealand Bellbirds Anthornis melanura (Brunton et al. Citation2008), Rufous-and-white Wrens Thryophilus rufalbus (Mennill Citation2006) and Barred Antshrikes Thamnophilus doliatus (Koloff & Mennill Citation2011), males preferentially responded to the playback of male song over female song; in Rufous-and-White Wrens (Mennill Citation2006) and House Wrens Troglodytes aedon (Krieg & Getty Citation2016), females responded more strongly to the playback of female song than male song. However, very few experiments which have investigated the role each sex plays in territoriality have tested all possible responses. In some cases, only one sex of intruder was simulated (Brunton et al. Citation2008, Cain & Langmore Citation2015); intruders of both sexes were simulated but only the responses from one sex were observed (Krieg & Getty Citation2016); the stimuli used were a duetting pair (Mennill Citation2006); or the playback trial was only conducted during one part of the breeding season (Koloff & Mennill Citation2011). Responses may differ before and after breeding depending on song function. If female song is used primarily in mate defence, for example, then females may be less inclined to defend their partner once they have offspring together or once paternal care has been provided. By contrast, if the main function of female song is territorial defence then the response to an intruder may not change across the breeding season (Cain & Langmore Citation2015). More experiments involving all combinations of responder and intruder by sex are therefore needed to further understand the function of female song and the relative contributions of males and females to territorial defence, especially in Northern temperate species.
The White-throated Dipper Cinclus cinclus (hereafter Dipper) is a sexually monochromatic, riverine songbird in which both sexes defend linear territories along rivers and streams and sing throughout much of the year (Tyler & Ormerod Citation1994, Magoolagan et al. Citationin press). Pairs are socially monogamous, with extra-pair paternity reportedly rare in this species (Øigarden et al. Citation2010). Only females incubate the eggs and brood chicks, but both parents provision the offspring (Tyler & Ormerod Citation1994). Magoolagan et al. (Citationin press) reported no structural differences between male and female songs, however, singing by females was less frequent once eggs had been laid, presumably due to the impact that parental care has on their time and energy budget (Brunton & Li Citation2006) and the effect that singing could have on the risk of offspring predation (Kleindorfer et al. Citation2016). This decline in song frequency suggests that female song is unlikely to be used for coordinating parental care of offspring, but rather for territory or mate defence, pair bonding or signalling fertility. A role in mate attraction also seems unlikely because song is most frequent in females which have already found a mate (Magoolagan et al. Citationin press). Here, we investigate the responses of both sexes to playback of unfamiliar male and female song (i.e. simulated intruders) in order to test whether the likelihood of responding is influenced by: (1) the sex of the focal individual; (2) the sex of the simulated intruder and (3) the timing of playback relative to the onset of breeding (i.e. before or after). Finally, we compare the latency to respond to playback between males and females.
Methods
Study site
This study was conducted in 2016 using an individually marked population of 40–50 pairs of Dippers in the River Lune catchment near Sedbergh, Cumbria, UK (54°323′N, 2°528′W); the study area comprises the catchment’s four rivers and their associated streams within a 6 km radius of the centre of Sedbergh. This population is the subject of a long-term study and a pedigree has been established from observational and ringing data (Magoolagan et al. Citationin press). The nesting attempts of all pairs were closely monitored each year. The playback experiment was conducted between February, when the first pairs began nest-building, and June, by which time most pairs had finished breeding.
Song recordings and playback files
Songs used for playback were recorded between January and June in 2014 and 2015. Songs were recorded from distances of 10–15 m using a Sennheiser ME66-K6 shotgun microphone with a Rycote Softie windshield and a standard pistol grip connected to a Marantz PMD661 MKII solid state recorder (for more details of the recording methods, see Magoolagan et al. Citationin press). Eight songs from each of five males and five females were used to create playback files with which to simulate an unfamiliar intruder to any pair’s territory within the study site. Files were constructed using Avisoft SASLab Pro, version 5.2.08 (Specht Citation1993), taking care to select recordings based on clarity and thereby maximize the signal-to-noise ratio during playback. One playback file per individual was created (i.e. ten files in total), each consisting of a looped sequence of the eight songs from that bird. Pauses between songs were determined using the average duration between songs for that individual, measured across a sample of ten songs using Avisoft.
Playback design
All trials took place before noon on days when there was little or no wind to interfere with the sound. Breeding pairs were considered to be ‘established’ when they had been observed foraging or nest-building together on two or more separate occasions during weekly censuses; once established, the trials could commence. Each of 11 focal pairs underwent four trials, two before egg laying (‘pre-incubation’) and two after the pair had reached the nestling stage and were no longer brooding (‘post-incubation’). Trials involving the same pair within the same breeding stage were separated by at least one day, one involving playback of male song and the other playback of female song. Focal pairs were assigned one male and one female playback file for each breeding phase using a random number generator. For the first trial, each playback file was given a number between one and ten. Playback files were removed from the random draw if they contained songs of an individual considered to be familiar to the focal pair; individuals were classified as familiar if they had been observed on the same river as the pair or if they were a close relative of either bird (i.e. a parent, offspring or sibling). The playback file for the second trial was selected from playback files of the opposite gender to the first, labelled 1-5 and again drawn using a random number generator. The third and fourth trials followed the same pattern of alternating sexes, each time removing the playback files played in previous trials. This system ensured that the sex of the first playback file in each pair of trials was randomized for each focal pair and order effects were minimized.
All playback trials were conducted by the same observer for consistency in measurements. For the two pre-incubation trials, the speaker was situated in a part of the territory near to where the pair would typically forage together that was also visually accessible to the observer. For the post-incubation trials, the speaker was placed 10–20 m away from the nest. For all trials the distance between the speaker, focal birds and observer varied according to the landscape, but the birds were always between 10 and 30 m away from both the speaker and observer. Playback of song was broadcast through a FoxPro Inferno speaker at a volume which best mimicked natural song; this was determined in preliminary trials and confirmed by comparing the average peak amplitude of five songs (from one individual) broadcast through the speaker to that of five songs (from the same individual) recorded in situ, all recorded from a distance of 10 m (playback song: mean (±sd) peak amplitude = −36.94 ± 1.93 dB; natural song: mean peak amplitude = −39.59 ± 1.91 dB; Mann Witney U test: W = 4, P = 0.09). Before each trial, the speaker was positioned and left in place for a minimum of five minutes with no playback. Playback trials commenced once birds were present near the speaker within clear view of the observer and were noted to be feeding or resting. In all trials, at least one focal bird looked at the speaker during playback. Both members of the pair were usually present, but some pairs rarely fed or rested together during the experiment and trials were therefore conducted with a single focal bird (n = 8 pre-incubation trials, n = 9 post-incubation trials). Two minutes of continuous song playback were followed by two minutes of quiet; preliminary trials indicated that these timings were ample for the focal individuals to respond before resuming ‘normal’ behaviours such as foraging or resting.
The responses of focal birds during each four-minute trial were recorded as behavioural measures or latency to respond in seconds from the start of the trial. A behavioural response occurred if the focal bird approached the speaker (i.e. hopped or flew towards it) or sang. The latency to respond was recorded by measuring the time (in seconds) from the start of the playback until the focal bird either first approached the speaker or first sang i.e. the latency to respond in either way. The behavioural responses of each individual were documented throughout the trial using a voice recorder to ensure that no behaviours were missed and all timings of responses were accurate relative to the start of the trial.
Statistical analysis
All analyses were carried out using R, version 3.2.2 (R Core Team Citation2015). Generalized linear mixed models with binomial error structure were used to investigate the factors which predicted whether or not an individual responded to playback (n = 71). The following explanatory variables were included in the full model as fixed effects: the sex of the focal individual (‘focal sex’); the sex of the simulated intruder (‘playback sex’ i.e. male or female song); the breeding stage of the focal individual (‘breeding stage’ i.e. pre-incubation or post-incubation); and trial order (i.e. whether playback of male song or female song was used first within the given breeding stage). All two-way interactions between focal sex, playback sex and breeding stage were also included. To control for pseudoreplication, the identities of the focal individual, focal pair (i.e. territory) and simulated intruder were fitted as random effects; focal individual identity was nested within pair identity. The full model was subjected to the ‘dredge’ function in the package ‘MuMIn’ (Barton Citation2016) to rank all sub-models by Akaike's Information Criterion, with the Hurvich and Tsai (Citation1989) correction for small sample size (AICc). If ΔAICc ≤ 2 between two or more of the most parsimonious models, model averaging was performed using MuMIn. Models were checked for overdispersion and validated following Zuur et al. (Citation2009).
To investigate whether males and females differ in their latencies to respond, the single quickest response time from each focal individual (i.e. across pre- and post-incubation trials) was compared between playback trials of male song (n = 10 males, n = 3 females) and female song (n = 8 males, n = 7 females); latencies to respond to male song were not compared statistically due to the small sample of female subjects. The quickest response by males and females during any trials, irrespective of stimulus, were also compared (n = 14 males, n = 9 females). All data were non-normally distributed and so latencies to respond were compared using Mann–Whitney U tests.
Results
Likelihood of responding
The results from all playback trials are provided in supplementary online Table S1. The sex of a focal individual was an important predictor of whether or not the bird responded to playback, with males more likely to respond than females (, ). The best-fitting models also contained breeding stage; individuals were more responsive before the onset of breeding than after (, ). No other variables were contained within the best-fitting models (online Table S2).
Figure 1. The probability of male and female Dippers responding to the playback of unfamiliar song. The points show the predicted probabilities for playback before incubation (filled triangles) and after incubation (filled circles) obtained from generalized linear mixed models; whiskers show the 95% confidence intervals.
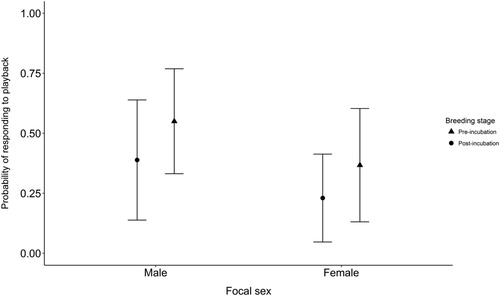
Table 1. The best-fitting generalized linear mixed models of the factors associated with responding to playback of song in Dippers: glmer(response ∼focal sex + playback sex + breeding stage + trial order + focal sex: playback sex + focal sex: breeding stage + playback sex: breeding stage + (1 + individual ID | pair ID) + (1 | playback ID), family = ‘binomial’) Only those models with an AICc value within 2 of that of the best-fitting model are shown, apart from the null model which is included by way of comparison. For the full list of models see Table S2.
Latency to respond
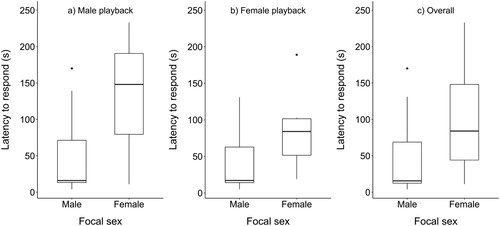
Males were quicker to respond to the playback of male song than females, although this difference could not be analysed statistically due to the small sample size for females (males = 49.9s ± 60.7; females = 130.7s ± 122.0; (a)). Males also responded more quickly than females to the playback of female song, but this difference was non-significant (males = 42.1 s ± 45.3; females = 85.4 s ± 54.9; W = 21, P = 0.64; (b)). Across all trials, however, males responded significantly more quickly to playback than females (males = 42.8 s ± 53.6; females = 98.6 ± 77.0; W= 95.5; P = 0.04; (c)).
Figure 2. The latencies with which male and female Dippers responded (approached the speaker, sang or both) to the playback of (a) male song (male: n = 10; female: n = 3); (b) female song (male: n = 8; female: n = 7); or (c) across all trials, i.e. the quickest response recorded from the individual to either male or female song. Boxes show the median, first and third quartiles, and the upper and lower whiskers extend to the highest and lowest values that are within 1.5 times the interquartile range. Data beyond the end of the whiskers are outliers and plotted as individual points.
Discussion
Male and female Dippers sing and defend territories throughout much of the year, often in pairs, but the function of female song is unknown and territorial behaviour has rarely been compared between the sexes (Tyler & Ormerod Citation1994, Magoolagan et al. Citationin press). In our study of breeding pairs, males were more likely and generally quicker to respond to the playback of song than females; this suggests that males take a dominant role in territorial defence. Males are larger than females (Tyler & Ormerod Citation1994), and it may therefore be less costly for them to engage in territorial conflict. Previous studies have shown that males have higher social dominance after controlling for age (Bryant & Newton Citation1996) and are generally more aggressive than females in the winter (Cousins Citation1985). However, females may also invest less in territorial defence due to the energetic constraints imposed by breeding and the amount of time spent on the nest during incubation and brooding (Brunton & Li Citation2006, Lattin & Ritchison Citation2009). Indeed, females sing less frequently once breeding is underway (Magoolagan et al. Citationin press), and in our study population, very rarely on or near the nest once it contains eggs or nestlings (Stuart Sharp unpublished data, but see Villain et al. Citation2017).
Neither male nor female Dippers differed in how likely they were to respond to the playback of male or female song. It may be that all unfamiliar song (i.e. not that of a partner or neighbour) is treated as intruder song by territorial pairs, or that Dippers cannot discriminate between the songs of males and females. Either way, this would support the suggestion of a territorial function for female song. However, it is worth noting that focal females were never observed singing in response to the playback of male song, and in all trials during which females responded to male song by approaching the speaker, their partner had also responded. Males, by contrast, sang in response to the song of both sexes and often approached the speaker when their partner did not. These observations suggest that females do not commonly behave aggressively towards male intruders and aggression in females may be largely intrasexual. Females might respond primarily to deter other females from mating with their partner and to defend the parental care they receive from him (Yasukawa & Searcy Citation1982), whereas males may not be perceived as a direct threat to their resources unless they interfere with breeding, something which has only very rarely been observed in the study population (Stuart Sharp, unpubl. data).
Individuals of both sexes were more likely to respond prior to the onset of breeding. This is typical of temperate species, in which the intensity of territorial behaviour often declines after nesting has commenced (Morton Citation1996, Catchpole & Slater Citation2008). Song rates have also been found to decrease during the nestling and fledgling stages, perhaps to minimize the risk of nest predation (Kleindorfer et al. Citation2016) but also because of the energetic demands of parental care (Geberzahn et al. Citation2009, Lattin & Ritchison Citation2009). Both male and female Dippers provision their young (Tyler & Ormerod Citation1994), so a reduced response to playback may be due to their parental responsibilities. Alternatively, if the response to playback reflects the defence of a mate rather than the territory, a decline in response rates might be expected once partners have invested sufficiently in offspring care (Cain & Langmore Citation2015). This may be particularly true for female dippers, in which song is not only more frequent before breeding but also seems to be associated with aggression towards other females (Magoolagan et al., Citationin press). In socially monogamous species with biparental care, it is often hard to differentiate between females defending a partner, the parental care they receive, the territory, or a mix of the three (Cain Citation2014), and further playback experiments with larger samples of focal females in a range of contexts are needed.
Overall, the results presented here suggest that female song is perceived similarly to male song by both sexes when the presence of an intruder is simulated using playback. The evidence also suggests that males are more likely and quicker to respond, indicating a dominant role in defence of the territory. However, the fact that females do sometimes respond indicates that they may also engage in territorial defence; this might be particularly important in Dippers due to their year-round territoriality in stretches of river that can be over 2500 m in length (Tyler & Ormerod Citation1994). The decline in responses to playback over the breeding season suggests that singing and responding may also function in mate defence, and further work is clearly needed to better understand both territoriality and song function in males and females. Data collected outside of the breeding season, especially from males and females defending separate winter territories, would be particularly valuable; including a scaled response in future playback experiments would help to better define the level of aggression shown by each sex. Finally, it may be that playback alone is not a strong enough stimulus to elicit typical responses; while more than half of all trials produced a response in at least one of the focal birds, further experiments with decoys might prove insightful.
Supplemental Material
Download MS Word (45.8 KB)Supplemental Material
Download MS Word (19.8 KB)Acknowledgements
We would like to thank the many local landowners and Paul Wilkinson at Yorkshire Dales National Park for providing access and supporting our work; Peter Mawby and Flora Whitehead for assistance with fieldwork; and Thomas Mondain-Monval, Mark Mainwaring, Rupert Marshall and Jos Barlow for comments on an earlier draft.
ORCID
Lucy Magoolagan http://orcid.org/0000-0002-1961-1114
Stuart P. Sharp http://orcid.org/0000-0002-3059-2532
Additional information
Funding
References
- Barton, K. 2016. MuMIn: multi-model inference. R Package Version 0.12.2/r18. http://R-Forge.R-project.org/projects/mumin/.
- Beletsky, L. 1982. Aggressive response to ‘self’ songs by female Red-winged Blackbirds, Agelaius phoeniceus. Can. J. Zool. 61: 462–465. doi: 10.1139/z83-062
- Brunton, D.H. & Li, X. 2006. The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J. Ethol. 24: 17–25. doi: 10.1007/s10164-005-0155-5
- Brunton, D.H., Evans, B., Cope, T. & Ji, W. 2008. A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19: 791–798. doi: 10.1093/beheco/arn027
- Buchanan, K.L. & Catchpole, C.K. 1997. Female choice in the sedge warbler Acrocephalus schoenobaenus: multiple cues from song and territory quality. Proc. R. Soc. B 264: 521–526. doi: 10.1098/rspb.1997.0074
- Bryant, D.M. & Newton, A.V. 1996. Dominance and survival of Dippers Cinclus cinclus. Behav. Ecol. Sociobiol. 38: 173–181. doi: 10.1007/s002650050230
- Cain, K.E. 2014. Mates of competitive females: the relationships between female aggression, mate quality and parental care. Adv. Zool. 2014: 319567. doi: 10.1155/2014/319567
- Cain, K.E. & Langmore, N.E. 2015. Female and male song rates across breeding stage: testing for sexual and nonsexual functions of female song. Anim. Behav. 109: 65–71. doi: 10.1016/j.anbehav.2015.07.034
- Catchpole, C. 1980. Sexual selection and the evolution of complex songs among European warblers of the genus Acrocephalus. Behaviour 74: 149–166. doi: 10.1163/156853980X00366
- Catchpole, C.K. 1996. Song and female choice: good genes and big brains? Trends Ecol. Evol. 11: 358–360. doi: 10.1016/0169-5347(96)30042-6
- Catchpole, C.K. & Slater, P.J.B. 1995. Bird Song, 1st edn. Cambridge Universty Press, Cambridge.
- Catchpole, C.K. & Slater, P.J.B. 2008. Bird Song-Biological Themes and Variations. Cambridge University Press, Cambridge.
- Cousins, S.A. 1985. Non-breeding territoriality of Dippers (Cinclus cinclus) and the influence of environmental conditions on their behaviour. Undergrad Thesis, University of Stirling.
- Farabaugh, S.M. 1982. The ecological and social significance of duetting. In Kroodsma, D.E. & Miller, E.H. (eds) Acoustic Communication in Birds, Vol. 2: 85–124. Cornell University Press, New York.
- Geberzahn, N., Goymann, W., Muck, C. & ten Cate, C. 2009. Females alter their song when challenged in a sex-role reversed bird species. Behav. Ecol. Sociobiol. 64: 193–204. doi: 10.1007/s00265-009-0836-0
- Hall, M.L. & Langmore, N.E. 2017. Fitness costs and benefits of female song. Front. Ecol. Evol. 5: 6–8. doi: 10.3389/fevo.2017.00048
- Hall, M. & Peters, A. 2008. Coordination between the sexes for territorial defence in a duetting fairy-wren. Anim. Behav. 76: 65–73. doi: 10.1016/j.anbehav.2008.01.010
- Hiebert, S.M., Stoddard, P.K. & Arcese, P. 1989. Repertoire size, territory acquisition and reproductive success in the song sparrow. Anim. Behav. 37: 266–273. doi: 10.1016/0003-3472(89)90115-2
- Hoelzel, A.R. 1986. Song characteristics and response to playback of male and female Robins Erithacus rubecula. Ibis 128: 115–127. doi: 10.1111/j.1474-919X.1986.tb02098.x
- Hurvich, C.M. & Tsai, C. 1989. Biometrika trust regression and time series model selection in small samples. Biometrika 76: 297–307. doi: 10.1093/biomet/76.2.297
- Kleindorfer, S., Evans, C. & Mahr, K. 2016. Female in-nest chatter song increases predation. Biol. Lett. 12: 18–21. doi: 10.1098/rsbl.2015.0513
- Koloff, J. & Mennill, D. 2011. Aggressive responses to playback of solos and duets in a Neotropical antbird. Anim. Behav. 82: 587–593. doi: 10.1016/j.anbehav.2011.06.021
- Krieg, C.A. & Getty, T. 2016. Not just for males: females use song against male and female rivals in a temperate zone songbird. Anim. Behav. 113: 39–47. doi: 10.1016/j.anbehav.2015.12.019
- Lambrechts, M. & Dhondt, A.A. 1986. Male quality, reproduction, and survival in the Great Tit (Parus major). Behav. Ecol. Sociobiol. 19: 57–63. doi: 10.1007/BF00303843
- Langmore, N.E. 1998. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13: 136–140. doi: 10.1016/S0169-5347(97)01241-X
- Langmore, N. & Davies, N. 1997. Female dunnocks use vocalizations to compete for males. Anim. Behav. 53: 881–890. doi: 10.1006/anbe.1996.0306
- Lattin, C. & Ritchison, G. 2009. Intra- and intersexual functions of singing by male Blue Grosbeaks: the role of within- song variation. W. J. Ornithol. 121: 714–721. doi: 10.1676/09-026.1
- Logue, D.M. & Krupp, D.B. 2016. Duetting as a collective behavior. Front. Ecol. Evol. 4: 7. doi: 10.3389/fevo.2016.00007
- Magoolagan, L., Mawby, P., Whitehead, F. & Sharp, S.P. In press. The structure and context of male and female song in dippers. J. Ornithol.
- Manica, L.T., Maia, R., Dias, A., Podos, J. & Macedo, R.H. 2014. Vocal output predicts territory quality in a neotropical songbird. Behav. Processes 109: 21–26. doi: 10.1016/j.beproc.2014.07.004
- McGregor, P.K., Krebs, J.R. & Perrins, C.M. 1981. Song repertoires and lifetime reproductive success in the Great Tit (Parus major). Am. Soc. Nat. 118: 149–159. doi: 10.1086/283811
- Mennill, D.J. 2006. Aggressive responses of male and female rufous-and-white wrens to stereo duet playback. Anim. Behav. 71: 219–226. doi: 10.1016/j.anbehav.2005.05.006
- Morton, E.S. 1996. A comparison of vocal behaviour among tropical and temperate passerine birds. In Kroodsma, D.E. & Miller, E.H. (eds) Ecology and Evolution of Acoustic Communication in Birds, 258–268. Cornell University Press, New York.
- Najar, N. & Benedict, L. 2015. Female song in New World Wood-Warblers (Parulidae). Front. Ecol. Evol. 3: 139. doi: 10.3389/fevo.2015.00139
- Odom, K.J. & Benedict, L. 2018. A call to document female bird songs: applications for diverse fields. Auk 135: 314–325. doi: 10.1642/AUK-17-183.1
- Odom, K.J., Hall, M.L., Riebel, K., Omland, K.E. & Langmore, N.E. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5: 3379. doi: 10.1038/ncomms4379
- Øigarden, T., Borge, T. & Lifjeld, J.T. 2010. Extrapair paternity and genetic diversity: the white-throated dipper Cinclus cinclus. J. Avian Biol. 41: 248–257. doi: 10.1111/j.1600-048X.2009.04847.x
- Potvin, D.A., Crawford, P.W., Macdougall-Shackleton, S.A. & MacDougall-Shackleton, E.A. 2013. Song repertoire size, not territory location, predicts reproductive success and territory tenure in a migratory songbird. Can. J. Zool. 93: 627–633. doi: 10.1139/cjz-2015-0039
- Price, J., Yunes-Jiménez, L., Osorio-Beristain, M., Omland, K.E. & Murphy, T.G. 2008. Sex-role reversal in song? Females sing more frequently than males in the Streak-Backed Oriole. Condor 110: 387–392. doi: 10.1525/cond.2008.8430
- R Core Team and R Development Core Team, R. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, edited by R. D. C. Team. R Foundation for Statistical Computing (R Foundation for Statistical Computing), Vienna.
- Slater, P. and Mann, N. 2004. Why do the females of many bird species sing in the tropics? J. Avian Biol. 35: 289–294. doi: 10.1111/j.0908-8857.2004.03392.x
- Specht, R. 1993. Avisoft SAS Lab pro version 5.2. Berlin.
- Tobias, J.A., Sheard, C., Seddon, N., Meade, A., Cotton, A.J. & Nakagawa, S. 2016. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4: 74. doi: 10.3389/fevo.2016.00074
- Topp, S.M. & Mennill, D. J. 2008. Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 62: 1107–1117. doi: 10.1007/s00265-007-0538-4
- Tyler, S.J. & Ormerod, S.J. 1994. The Dippers. First. T & AD Poyser Ltd., Calton, Staffordshire.
- Villain, A.S., Mahamoud-Issa, M., Doligez, B. & Vignal, C. 2017. Vocal behaviour of mates at the nest in the White-throated Dipper Cinclus cinclus: contexts and structure of vocal interactions, pair-specific acoustic signature. J. Ornithol. 158: 897–910. doi: 10.1007/s10336-017-1449-4
- Webb, W.H., Brunton, D.H., Aguirre, J.D., Thomas, D.B., Valcu, M. & Dale, J. 2016. Female song occurs in songbirds with more elaborate female coloration and reduced sexual dichromatism. Front. Ecol. Evol. 4: 22. doi: 10.3389/fevo.2016.00022
- Yasukawa, K. & Searcy, W.A. 1982. Blackbirds : aggression in female red-winged a strategy to ensure male parental investment. Behav. Ecol. Sociobiol. 11: 13–17. doi: 10.1007/BF00297660
- Zuur, A.F., Ieno, E.N., Walker, N., Saveliev, A.A. & Smith, G.M. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
