ABSTRACT
Capsule: Individual Eurasian Teal Anas crecca with white neck-rings have been found in Italy and an extensive museum survey found this plumage character occurring with a low frequency, although no genetic difference was observed with respect to the normal phenotype.
Aims: To estimate the relative frequency and distribution of this plumage variation and to provide a first genetic assessment to verify if this character could have a taxonomic basis or if it occurs by chance among populations.
Methods: An extensive museum survey was conducted to check for the occurrence of white neck-ringed Eurasian Teals in preserved specimens from its distributional range. A genetic analysis was conducted at standard mitochondrial loci (D-loop and COI) of ten white neck-ringed Eurasian Teals from Venice lagoon, Italy. Haplotypes were compared with genetic data from Holarctic populations.
Results: The museum survey uncovered additional cases of white neck-ringed Eurasian Teals in Italy and Europe. Moreover, this mutation has also been documented for the first time in the North American Green-winged Teal Anas carolinensis. The white neck-ring was found to be variable in size and occurred in both sexes and in different age classes in about 1.5% of examined individuals. The genetic analysis did not show any significant lineage differentiation between them and the normal phenotype, but the Italian samples showed high values of haplotype diversity indicating a key role of northeast Italy in attracting different migratory populations in winter.
Conclusions: While the same variation occurs in many other species of the tribe Anatini, none of these cases clearly suggested the occurrence of hybridization, progressive greying, environmental causation or another kind of locally frequent mutation. It is most likely this plumage trait is a form of leucism or the expression of an ancestral phenotype, possibly occurring in different populations within the species’ range.
Birds are perhaps the most colourful of all vertebrates and are known to display an impressive amount of intra-specific variation in plumage colour, ranging from sexual dichromatism or age and seasonal colour changes to true colour polymorphism and aberrant colourations (Roulin Citation2004, Mundy Citation2006). Colour polymorphism is defined as the occurrence of multiple discrete colour phenotypes within populations, with frequencies that presumably result directly from genetic variation and heritability dynamics rather than from recurrent random mutation (Ford Citation1945, White & Kemp Citation2016). Colour polymorphism is found in 3.5% of all bird species and involves 61% of the 23 orders and 37% of the 143 families. Polymorphism is particularly frequent in Strigiformes (owls and nightjars, 33.5% of the species are polymorphic), Cuculiformes (11.9%), Galliformes (9.5%), and Ciconiiformes (8.8%). Conversely, the orders with the lowest incidence of polymorphism are Piciformes (0.6%), Passeriformes (0.9%), Psittaciformes (1.1%) and Anseriformes (1.1%) (Galeotti et al. Citation2003). The functional significance of colour polymorphism may be linked to the mechanism maintaining it, ranging from sexual selection to frequency-dependent and disruptive selection (Widemo Citation1998). However, discrete colour signals, such as those found in polymorphic species, may represent non-adaptive correlates of physiological or fitness characters of selective value (Huxley Citation1955), or may simultaneously accomplish different functions, such as status signalling, crypsis, mimicry, thermoregulation and habitat use (Cooper & Burns Citation1987, Thompson et al. Citation1993, Martin & Forsman Citation1999, Andrés et al. Citation2002, Galeotti et al. Citation2003, Galeotti & Rubolini Citation2004, Lopez et al. Citation2004, Stuart-Fox et al. Citation2006). Finally, discrete colour morphs may advertise different life history and behavioural traits (Lepetz et al. Citation2009), for example alternative reproductive strategies (Sinervo Citation2000).
Plumage aberrations are not uncommon in wild birds (Gross Citation1965, Hosner & Lebbin Citation2006, Guay et al. Citation2012, Mahabal et al. Citation2016). These phenotypes can take on many forms, but albinism and leucism (sometimes wrongly called ‘partial albinism’, see van Grouw Citation2006) have traditionally been considered the most common plumage aberrations in birds (Sage Citation1963, Gross Citation1965). However, recent accurate revision of published literature, revealed that most of these cases should be treated as ‘progressive greying’ (van Grouw Citation2012, Citation2013, Citation2018, van Grouw et al. Citation2016). Albinism results from the complete lack of all melanin in both plumage and skin due to an inherited lack of tyrosinase enzyme in pigment cells (Fox & Vevers Citation1960). Albinistic birds thus have completely white plumage, pinkish-red eyes and pale skin. Leucism results from the complete lack of melanin from all or part of the plumage, but not necessarily from the soft parts. Leucistic birds have a varying proportion of white feathers but have normally coloured eyes. Progressive greying results from a progressive loss of melanin pigment with each successive moult. This condition could be due to the loss of pigment cells or to the gradual reduction of their tyrosinase activity. Usually, at the initial stage, affected feathers appear, but finally the entire plumage can become white. Although this phenomenon is rather frequent, its causes are still unknown and could involve both heritable disorders, environmental factors and diet imbalance. The frequency of albinism, leucism and progressive greying is highly variable among bird species, and the occurrence and extent of these phenomena can also vary between and within different populations of the same species (van Grouw Citation2012, Citation2013). Leucism seems to have been selected during domestication, as many domestic birds, including chickens, ducks, pigeons, geese and turkeys, have leucistic breeds (Crawford Citation1990). Leucism (or more likely progressive greying, see van Grouw Citation2013, Citation2018 and Mahabal et al. Citation2016), has long been considered the commonest plumage aberration in wild birds, showing a wide range of incidence and variation (e.g. >40% in Brewer’s Blackbird Euphagus cyanocephalus, Edson Citation1928 and less than 1% in the Chinstrap Penguin Pygoscelis antarcticus, Forrest & Naveen Citation2000). Within a species, important variation can also occur between populations (Enders & Post Citation1971, Bensch et al. Citation2000), and leucism (and in some cases progressive greying) seem to be more frequent in urban than in rural populations (Rollin Citation1953) and in small, potentially inbred, ones (Bensch et al. Citation2000).
White neck-rings or spots are examples of aberrant plumage observed in multiple dabbling duck species (). Unusual white neck-rings or spots have been described (mainly in drakes) in Eurasian Teal Anas crecca (Arrigoni degli Oddi Citation1929, Kuroda Citation1937, Harrison & Harrison Citation1959a, Citation1962), Speckled Teal Anas flavirostris (Harrison & Harrison Citation1958), Blue-winged Teal Spatula discors (Trauger Citation1976), Northern Shoveler Spatula clypeata (Harrison & Harrison Citation1959b) and Gadwall Mareca strepera (Harrison & Harrison Citation1959c, Citation1963), and, more recently, in single individuals of Yellow-billed Pintail, Anas georgica spinicauda (Wilson et al. Citation2006) and Chestnut-breasted Teal Anas castanea (Guay Citation2011). This feature has been suggested to result from different biological phenomena such as hybridization (Bonhote Citation1907), expression of ancestral polymorphisms (Harrison & Harrison Citation1963) or even mutation due to habitat alteration, but the frequency, extent and heritability of such traits are largely unknown for many species.
Figure 1. Examples of dabbling ducks showing the white neck-ring plumage trait. (a) Eurasian Teal, adult male, showing a white neck-ring, between two males showing normal plumage (ISPRA collection, Ozzano, Italy); (b) Eurasian Teal, adult female, the same bird was genetically analysed in this study (Carmagnola collection, Carmagnola Museum, Italy); (c) Green-winged Teal, adult male (Smithsonian collection, Washington Museum – US); Green-winged Teal, juvenile male (Smithsonian collection, Washington Museum – US); (e) Gadwall, female (Scagliarini collection, Jesolo Museum, Italy) and (f) Eurasian Wigeon, male (Scagliarini collection, Jesolo Museum, Italy).

In this study, we reported on a number of cases of a more or less extensive partial white neck-ring (neck collars/neck spots) occurring in both male and female Eurasian Teal ((a,b)). To estimate the relative frequency and distribution of this peculiar plumage variation we surveyed multiple natural history museum collections and performed thorough literature and internet searches. We focused our search on the Eurasian Teal Anas crecca as the most recently documented cases of white neck-plumage variation pertains to a large population of this species, wintering in the Venice Lagoon, northeastern Italy (Bon & Scarton Citation2012). Secondly, we present the first genetic assessment of specimens showing this plumage trait by measuring mitochondrial DNA (mtDNA) sequence variation between Eurasian Teals with aberrant and typical plumage. Since the Italian Eurasian Teals are typically migrating and/or wintering birds, the genetic survey was conceived to determine if the sampled white neck-ringed individuals represented a distinct genetic lineage (or subpopulation) of the species where there has recently been a high occurrence of this plumage trait, or more likely, a rarer morph occurring by chance in different populations.
Methods
Specimen inquiry
Due to the observation of a number of Eurasian Teals showing a clear white neck-ring ((a,b)) among the hunting bags during 2014–15 in the Venice Lagoon (northeast Italy), we contacted several European public and private collections. We specifically inquired at natural history museums having large wildfowl collections and Italian private collections where they possessed teal specimens with a white neck-ring in their archives ( & ). In addition, we gathered information from specialized hunters and duck ringers, and performed a thorough literature search regarding the occurrence of this aberrant plumage trait in all duck species.
Table 1. Documented white neck-ringed Eurasian Teal in the Italian peninsula. The number of birds, their sex, sampling locality, collection date, specimen typology and archive number are reported. Birds genetically analysed in this study are indicated with *.
Table 2. Results of museums inquiry to identify colections with examples of white neck-ringed and normal Eurasian Teal, and Green-winged Teal for GB-Tring (– number not given). CH: Switzerland; GB: Great Britain; I: Italy; R: Russia.
The Eurasian Teal breeds in northern Eurasia and the Aleutian Islands, and the closely related Green-winged Teal Anas carolinensis inhabits North America (Dickinson & Remsen Citation2013, Del Hoyo et al. Citation2014). Both taxa are highly migratory, and their taxonomic status has been extensively debated. The two taxa are strongly differentiated at mitochondrial DNA loci with a genetic distance of 5.9% which is similar to genetic divergence estimates between closely related species such as Mallard (Anas platyrhynchos) and Pintail (Anas acuta) (i.e. 5.8%, Peters et al. Citation2012). Due to this marked genetic divergence, some authors (e.g. Sangster et al. Citation2001, Citation2002) have treated Eurasian Teal and Green-winged Teal as separate species, with the former including the subspecies nimia of the Aleutian Islands. Given the phenotypic similarity of the two teal species, we extended our survey also to Green-winged Teal, for which, to date, there is no information about white neck-ring variants. Finally, we considered similar cases of anomalous colour traits in other Anas species.
DNA extraction, amplification and sequencing
A total of ten tissue samples (tongue) were collected from Eurasian Teals showing a distinct white neck-ring (eight males and two females) legally shot in a wetland area (45°50′N 12°46′E) near Venice, northeast Italy during winter 2014/15. All tissues were stored in 99% ethanol at −20°C (n = 10, online Appendix S1). Genomic DNA was extracted and purified using the DNeasy Blood & Tissue Kit (Qiagen, Milan, Italy) following the manufacturer’s instructions.
We amplified two mitochondrial (mtDNA) genes: COI (749 basepairs [bp]) and control region (D-loop) (987 bp). These two markers were chosen due to their high availability in public genetics repositories (e.g. Genbank, European Nucleotide Archive). In addition, these markers have proven valuable in integrative taxonomy for species identification (Kerr et al. Citation2009) as well as to investigate population level differences including the teal complex (Peters et al. Citation2012, Winker & Peters Citation2013, Peters et al. Citation2014) due to their fast mutation rates compared to other mitochondrial genes (Baker & Marshall Citation1997). The COI fragment was amplified using primers BirdF1 and BirdR1 with the polymerase chain reaction (PCR) conditions described in Hebert et al. (Citation2004) and amplification of the D-loop region was performed with primer pairs L78–H774 and L736–H1251 (Sorenson & Fleischer Citation1996, Sorenson et al. Citation1999) with PCR conditions described in (Peters et al. Citation2012). PCRs were conducted in a 25 μL reaction (including 10 ng of DNA as template) using puReTaq Ready-To-Go PCR beads (Amersham Bioscience, Freiburg, Germany), according to the manufacturer’s instructions. PCR product amplification success was checked on a 1.5% agarose gel and sequencing conducted on a gene sequencer (ABI 155 3730XL, Macrogen, Seoul, Korea), with the same amplification primers. Sequence from opposite strands were reconciled in BIOEDIT 7.1 (Hall Citation1999).
As the occurrence of nuclear pseudogenes is common in avian species (Sorenson & Quinn Citation1998), codons were translated into amino acid sequences for COI to check for the presence of nuclear pseudogenes using MEGA6 (Tamura et al. Citation2013). To avoid the inclusion of nuclear sequences of mitochondrial origin (i.e. NUMTs, nuclear mitochondrial DNAs, Stoeckle & Kerr Citation2012), we also followed the guidelines proposed in Song et al. (Citation2008) and Buhay (Citation2009). Sequence data were submitted to the European Bioinformatics Institute of the European Molecular Biology Laboratory (EMBL-EBI) (accession numbers: LT899903–LT899922, see online Appendix S1).
Genetic data analysis
We used previously published genetic datasets from Eurasian and Green-winged Teal to compare to our samples showing an aberrant white neck-ring. The resulting dataset included the 10 samples from this study and 32 publicly available COI sequences from specimens found throughout Europe (online Appendix S1) and 111 D-loop sequences from North American and Eurasian origins (Yoo et al. Citation2006, Kerr et al. Citation2009, Johnsen et al. Citation2010, Lee et al. Citation2010, Jin et al. Citation2012, Peters et al. Citation2012, Winker & Peters Citation2013, Saitoh et al. Citation2015). As there were very few reference public accessions that had both COI and D-loop sequences, analyses were conducted separately on the two mitochondrial markers. All sequences of each marker were aligned using MUSCLE online (http://www.ebi.ac.uk/Tools/msa/muscle/; Edgar Citation2004) with default options. Due to different lengths of publicly available GenBank sequences, we trimmed the alignments to the same final length, yielding a final sequence length of 633 and 983 bp for COI and D-loop, respectively.
For both datasets, the number of haplotypes and nucleotide diversity per site and per morph (normal and aberrant white neck-ring) were computed with DnaSP v5.10.1 software (Librado & Rozas Citation2009). In addition, the average genetic sequence divergences (and relative standard errors, se) between and within white neck-ringed teals and the reference Genbank samples were calculated using MEGA 6. In order to estimate the gene genealogy of Eurasian Teal haplotypes and to verify the occurrence of a putative lineage including white neck-ringed individuals collected in northeast Italy, an unrooted minimum-spanning network, obtained using the median-joining algorithm (Bandelt et al. Citation1999) implemented in Network 5.1 (www.fluxus-engineering.com – default settings), was constructed for the D-loop datasets. This choice was due to the fact that only a few public COI sequences were available for the teal and they covered the same geographic regions of D-loop accessions.
Results
Specimens survey
We found anterior white neck-rings or spots in 26 Eurasian Teal specimens (4 females and 22 males), collected in Italy and preserved in museum or private bird collections (). In addition to these Italian specimens, we also found this trait occurring in Japan (n = 14), United Kingdom (n = 9), Central Siberia (n = 1), Hungary (n = 1) and Korea (n = 1). All but two white neck-ringed teals found in Europe were recovered from presumably wintering grounds or during active post-reproductive migration (October to February).
White neck-rings or spots invariably were situated at the junction of the chestnut with the grey and white barring of the upper breast and varied in extent, from a little anterior spot (the usual appearance in females) to a large quite complete ring (commonest in males) (). This variant occured in both sexes, different age classes and also in eclipse plumage. The trait was never associated with other leucistic features or with evidence of hybridization. Among the Italian specimens, males (n = 22) outnumbered females (n = 5) similarly to the complete dataset considered for our survey (11 females vs. 30 males).
Overall, this aberrant plumage trait occurred in 1.47% of teals recorded in the museum survey. However, this number might overestimate its frequency in the wild, as collectors pay particular attention to abnormal specimens especially plumage peculiarities, although an inquiry with skilled hunters with extensive experience in northeastern Italy and Hungary reported similar percentages (1–3%) (G. Scagliarini pers. comm.). Conversely, data from a ringing station near Naples (southern Italy), where at least 300 Eurasian Teals are processed per year, showed only 1 young male with a partial white neck-ring in the last seven years (i.e. less than 0.33%, V. Cavaliere pers. comm.). An internet search revealed only one confirmed occurrence via a photograph in Korea from over 300 high quality (over 640 pixel) pictures available on google images (see photo Tim Edelsten, Anyang Stream, Seoul, on 14 November 2009, searched key words; Anas crecca, teal). These latter data were not taken into account to calculate the percentage of white neck-ringed birds due to the uncertainties about the total of observed animals at the moment of photography. A survey of 70 specimens of Green-winged Teal in the Tring Natural History Museum revealed no specimens with a white neck-ring. Nevertheless, this trait occurred in two specimens (one adult and one young male) of this species preserved at the Smithsonian Institution of Washington, DC (Brian Schmidt, pers. obs.) ((c,d)).
Interestingly, during our museum and literature/internet searches, we uncovered additional cases of the plumage peculiarity in other duck species, including one female and four male Gadwall with well-defined neck-rings and one male Eurasian Wigeon Mareca penelope ((e,f)).
Molecular survey
The COI DNA barcoding dataset contained no insertion/deletions (indels) or stop codons. In the 42 total samples examined, we identified 8 unique mtDNA haplotypes from COI characterized by 7 variable sites of which 2 were parsimony-informative. For D-loop, we obtained 65 total haplotypes containing 7 indel positions and 58 variable sites, of which 32 were parsimony-informative. Online Appendix S1 shows the number and distribution of haplotypes. The values of nucleotide diversity (Π) (Nei Citation1987) for the white neck-ringed teals analysed in this study were low (0.00119 ± 0.00048) as well as the overall Π value for Eurasian Teal (0.00052 ± 0.00017).
Among the ten white ring-necked Eurasian Teal samples, four and nine different haplotypes were found at COI and D-loop markers respectively (online Appendix S1). Of these, two COI and four D-loop haplotypes were shared between white neck-ringed teals and other teals (presumed normal phenotype) archived in Genbank. The network reconstruction of D-loop sequences () clearly shows that these shared haplotypes do not follow a specific geographic pattern as they belong to different breeding and wintering localities. For example, the two haplotypes showing the highest frequency in the overall dataset (i.e. CO1 and DL26) were found in six and four different countries belonging to different continents (online Appendix S1 and ). Except for the D-loop haplotype DL26, all the other white neck-ringed teal haplotypes were exclusive or at least shared with other (presumably) normal phenotype birds, thus confirming that the individuals found in northeast Italy belonged to different maternal lineages and not from the same family group.
Figure 2. Median-joining network for the D-loop haplotypes identified in the Eurasian Teal complex analysis (see online Appendix S1). Each circle represents a haplotype and its size is proportional to haplotype frequency. Colours indicate different sampling countries. A different colour (green) was used in the case of the haplotypes belonging to the ten white ring-necked Eurasian Teals collected in northeast Italy and analysed in this study. Small black nodes represent possible median vectors.
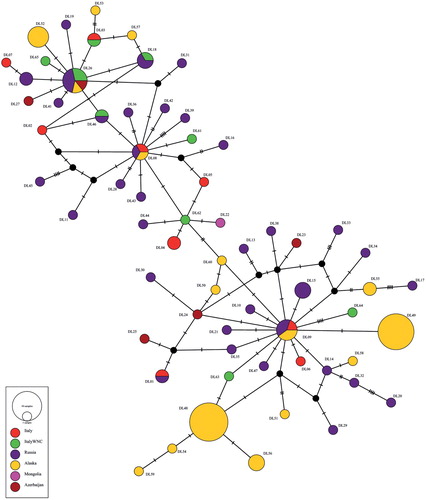
Finally, the genetic divergence (uncorrected p-distance %) among samples at both markers revealed maximum values of 0.5% (se = 0.3%) and 1.5% (se = 0.4%) for COI and D-loop respectively. Such values fall below the ‘alert’ threshold values usually adopted to hypothesize robust taxonomic differences in birds (Hebert et al. Citation2004, Kerr et al. Citation2009). Although the use of thresholds is a topic of current debate, it is important to underline that in the largest dataset (i.e. D-loop), the ‘highly divergent’ haplotypes did not include the white ring-necked teals analysed in our study.
Discussion
The occurrence of a white neck-ring or neck-spot is a relatively well-known plumage aberration in the dabbling ducks (Wilson et al. Citation2006). Although extensive work has been conducted to assess the frequency of plumage whitening phenomena (i.e. albinism, leucism and progressive greying) dating back to Sage (Citation1963) and Gross (Citation1965) until the most recent studies by Mahabal et al. (Citation2016) and van Grouw (Citation2013, Citation2018), the occurrence of white neck-rings in ducks was mainly known from single species reports or from brief reviews citing these reports. The present study expands on these reports of the occurrence of this feature in detail for a common Eurasian species and documents new cases in Green-winged Teal and Eurasian Wigeon. The surprising number of recent cases detected in Eurasian Teals in hunters’ bags in northeast Italy led us to evaluate the frequency and location of occurrences, and possible causes of this plumage colour aberration.
Our survey showed that white neck-rings occur in both sexes and age classes, with an overall frequency of 1.5%. Admittedly, the current frequency in the wild of this phenotype might be overrepresented in collections, due to possible active targeting of abnormal individuals by collectors; primarily in private mounted collections rather than in large museum skin collections. Interestingly, a report from a duck hunting area in Japan (now the Tokyo International Airport) also showed a similar frequency occurrence (1.4%) where at least 14 Eurasian Teals had a white neck-ring (or spot) out of 1000 examined (Kuroda Citation1937). To the best of our knowledge, our finding of female Eurasian Teals with white neck-rings, as well as one female Gadwall are the first since Trauger (Citation1976) reported the feature in Blue-winged Teal Spatula discors. Although, in general, the extent of the white neck-ring or spot varied among specimens, it was either more complete (if a ring) or larger (if a spot) in males than in females, where the white plumage was reduced to an anterior spot. Based on the widest geographical dataset ever considered for the Eurasian Teal, the occurrence of white-necked individuals seems more widespread than originally thought, with records distributed from the United Kingdom, Russia, Hungary, Italy and Korea.
The Northern Adriatic region (northeast Italy) has one of the greatest wintering Eurasian Teal populations in Italy. A recent satellite tracking study on Italian populations of this species, suggested that the routes followed by the birds wintering on the Northern Adriatic coast were all directed towards multiple localities scattered throughout central and eastern Europe (CitationGiunchi et al. 2019). Therefore, it may not be surprising that the highest frequency of white ring-necked Eurasian Teals was found there but it also has a high hunting pressure that in turn could have biased collections toward plumage-aberrant individuals. We may hypothesize a common geographical origin for these wintering birds, although our DNA analysis did not find any significant clustering of these individuals or any differences between normal or aberrant plumaged teal. However, it should be noted that of the sampled individuals, only two shared a mtDNA D-loop haplotype suggesting the analysed samples were not closely related individuals, or at least did not come from the same maternal lineage.
Multiple causes of plumage aberrations in bird species have been proposed so far. These include injury, disease, diet, partial leucism, low gene expression, pigment concealment, or atavistic resilience of ancestral traits (Hubbs & Bartholomew Citation1951, Nero Citation1954, Harrison & Harrison Citation1963, Gross Citation1965, Enders & Post Citation1971, Slagsvold et al. Citation1988, van Grouw Citation2013). Genetic mutations have long been associated with plumage aberrations. Such mutations have indeed been ‘explored’ for their inheritance and actively selected by breeders to preserve those plumage colourations appreciated for ornamental purposes. Albinism, for example, is caused by a genetic mutation resulting in the absence of the enzyme tyrosinase, which is essential for the synthesis of both eumelanin and phaeomelanin (Fox & Vevers Citation1960). As early as 1941, experiments linked complete albinistic plumage with a single autosomal recessive gene (Sage Citation1962). Conversely, leucism may be caused by a panel of possible genetic factors ranging from dominant to recessive allelic conditions but it usually affects one or more distinct parts of bird plumage (Sage Citation1962). This is the case of Blue-winged Teal for which it was suggested that the occurrence of a white neck-ring was due to leucism because it was found in both males and females along with unusual white feathering spread on other body parts (Trauger Citation1976). Our mtDNA-based investigation is obviously unsuitable to determine the genetic control of plumage traits, but it does provide some insight in that the gene(s) responsible for this trait expression in our sample is not localized in a single family group of birds. Although in most cases the evolutionary history of mutations causing leucism or progressive greying is unknown, environmental factors could also play a key role in shaping the appearance or heritability of such traits. For example, Ellegren et al. (Citation1997) found that a population of Barn Swallows Hirundo rustica exposed to high levels of radioactivity at their breeding site around Chernobyl, Ukraine, in the years following the nuclear catastrophe in 1987, had an unusually high frequency of leucism or progressive greying.
Distinct leucistic patterns may be observed among a number of separate but related species (e.g. white neck-rings in waterfowl, Guay Citation2011) and may be considered an atavistic phenotype reappearing in some species, such as the drake mallard (Harrison & Harrison Citation1959b, Citation1963, Campbell & Lack Citation1985). Besides this species, the males of only three other ducks normally display a white neck-ring in their breeding plumage: Baikal Teal Sibirionetta formosa, Falcated Duck Mareca falcata and Brown Teal Anas chlorotis, the last being an interesting case as only some males in nuptial plumage have an evident white neck-ring. Finally, less phylogenetically related species with well-marked neck-rings in both sexes are the Australian Shelduck Tadorna tadornoides and the Aleutian Cackling Goose Branta hutchinsii leucopareia (Todd Citation1997, Del Hoyo et al. Citation2014).
One final possibility about the occurrence of white neck-rings relies on gene transfer and fixation of the trait due to hybridization between a species normally showing the neck ring (e.g. mallard) and other dabbling ducks. There are many duck species sharing a similar appearance and ecology and fertile hybrids frequently occur, yet these species remain distinct (Tubaro & Lijtmaer Citation2002). Recently, Kraus et al. (Citation2012) showed that the degree of shared single nucleotide polymorphisms (SNPs) in different Anas species is much higher than that previously reported between other groups of species having comparable evolutionary distances. Moreover, the same authors demonstrated that hybridization phenomena in dabbling ducks have led to consistent exchange of genetic material between different species without disintegrating species boundaries. All specimens having a white mark on the neck did not show any other white patch or feature of plumage and pattern different from the typical one suggesting these teals were at least not early generation hybrids. Furthermore, the low frequency of this trait and the fact that it seems to be more expressed in males than in females is intriguing, since it could also suggest that sexual selection (either in the form of ‘rare male effect’ or ‘status signalling trait’) could be involved as an evolutionary mechanism driving its fixation and heritability. For example, the white neck-ring could contribute to confer fitness benefits to bearers in mate-choice contexts, and fixation of the trait in only some populations could be at an early stage due to rarity of hybridization events previously suggested.
These hypotheses could be tested by two-choice experiments manipulating male appearance or his digital image to prospecting real females. Moreover, considering the recent advances in high throughput sequencing techniques, further studies should be devoted to the genomic differences occurring in these plumage aberrant birds, in order to shed more light on determining the genome regions related to this trait and its heritability in teals and other duck species. At the same time, national or global campaigns or monitoring could be launched by exploiting public citizen science platforms to achieve a better understanding of the real occurrence, phenological and geographical extent of this phenomenon.
Acknowledgements
We are particularly grateful to G. Bergamo, A. Blancato, F. Bosio, V. Cavaliere, N. Celeghin, S. Del Carlo, S. Farina, E. Felce, S. Laurenti, M. Leoni, G. Mainstrello, P. Monti, G. Rallo, M. Rossi, G. Scagliarini, L. Simeoni, S. Simeoni. We are also deeply indebted with the curators of the Ornithological Collections of different Italian and European Museum, which kindly provided information on their preserved teals: B. Schmidt (Smithsonian, Washington, US), H. van Grouw (NH, Tring, GB), E. Koblik (Moscow, Russia), I. Fadev (Darwin, Moscow), L. Vallotton (Geneve, CH), E. Borgo (Genoa, Italy), G. Chiozzi (Milano Italy), N. Baccetti and A. de Faveri (ISPRA, Ozzano BO, Italy), C. Marangoni (Roma Museum, Italy), S. Farina (Pisa, Italy). Cheryl Ames and Masao Migita kindly translate from Japanese the relevant parts of the Kuroda paper.
ORCID
Andrea Galimberti http://orcid.org/0000-0003-3140-3024
References
- Andrés, J.A., Sanchez-Guillén, R.A. & Cordero-Rivera, A. 2002. Evolution of female colour polymorphism in damselflies: testing the hypotheses. Animal Behav. 63: 677–685. doi: 10.1006/anbe.2001.1948
- Arrigoni degli Oddi, E. 1929. Ornitologia Italiana. Hoepli, Milano.
- Baker, A.J. & Marshall, H.D. 1997. Mitochondrial control region sequences as tools for understanding evolution. In Mindell, D.P. (ed) Avian Molecular Evolution and Systematics, 51–82. Academic Press, San Diego.
- Bandelt, H.J., Forster, P. & Röhl, A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16: 37–48. doi: 10.1093/oxfordjournals.molbev.a026036
- Bensch, S., Hansson, B., Hasselquist, D. & Nielsen, B. 2000. Partial albinism in a semi-isolated population of Great Reed Warblers. Hereditas 133: 167–170. doi: 10.1111/j.1601-5223.2000.t01-1-00167.x
- Bon, M. & Scarton, F. 2012. Lo svernamento degli uccelli acquatici in provincia di Venezia (1993–2012). Assessorato alla Caccia, Provincia di Venezia.
- Bonhote, J.L. 1907. Some notes on the hybridising of ducks. Proc. Int. Ornithol. Cong. 4: 235–264.
- Buhay, J.E. 2009. ‘COI-like’ sequences are becoming problematic in molecular systematic and DNA barcoding studies. J. Crustacean Biol. 29: 96–110. doi: 10.1651/08-3020.1
- Campbell, B. & Lack, E. 1985. A Dictionary of Birds. Poyser, London.
- Cooper, W.E. & Burns, N. 1987. Social significance of ventrolateral coloration in the fence lizard, Sceloporus undulatus. Animal Behav. 35: 526–532. doi: 10.1016/S0003-3472(87)80277-4
- Crawford, R.D. 1990. Poultry Breeding and Genetics. Elsevier, Oxford, UK.
- Del Hoyo, J., Collar, N.J., Christie, D.A., Elliott, A. & Fishpool, L.D.C. 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World. 1. Non-Passeriformes. Lynx, Barcelona.
- Dickinson, E.C. & Remsen, J.V., Jr. (eds) 2013. The Howard and Moore Complete Checklist of the Birds of the World, 4th edn, Vol. 1. Aves Press, Eastbourne, UK.
- Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. doi: 10.1093/nar/gkh340
- Edson, J.M. 1928. An epidemic of albinism. Auk 45: 377–378. doi: 10.2307/4076054
- Ellegren, H., Lindgren, G., Primmer, C.R. & Møller, A.P. 1997. Fitness loss and germline mutations in barn swallows breeding in chernobyl. Nature 389: 593–596. doi: 10.1038/39303
- Enders, F. & Post, W. 1971. White-spotting in the genus Ammospiza and other grassland sparrows. Bird-banding 42: 210–219. doi: 10.2307/4511773
- Ford, E.B. 1945. Polymorphism. Biol. Rev. Biol. Proc. Cambridge Philosophical Soc. 20: 73–88. doi: 10.1111/j.1469-185X.1945.tb00315.x
- Forrest, S.C., & Naveen, R. 2000. Prevalence of leucism in pygocelid penguins of the Antarctic peninsula. Waterbirds 23: 283–285.
- Fox, H.M. & Vevers, G. 1960. The Nature of Animal Colours. Sidgwick & Jackson, London.
- Galeotti, P. & Rubolini, D. 2004. The niche variation hypothesis and the evolution of colour polymorphism in birds: a comparative study of owls, nightjars and raptors. Biol. J. Linn. Soc. 82: 237–248. doi: 10.1111/j.1095-8312.2004.00355.x
- Galeotti, P., Rubolini, D., Dunn, P.O. & Fasola, M. 2003. Colour polymorphism in birds: causes and functions. J. Evol. Biol. 16: 635–646. doi: 10.1046/j.1420-9101.2003.00569.x
- Giunchi, D., Baldaccini, N.E., Lenzoni, A., Luschi, P., Sorrenti, M., Cerritelli, G. & Vanni, L. 2019. Spring migratory routes and stopover duration of satellite-tracked Eurasian Teals Anas crecca wintering in Italy. Ibis 161: 131–146.
- Gross, A.O. 1965. The incidence of albinism in North American birds. Bird-banding 36: 67–71. doi: 10.2307/4511145
- Guay, P.J. 2011. An aberrant plumaged Chestnut Teal Anas castanea with a white neck- ring. Corella 35: 57–58.
- Guay, P.J., Potvin, D.A. & Robinson, R.W. 2012. Aberrations in plumage coloration in birds. Aust. Field Ornithol. 29: 23–30.
- Hall, T.A. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symp. Ser., Vol. 41: 95–98. Oxford University Press, Oxford, UK.
- Harrison, J.M. & Harrison, J.G. 1958. The white neck spot variant in the European Green-winged Teal and in the Yellow-billed Teal. Bull. British Ornithol. Club 78: 104–105.
- Harrison, J.M. & Harrison, J.G. 1959a. Further remarks on the white neck-spot variant in the European Green-winged Teal. Bull. British Ornithol. Club 79: 25–27.
- Harrison, J.M. & Harrison, J.G. 1959b. Evolutionary significance of certain plumage sequences in Northern Shoveler. Bull. British Ornithol. Club 79: 135–142.
- Harrison, J.M. & Harrison, J.G. 1959c. Plumage variants in drake Gadwall. Bull. British Ornithol. Club 79: 78–79.
- Harrison, J.M. & Harrison, J.G. 1962. A European Green-winged Teal with a white neck-ring. Bull. British Ornithol. Club 82: 88–90.
- Harrison, J.M., & Harrison, J.G. 1963. A Gadwall with a white neck ring and a review of plumage variants in wildfowl. Bull. British Ornithol. Club 83: 101–108.
- Hebert, P.D., Stoeckle, M.Y., Zemlak, T.S. & Francis, C.M. 2004. Identification of birds through DNA barcodes. PLoS Biol. 2: e312. doi: 10.1371/journal.pbio.0020312
- Hosner, P.A. & Lebbin, D.J. 2006. Observations of plumage pigment aberrations of birds in Ecuador, including Ramphastidae. Boletín Sociedad Antioqueña de Ornithologia 16: 30–43.
- Hubbs, C. & Bartholomew, G. 1951. Persistence of a rare color aberration in the Heermann Gull. Condor 53: 221–227. doi: 10.2307/1364955
- Huxley, J. 1955. Morphism in birds. Acta. Int. Congr. Ornithol. 11: 309–328.
- Jin, S.D., Rashedul Hoque, M., Seo, D.W., Kim, I.K., Jo, C., Paek, W.K. & Lee, J.H. 2012. Phylogenetic relationships among dabbling duck species in Korea using COI gene variations in mtDNA. J. Poultry Sci. 49: 163–170. doi: 10.2141/jpsa.011102
- Johnsen, A., Rindal, E., Ericson, P.G., Zuccon, D., Kerr, K.C., Stoeckle, M.Y. & Lifjeld, J.T. 2010. DNA barcoding of Scandinavian birds reveals divergent lineages in trans-Atlantic species. J. Ornithol. 151: 565–578. doi: 10.1007/s10336-009-0490-3
- Kerr, K.C., Birks, S.M., Kalyakin, M.V., Red’kin, Y.A., Koblik, E.A. & Hebert, P.D. 2009. Filling the gap-COI barcode resolution in eastern Palearctic birds. Front. Zool. 6: 29. doi: 10.1186/1742-9994-6-29
- Kraus, R.H.S., Kerstens, H.H.D., van Hooft, P., Megens, H.J., Elmberg, J., Tsvey, A., Sartakov, D., Soloviev, S.A., Crooijmans, R.P.M.A., Groenen, M.A.M., Ydenberg, R.C. & Prins, H.H.T. 2012. Widespread horizontal genomic exchange does not erode species barriers among sympatric ducks. BMC Evol. Biol. 12: 45. doi: 10.1186/1471-2148-12-45
- Kuroda, N. 1937. An examination on the individual variations among 1,000 Teals. Japanese J. Ornithol. 9: 273–299. doi: 10.3838/jjo1915.9.273
- Lee, D.H., Lee, H.J., Lee, Y.J., Kang, H.M., Jeong, O.M., Kim, M.C., Kwon, J.S, Known, K.C.B., Lee J.B., Park S.J., Choi I.S. & Song, C.S. 2010. DNA barcoding techniques for avian influenza virus surveillance in migratory bird habitats. J. Wildl. Dis. 46: 649–654. doi: 10.7589/0090-3558-46.2.649
- Lepetz, V., Massot, M., Chaine, A.S. & Clobert, J. 2009. Climate warming and the evolution of morphotypes in a reptile. Glob. Change Biol. 15: 454–466. doi: 10.1111/j.1365-2486.2008.01761.x
- Librado, P. & Rozas, J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. doi: 10.1093/bioinformatics/btp187
- Lopez, P., Martin, J. & Cuadrado, M. 2004. The role of lateral blue spots in intrasexual relationships between male Iberian rock-lizards, Lacerta monticola. Ethology 110: 543–561. doi: 10.1111/j.1439-0310.2004.00996.x
- Mahabal, A., van Grouw, H., Sharma, R.M. & Thakur, S. 2016. How common is albinism really? Colour aberrations in Indian birds reviewed. Dutch Bird 38: 301–309.
- Martín, J. & Forsman, A.J. 1999. Social costs and development of nuptial coloration in male Psammodromus algirus lizards: an experiment. Behav. Ecol. 10: 396–400. doi: 10.1093/beheco/10.4.396
- Mundy, N.Y. 2006. Genetic basis of color variation in wild birds. In Hill, G. E., & McGraw K. J. (eds) Bird Coloration, Mechanisms and Measurements, 469–506. Harvard University Press, Cambridge, USA.
- Nei, M. 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Nero, R. 1954. Plumage aberrations of the Redwing (Agelaius phoeniceus). Auk 71: 137–155. doi: 10.2307/4081568
- Peters, J.L., McCracken, K.G., Pruett, C.L., Rohwer, S., Drovetski, S.V., Zhuravlev, Y.N. & Winker, K. 2012. A parapatric propensity for breeding precludes the completion of speciation in common teal (Anas crecca, sensu lato). Mol. Ecol. 21: 4563–4577. doi: 10.1111/j.1365-294X.2012.05711.x
- Peters, J.L., Winker, K., Millam, K.C., Lavretsky, P., Kulikova, I., Wilson, R.E. & McCracken, K.G. 2014. Mito-nuclear discord in six congeneric lineages of Holarctic ducks (genus Anas). Mol. Ecol. 23: 2961–2974. doi: 10.1111/mec.12799
- Rollin, N. 1953. A note on abnormally marked Song Thrushes and Blackbirds. Trans. Nat. Hist. Soc. Northumberland 10: 183–184.
- Roulin, A. 2004. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol. Rev. 79: 815–848. doi: 10.1017/S1464793104006487
- Sage, B.L. 1962. Albinism and melanism in birds. Br. Birds 55: 201–225.
- Sage, B.L. 1963. The incidence of albinism and melanism in British birds. Br. Birds 56: 409–416.
- Saitoh, T., Sugita, N., Someya, S., Iwami, Y., Kobayashi, S., Kamigaichi, H., Higuchi, A., Asai, S., Yamamoto, Y. & Nishiumi, I. 2015. DNA barcoding reveals 24 distinct lineages as cryptic bird species candidates in and around the Japanese Archipelago. Mol. Ecol. Resour. 15: 177–186. doi: 10.1111/1755-0998.12282
- Sangster, G., Collinson, M., Helbig A.J., Knox, A.G., Parkin D.T. & Prater T. 2001. The taxonomic status of Green-winged Teal Anas carolinensis. Br. Birds 94: 218–226.
- Sangster, G., Knox, A.G., Helbig, A.J. & Parkin, D.T. 2002. Taxonomic recommendations for European birds. Ibis 144: 153–159. doi: 10.1046/j.0019-1019.2001.00026.x
- Sinervo, B. 2000. Adaptation, natural selection, and optimal life history allocation in the face of genetically-based trade-offs. In Mousseau, T., Sinervo, B. & Endler J.A. (eds) Adaptive Genetic Variation in the Wild, 41–64. Oxford University Press, Oxford.
- Slagsvold, T., Rofstad, G. & Sandvik, J. 1988. Partial albinism and natural selection in the hooded crow Corvus corone cornix. J. Zool. 214: 157–166. doi: 10.1111/j.1469-7998.1988.tb04993.x
- Song, H., Buhay, J.E., Whiting, M.F. & Crandall K.A. 2008. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Nat. Acad. Sci. 105: 13486–13491. doi: 10.1073/pnas.0803076105
- Sorenson, M.D. & Fleischer, R.C. 1996. Multiple independent transpositions of mitochondrial DNA control region sequences to the nucleus. Proc. Nat. Acad. Sci. 93: 15239–15243. doi: 10.1073/pnas.93.26.15239
- Sorenson, M.D. & Quinn, T.W. 1998. NUMTS: a challenge for avian systematics and population biology. Auk 115: 214–221. doi: 10.2307/4089130
- Sorenson, M.D., Ast, J.C., Dimcheff, D.E., Yuri, T. & Mindell, D.P. 1999. Primers for a PCR-based approach to mitochondrial genome sequencing in birds and other vertebrates. Mol. Phylogenet. Evol. 12: 105–114. doi: 10.1006/mpev.1998.0602
- Stoeckle, M.Y. & Kerr, K.C. 2012. Frequency matrix approach demonstrates high sequence quality in avian BARCODEs and highlights cryptic pseudogenes. PloS One 7: e43992. doi: 10.1371/journal.pone.0043992
- Stuart-Fox, D., Whiting, M.J. & Moussalli, A. 2006. Camouflage and colour change: antipredator responses to bird and snake predators across multiple populations in a dwarf chameleon. Biol. J. Linnean Soc. 88: 437–446. doi: 10.1111/j.1095-8312.2006.00631.x
- Tamura, K., Stecher G., Peterson D., Filipski, A. & Kumar, S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. doi: 10.1093/molbev/mst197
- Thompson, C.W., Moore, I.T. & Moore, M.C. 1993. Social, environmental and genetic factors in the ontogeny of phenotypic differentiation in a lizard with alternative male reproductive strategies. Behav. Ecol. Sociobiol. 33: 137–146. doi: 10.1007/BF00216593
- Todd, F.S. 1997. Natural History of the Waterfowl. Ibis Publishing Company, Vista, USA.
- Trauger, D.L. 1976. Plumage aberrancy in Blue-winged Teal. Auk 93: 646–650.
- Tubaro, P.L. & Lijtmaer, D.A. 2002. Hybridization patterns and the evolution of reproductive isolation in ducks. Biol. J. Linnean Soc. 77: 193–200. doi: 10.1046/j.1095-8312.2002.00096.x
- van Grouw, H. 2006. Not every white bird is an albino: sense and nonsense about colour aberrations in birds. Dutch Birding 28: 79–89.
- van Grouw, H. 2012. What colour is that sparrow? A case study: colour aberrations in the house sparrow Passer domesticus. Int. Stud. Sparrows 36: 30–55. doi: 10.1515/isspar-2015-0012
- van Grouw, H. 2013. Aberrations in birds. Br. Birds 106: 17–29.
- van Grouw, H. 2018. White feathers in black birds. Br. Birds 111: 250–263.
- van Grouw, H., Mahabal, A., Sharma, R.M. & Thakur, S. 2016. How common is albinism really? Colour aberrations in. Dutch Birding 38: 301–309.
- White, T.E. & Kemp, D.J. (2016). Colour polymorphism. Curr. Biol. 26: 517–518. doi: 10.1016/j.cub.2016.03.017
- Widemo, F. 1998. Alternative reproductive strategies in the ruff, Philomachus pugnax: a mixed ESS? Anim. Behav. 56: 329–336. doi: 10.1006/anbe.1998.0792
- Wilson, R.E., Valqui, T.H. & McCracken, K.G. 2006. Aberrant plumage in the Yellow-billed Pintail Anas georgica. Wildfowl 56: 192–196.
- Winker, K. & Peters, J. 2013. Heteropatric speciation in a duck, Anas crecca. Mol. Ecol. 22: 5922–5935. doi: 10.1111/mec.12525
- Yoo, H.S., Eah, J.Y., Kim, J.S., Kim, Y.J., Min, M.S., Paek, W.K., Lee, H. & Kim, C.B. 2006. DNA barcoding Korean birds. Mol. Cell. 22: 323–327.
