ABSTRACT
Capsule: Trophic status of a deep-water lake was the main driver of changes in breeding population size of Great Crested Grebes Podiceps cristatus while reproductive success was also strongly affected by weather parameters.
Aims: To determine the effects of changes in nutrient status of a formerly highly-eutrophicated deep-water lake and other environmental parameters on a Great Crested Grebe population during a phase of re-oligotrophication.
Methods: Annual surveys were carried out on a natural lake in Switzerland over a period of 25 years to determine breeding population size and reproductive success. The effects of phosphorus content, other limnological parameters and weather variables were analysed with quasi-Poisson models.
Results: The breeding population increased from 80 pairs in 1992 to 417 pairs in 2001, after which numbers showed strong fluctuations. Total phosphorus content in the lake had a strong negative effect on breeding population size. A significant positive correlation was found with the national population index. Reproductive success fluctuated strongly but showed an overall decline. The model indicated positive effects on reproductive success of phosphorus and negative effects of the number of days with strong wind. Rapid water-level increases in early summer and water transparency in June led to higher proportions of late broods.
Conclusion: Phosphorus concentration was identified as the main driver affecting the breeding population of Great Crested Grebes during the phase of recovery of the lake from a highly-eutrophic state. Results indicate that mesotrophic to eutrophic conditions enabled a large population and high breeding success. Reproductive output was further negatively affected by strong wind during a critical breeding phase.
Nutrient level is one of the most important parameters determining productivity of aquatic habitats. In freshwater lakes, where phosphorus is usually the main factor limiting productivity, phosphorus input from anthropogenic sources can lead to increased algal growth which can cause massive changes in limnological parameters such as lower oxygen levels and reduced water transparency (Smith et al. Citation1999). This affects various aspects of fish biology as well as the composition of communities of fish and other taxonomic groups in the food web of lakes (Müller Citation1992, Eckmann & Rösch Citation1998, Smith et al. Citation1999).
The effects of long-term anthropogenic changes in aquatic habitats on waterbirds have rarely been studied at individual waterbodies over decades, an example being Virkkala (Citation2016), who compared population trends of waterbirds in a boreal lake complex in Finland. The relationships between trophic status of the lake and species richness or population sizes of birds were usually studied by comparing lakes with different nutrient levels within a region (Nilsson Citation1978, Nilsson & Nilsson Citation1978, Kerekes Citation1990, Hoyer & Canfield Citation1994, Suter Citation1994, Paszkowski & Tonn Citation2006). In coastal or marine habitats effects of eutrophication on waterbird abundance was shown, for instance, for the Northern Baltic Sea (Rönkä et al. Citation2005) or for Denmark, where it was related to fertilizer use in agriculture (Møller & Laursen Citation2015). Eutrophication has been put forward as one of the reasons for the increase in the European populations of fish-eating birds, for example, Great Cormorants Phalacrocorax carbo (van Eerden & Gregersen Citation1995). Great Crested Grebes Podiceps cristatus were found to increase their wintering numbers in response to an increase in nutrient levels in inland waters (Utschick Citation1976, Suter Citation1994) as well as in coastal habitats (Fernández et al. Citation2005).
In many parts of Europe restoration projects or changes in land-use have reduced nutrient input to lakes but the effects on waterbirds has received little attention, focusing mainly on herbivorous waterbirds in relation to the return of macrophytes (Keller Citation2000, Noordhuis et al. Citation2002). Long-term counts of moulting waterbirds at Ismaning reservoir and adjacent fishponds in Germany also indicated increases in herbivorous waterbirds with decreasing nutrient levels, while species preferring animal food declined (Krosigk & Köhler Citation2000). That effects of eutrophication in relation to fertilizer use can be reversible has been shown by Møller & Laursen (Citation2015). Otherwise, the effects of reduced nutrient inputs on inland waters have mostly been studied with regard to plankton, macrophytes or fish (Eckmann & Rösch Citation1998, Bürgi & Stadelmann Citation2002, Jeppesen et al. Citation2005, Eigemann et al. Citation2016).
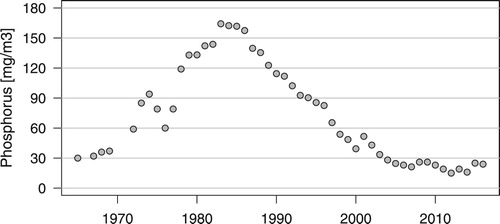
During the course of the twentieth century, anthropogenic eutrophication has affected most lakes in Switzerland (Liechti Citation1994). The majority of these lakes are of glacial origin, deep and naturally oligotrophic or mesotrophic. Lakes on the Swiss plateau, where agriculture is intensive and human population density is high, were particularly affected. The introduction or improvement of sewage treatment plants from the 1970s, a ban on phosphate in textile detergents in 1986 and measures to reduce phosphorus run-off from agricultural land resulted in a reduction of phosphorus concentrations and many lakes are gradually returning to a more natural oligo- or mesotrophic state (Eckmann Citation2013).
Among the many lakes in Switzerland impacted by eutrophication Lake Sempach was one of the most affected, reaching a hypertrophic state in the 1980s (Bürgi & Stadelmann Citation2002). Following a series of restoration measures phosphorus concentrations underwent a massive reduction. In parallel, a monitoring programme was implemented and provided data on phosphorus and oxygen concentration, water transparency and water temperature. Independently, the first author started annual counts of the breeding population of Great Crested Grebes in 1992. We here use the results from 25 years of monitoring to analyse how nutrient levels and other environmental parameters affected population size and reproductive success during the period of change from a highly-eutrophic to a mesotrophic state.
Methods
Study area
Lake Sempach, a lake formed after the last ice age, is situated at 504 m above sea level in the Canton of Lucerne in central Switzerland (47° 08’ N, 8° 09’ E). It has a surface area of 14.4 km2, a maximum depth of 87 m, a mean depth of 46 m and a water residence time of around 15 years (Bürgi & Stadelmann Citation2002). Due to its small catchment area (61.9 km2) with a pluvial regime and the regulation of the outflow, water levels do not show wide fluctuations and most reedbeds are flooded during the whole summer period. As a consequence of progressive enrichment with nutrients, the lake changed from an oligotrophic state prior to 1950 to a mesotrophic one around 1965 and reached hypertrophic conditions in the 1980s (Stadelmann Citation1988). The first measurements taken in 1952 showed a spring phosphorus content of around 15 mg/m3 (Thomas Citation1953). From 1965 to 1985, total phosphorus concentration rose from 30 to 160 mg/m3. Following a massive fish die-off in 1984 restoration measures were undertaken within the lake by artificial mixing in winter and providing pure oxygen in summer in order to prevent anoxic conditions in the hypolimnion. In 1997 these measures were replaced by bubbling compressed air at depths of about 85 m (Gächter Citation1987, Bürgi & Stadelmann Citation2002). Measures were also introduced to reduce phosphorus inflow from agricultural land. In addition to the country-wide measures mentioned above, these site-specific measures led to a decrease of total phosphorus concentrations to below 30 mg/m3, the target set by the restoration programme, from 2006 onwards ().
Whitefish Coregonus sp. are the most abundant fish species in the lake, while cyprinids Cyprinidae, Perch Perca fluviatilis and several other species were considered to be less abundant (Müller et al. Citation1993). Natural reproduction of whitefish was, however, made impossible during the eutrophication phase due to anoxic conditions at the lake bottom, and fish are artificially reared and restocked by professional fishermen (Müller Citation1992).
The shores of Lake Sempach consist of stretches with natural vegetation (around one third of total shore length) and others where the shores have been artificially altered, in particular close to settlements and individual houses. The lake is used for recreation. In 2003, several refuge areas where access by boat is restricted during the breeding season of waterbirds have been created in areas where reedbeds stretch along the shore. After strong declines most reedbeds are only a few metres wide (Hofer et al. Citation2010).
Grebe surveys
Counts of Great Crested Grebes were carried out annually from 1992 to 2016 from a boat, following the whole shore line. The first count within the season was scheduled for around 20 May (between 17 and 27 May), which corresponds to the time on Lake Sempach when most pairs start to build nests and are therefore concentrated close to the shore but not yet hidden in the reedbeds while incubating. Number of adults/2 was used as an estimate for the number of breeding pairs (see also Woollhead Citation1987). Based on an incubation period of four weeks and a brood-rearing period of 10–12 weeks the dates for a second survey to determine reproductive success was set to the beginning of August (between 2 and 12 August), which is before the first chicks fledged. A third count was carried out about three weeks later (between 25 August and 5 September). All adults, families, chicks in families and solitary chicks were recorded. During the third census at the end of August chicks younger than about three weeks, which would not have been recorded during the second census, were recorded separately. The maximum number of chicks counted on any of the second and third censuses correlated closely to the sum of the number of chicks counted during the second census plus the number of small chicks during the third census. We therefore used the maximum number of chicks from any of the censuses as a measure of reproductive output.
Limnologial and weather parameters
We used the following limnogical parameters, obtained from monitoring programmes by the environment agency of the Canton of Lucerne (): total phosphorus content in spring, water transparency measured monthly from April to August as Secchi disk depth, and water temperature in April at different depths (1, 2.5, 5, and 7.5 m). Water level was automatically measured by the Federal Office for the Environment; we used monthly means for May, June and July, the main nesting period. We also identified days with increases of water level of at least 5 cm compared to the preceding day (using an 8 cm threshold did not alter the conclusions). Such rapid water level changes happen mostly in periods with heavy rainfall during summer storms and might lead to nest failures. The same applies to strong wind and the resulting waves. We calculated the number of days with peak wind speeds of >70 km/h for the periods 1 June to 31 July and for a more restricted period of 10 June to 15 July, corresponding to the peak hatching period of Great Crested Grebes. We used data from the weather station at Egolzwil around 10 km west of Lake Sempach at a similar altitude. A few missing data were modelled using data from the weather station at Wynau, Canton of Bern. Data were provided by the Federal Office of Meteorology and Climatology MeteoSwiss. We also considered the estimated number of breeding pairs in May on Lake Sempach as a predictor in some models, and the national breeding population index for Great Crested Grebes which is calculated by the Swiss Ornithological Institute based on data from lakes of different sizes, including Lake Sempach (https://www.vogelwarte.ch/en/projects/population-trends/breeding-population-indices/). Data on fisheries yield were provided by the cantonal agency for nature, hunting and fisheries. As they were incomplete, especially with regard to species other than whitefish, and fishery effort had not been recorded, they were eventually excluded from the analysis. Other variables tested but finally not included were the number of juveniles in the previous year, European population index for Great Crested Grebes, and the installation of zones on Lake Sempach with restricted access for boats (i.e. before vs. after 2003), which seemed not to have a measurable effect.
Table 1. Predictors used in the final models, with their mean, standard deviation and range of observed values. Predictors in italics were tested but not retained in the final models. GCG = Great Crested Grebes.
Statistical analyses
For each outcome value (population size, breeding success and proportion of late broods) we defined a subset of 4–5 predictors from the predictors defined in the previous section () based on our expectations and interests (). These variables selected a-priori were kept in the model independent of their statistical significance. In addition, interactions and quadratic effects defined a-priori or indicated by the analysis of residuals were tested but eliminated again when the effect was not significant (P > 0.05). For each model, additional predictors considered potentially important were added one at a time. They were eliminated again when not statistically significant. For predictors that were available for different months (water transparency, water level) or different water depths (water temperature in April) we defined a-priori which one was judged to be most relevant for the particular parameter from a biological point of view. Values for other months or depths were, however, tested as well (see for details). We based our model selection on P-values (P > 0.05) because the quasi-models we used did not have a defined Akaike information criterion (AIC) for model comparison.
Table 2. Coefficient estimates for the final models of the three outcome variables. See for explanations of the variables. Variables were z-transformed; an ‘x’ represents an interaction. All variables were in the a-priori defined models, except for the national population index in the first model which was added and retained based on its significance. In model b) strong wind 1 June to 31 July (estimate −0.10, P = 0.19) was replaced by the corresponding value for 10 June to 15 July; in all other cases, the month specified in the a-priori models was retained.
Population size (number of breeding pairs) was modelled using a quasi-Poisson model because of overdispersion in the count data. We received the same conclusions when using a negative-binomial model instead of the quasi-Poisson (also regarding model selection based on the AIC available with a negative-binomial model), but we kept the quasi-Poisson model for coherence with the following two models. The number of chicks per breeding pair and the number of late broods among all families were modelled using a quasi-Poisson model with the corresponding offset (i.e. number of breeding pairs and total number of families, respectively; a negative-binomial model was not possible because the glm.nb function we used did not allow for an offset). All continuous variables were z-transformed to improve the comparability of the effect. Quadratic effects were studied using orthogonal polynomials. Model assumptions were tested by residual analysis. In particular we tested temporal autocorrelation (using autocorrelation function graphics), constant residual variance, qq plots of residuals, and graphs of residuals plotted against each predictor.
The effects of predictors are presented in partial effect graphs. Partial effects show the effects of a variable under the assumption that all other variables are constant, i.e. set to the mean of each predictor unless indicated otherwise. Uncertainties are indicated with 95% confidence intervals. Analyses were conducted with R3.4.3 (R Core Team Citation2017).
Results
Breeding population size
The number of Great Crested Grebes counted in May increased almost continuously from 160 (80 breeding pairs) in 1992 to a first peak of 833 (417 breeding pairs) in 2001 (). This increase was followed by a period of strong fluctuations until 2016. The number of breeding pairs was strongly related to total phosphorus (with a linear and quadratic influence) and the national population index (a, ).
Figure 2. Number of breeding pairs, number of chicks in late summer and reproductive success of Great Crested Grebes on Lake Sempach 1992–2016.
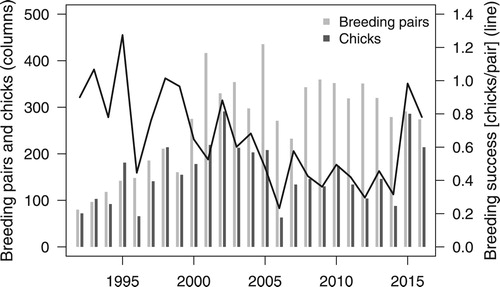
Figure 3. Partial effects of phosphorus (A) and national population index (B) on Great Crested Grebe population size in May. Circles indicate raw data, dotted lines the 95% confidence interval. Graphs show the effect of the parameter assuming constant values of the other parameters (set to their mean).
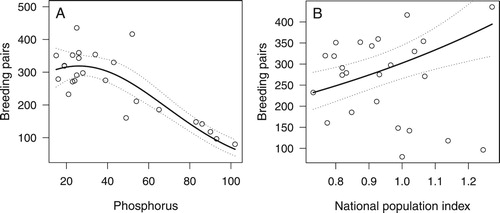
The final model showed good residual plots and no relevant temporal autocorrelation. The year 2001 was found to have a rather strong influence on the results (Cook’s distance approximately 0.6). Removing the year did not change the model much: the direction of the signs remained the same, water level in May became significant. We therefore kept the year 2001 in the model. Total phosphorus had a strong negative effect on breeding population size. A significant positive correlation was found with the national population index.
Reproductive success
The total number of Great Crested Grebe chicks counted varied between years from 63 to 291 (). Reproductive success (number of chicks per pair), fluctuated strongly but showed an overall decline until 2014, followed by two years with high values ().
The effect of environmental parameters on reproductive success was analysed with data from 1994 to 2016 because in 1992 and 1993 no consistent distinction between small chicks and those older than three weeks was made. The final model showed positive effects of phosphorus and negative effects of number of days with strong wind (b, ).
Figure 4. Partial effects of phosphorus (A), water transparency in June (Secchi disk depth) (B), and the number of days with strong wind 10 June to 15 July (C) on reproductive success of Great Crested Grebes (chicks/breeding pair).
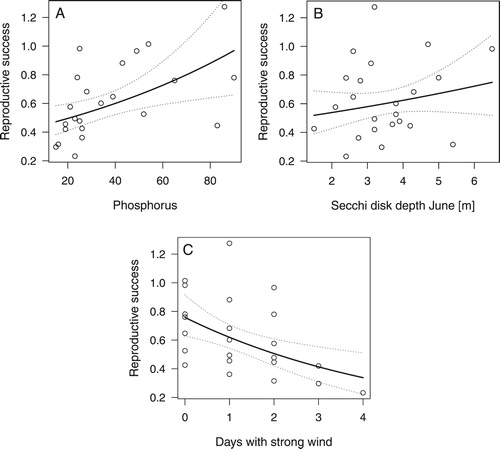
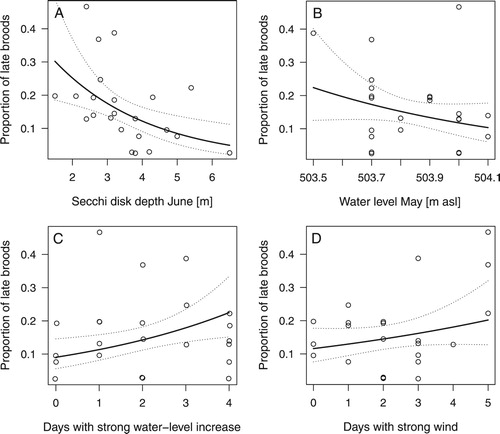
The number of days with strong wind had a negative effect on reproductive success, with the number of juveniles per pair decreasing from 0.7–0.8 without strong-wind days to 0.4–0.5 with four strong-wind days within the period mid-June to mid-July (). The effect was similar but somewhat stronger when population size was used instead of phosphorus (these two variables correlated (r = −0.70) and therefore were not used simultaneously in a model). There was no strong interaction effect of days with strong wind and increases in water level. Using the proportion of successful pairs, estimated as the number of families in August per number of pairs in May, as dependent variable instead of the number of juveniles per breeding pair resulted in essentially the same predictor effects.
Late broods
The proportion of late broods can be used as a proxy for the number of replacement clutches. The final model showed a significant negative effect of water transparency in June on the proportion of late broods, i.e. a decreasing proportion of late broods with a higher water transparency (c, ). The number of days with a rapid increase of water level led to an increase in the proportion of late broods (, ).
Discussion
Breeding population size and reproductive success of Great Crested Grebes were affected by phosphorus content and other limnological parameters. Reproductive success was negatively affected by strong wind in June.
The strong increase of the breeding population with decreasing phosphorus content has to be put in the long-term context. Although the Great Crested Grebe population was not continuously surveyed before our study began, there are indications that the population had undergone a massive decline in the 1980s, when the lake reached a hypertrophic state. In May 1975, 302 adult grebes were counted (Fuchs Citation1978), with 320 in 1978, 312 in 1979 and 236 in 1982 (Fuchs Citation1982). Phosphorus values at that time were similar to the values in the early 1990s when Great Crested Grebe numbers had reached a similar level again. From 1983 to 1991 numbers of Great Crested Grebes were very low (L. Schifferli pers. comm.), which was confirmed by counts of adults and juveniles in late summer from 1989 to 1991 (23–38 juveniles, H. Schmid unpubl.). The strong increase in the 1990s therefore indicates a recovery of the breeding population from a very low level. Water transparency may have played an important role, affecting hunting efficiency, although Secchi disk depth was rarely lower than 2 m and thus higher than the threshold of 0.4 m at which a large number of Great Crested Grebes no longer attended the moulting site at Ijsselmeer in the Netherlands (van Eerden et al. Citation1993). It is likely that the fast increase of the population in the early 1990s was also due to a recovery of fish populations after the high-eutrophication phase. The fish-kill in August 1984, which was attributed to the effect of toxins from a massive outbreak of cyanobacteria Aphanizomenon sp., affected mainly cyprinids, Perch and Pike Esox lucius but not the whitefish which occur in deeper water (Bürgi & Stadelmann Citation2002). Unfortunately, data on fish abundance in Lake Sempach are lacking and statistics of fishery yield are considered reliable only for whitefish, which are the most important fish for commercial fishery. According to one of the professional fishermen cyprinids in particular were only harvested irregularly, when time allowed it (J. Hofer, pers. comm.). Great Crested Grebes will hunt for whitefish and can reach water depths of 20 m or more at Lake Sempach (Hofer Citation1969) but during the breeding season they are more likely to concentrate on cyprinids and Perch as was found in a study on Lake Biel in western Switzerland with a similar fish community (Geiger Citation1957). Fishery statistics are also difficult to use because Great Crested Grebes, in particular when feeding chicks, tend to prey on young age classes of fish, which are not reflected in the statistics. Despite the difficulties with regard to fishery statistics (https://www.uzh.ch/wild/ssl-dir/fishst.5/?page=statistik#) a comparison of catches on Lake Sempach, as an indicator for fish population size, with the breeding population trend of Great Crested Grebes indicates possible effects of fish populations: during the early 1990s catches of cyprinids and Perch were again similar to the situation before the fish-kill. Perch catches reached a peak in 2002 and 2003, in the same period as the high population sizes of Great Crested Grebes with maximum numbers in 2001 and 2005.
The rapid increase of the Great Crested Grebe population in the 1990s cannot be explained by the production of young on the lake alone. Already in the 1970s breeding success was considered insufficient to maintain the population (Fuchs Citation1982). Fuchs estimated that around 1 chick/pair would be needed. This value was only reached in the first years of our study although our estimate has to be treated with caution and may have been underestimated (see below). Immigration probably played an important role during the phase of population increase, which is also indicated by the effect of the national population index, which is calculated on the basis of a range of lakes of different sizes. The fluctuation of the population after the period of increase indicates that the population approached carrying capacity. Density-dependent effects on the population cannot be excluded but Great Crested Grebes on Lake Sempach are not strongly territorial and the system is not closed. Numbers are therefore likely to be also affected by immigration and emigration.
The effect of changes in reedbed width or structure could not be integrated in the current study due to a lack of information. Reed die-back, for which eutrophication has been postulated as one of the causes (Ostendorp Citation1989), was noticed on Lake Sempach already before the strong eutrophication phase in the 1980s. During the study period, reedbeds seemed to have progressed in certain parts of the lake but not to a large extent. Especially along the northeastern shore of the lake reedbeds tended to become broader and after around the year 2000 more Great Crested Grebes were nesting on this side of the lake than before (pers. obs.).
The estimated number of chicks per breeding pair is suitable as a proxy for reproductive success for our analyses of change but as an absolute figure it should be treated with caution. On the one hand, we do not know the proportion of non-breeders among the individuals counted in May, on the other hand some breeders may have remained undetected and our count method did not allow to take detectability into account.
Reproductive success fluctuated strongly with phosphorus and weather conditions. Water transparency in June indicated a fairly high positive effect although not significant (). High phosphorus combined with clear water in June seemed to be positive, likely indicating high food abundance and good food accessibility.
An effect of storms in early summer on Great Crested Grebe breeding success has been suspected by the professional fisherman J. Hofer (pers. comm.) and has been confirmed by our analysis: grebe broods are susceptible to storms particularly at the end of the incubation period or when the chicks are newly hatched. Grebes build floating nests and can cope well with changes in water level (Keller Citation1992). Strong waves may, however, destroy nests (Ulfvens Citation1988, Allen et al. Citation2008) and are likely to increase mortality among small chicks. The mostly narrow reedbeds on Lake Sempach may exacerbate the effects of strong winds.
Replacement nests after nest losses are common among Great Crested Grebes (Fjeldså Citation2004). In years with high losses due to strong wind we would therefore expect a higher proportion of late broods, which can be considered as a proxy for replacement clutches. This was indeed shown by the increase in the proportion of late broods with days with rapid increases in water level. Strong wind, although not significant, may have had an additional effect as is indicated by the fairly large effect (). The fact that strong wind lowered the total number of chicks per breeding pair indicated, however, that replacement clutches could not increase the overall reproductive output to a level of years without storms.
Late broods can also be an indication of replacement clutches due to nest losses as a consequence of human disturbance (Keller Citation1989). The introduction of refuge areas where access by boats is restricted did not, however, seem to have a noticeable effect on overall population size or reproductive output on the lake. A possible effect may have been obscured by the strong effects of trophic status and wind.
Despite the fact that the relationships between environmental parameters and waterbird populations are complex our results indicate that trophic status of Lake Sempach was the main driver of breeding population trend of Great Crested Grebes in the last 25 years. In the 1990s, grebes probably benefitted from conditions that were still eutrophic and offered a good food supply for raising chicks but without the negative effects of low water transparency and therefore managed to increase their population from the low levels in the 1980s. Since the early 2000s the breeding population tends to fluctuate around 300 breeding pairs with a tendency of a decline over the last ten years. Population size is thus approaching the 150–200 pairs estimated around 1960 before the strong eutrophication phase (Glutz von Blotzheim Citation1962). Overall, the Great Crested Grebe population seems to thrive best under mesotrophic to eutrophic conditions. While the main drivers of population trend and reproductive output have been identified, the mechanisms behind remain unclear. More detailed studies including the role of fish populations in the food web and the role of the artificial stocking would be needed.
Eutrophication is often cited as major factor affecting waterbird populations. Our study is in line with other studies that showed that in contrast to insectivorous and herbivorous species fish-eating birds benefit from eutrophication (Virkkala Citation2016) but it also shows that the relationships are complex and that hypertrophic conditions may affect populations negatively. When discussing effects of eutrophication such non-linear effects should therefore be taken into account.
Acknowledgements
We thank the many people who helped with the surveys, in particular Matthias Kestenholz, Heinz Bachmann and Hans Schmid, and Isabelle Kaiser, Bruno Meyer and Lis Räber for digitizing the data. Josef Hofer provided ideas from his observations on Lake Sempach. Robert Lovas from the Office of Environment and Energy of the Canton of Lucerne provided the limnological data. Lukas Jenni, Thomas Sattler, Stefan Werner and Niklaus Zbinden provided comments to the manuscript.
ORCID
Verena Keller http://orcid.org/0000-0002-5905-8739
Additional information
Funding
References
- Allen, J.H., Nuechterlein, G.L. & Buitron, D. 2008. Weathering the storm: how wind and waves impact Western Grebe nest placement and success. Waterbirds 31: 402–410. doi: 10.1675/1524-4695-31.3.402
- Bürgi, H. & Stadelmann, P. 2002. Change of phytoplankton composition and biodiversity in lake Sempach before and during restoration. Hydrobiologia 469: 33–48. doi: 10.1023/A:1015575527280
- Eckmann, R. 2013. A review of the population dynamics of coregonids in European alpine lakes. Adv. Limnol. 64: 3–24. doi: 10.1127/1612-166X/2013/0064-0002
- Eckmann, R. & Rösch, R. 1998. Lake Constance fisheries and fish ecology. Adv. Limnol. 53: 285–301.
- Eigemann, F., Mischke, U., Hupfer, M., Schaumburg, J. & Hilt, S. 2016. Biological indicators track differential responses of pelagic and littoral areas to nutrient load reductions in German lakes. Ecol. Indic. 61: 905–910. doi: 10.1016/j.ecolind.2015.10.045
- Fernández, J.M., Selma, M.A.E., Aymerich, F.R., Sáez, M.T.P. & Fructuoso, M.F.C. 2005. Aquatic birds as bioindicators of trophic changes and ecosystem deterioration in the Mar Menor lagoon (SE Spain). Hydrobiologia 550: 221–235. doi: 10.1007/s10750-005-4382-0
- Fjeldså, J. 2004. The Grebes. Oxford University Press, Oxford.
- Fuchs, E. 1978. Bestand und Verbreitung des Haubentauchers Podiceps cristatus in der Schweiz. Ornithol. Beob. 75: 19–32.
- Fuchs, E. 1982. Bestand, Zugverhalten, Bruterfolg und Mortalität des Haubentauchers Podiceps cristatus auf dem Sempachersee. Ornithol. Beob. 79: 255–264.
- Gächter, R. 1987. Lake restoration. Why oxygenation and artificial mixing cannot substitute for a decrease in the external phosphorus loading. Schweiz. Z. Hydrol 49: 170–185. doi: 10.1007/BF02538501
- Geiger, W. 1957. Die Nahrung der Haubentaucher (Podiceps cristatus) des Bielersees. Ornithol. Beob. 54: 97–133.
- Glutz von Blotzheim, U.N. 1962. Die Brutvögel der Schweiz. Verlag Aargauer Tagblatt, Aarau.
- Hofer, J. 1969. Zur Tauchtiefe des Haubentauchers Podiceps cristatus. Ornithol. Beob. 66: 1–6.
- Hofer, J., Korner-Nievergelt, P. & Korner-Nievergelt, F. 2010. Auftreten und Herkunft der Wasservögel am Sempachersee. Ornithol. Beob., Beiheft 11: 1–187.
- Hoyer, M.V. & Canfield Jr., D.E. 1994. Bird abundance and species richness on Florida lakes: influence of lake trophic status, morphology, and aquatic macrophytes. Hydrobiologia 297/280: 107–119. doi: 10.1007/BF00027846
- Jeppesen, E., Søndergaard, M., Jensen, J.P., Havens, K.E., Anneville, O., Carvalho, L., Coveney, M.F., Deneke, R., Dokulil, M.T., Foy, B., Gerdeaux, D., Hampton, S.E., Hilt, S., Kangur, K., Köhler, J., Lammens, E.H.H.R., Lauridsen, T.L., Manca, M., Miracle, M.R., Moss, B., Nöges, P., Persson, G., Phillips, G., Portielje, R., Romo, S., Schelske, C.L., Straile, D., Tatrai, I., Willén, E. & Winder, M. 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 50: 1747–1771. doi: 10.1111/j.1365-2427.2005.01415.x
- Keller, V. 1989. Variations in the response of Great Crested Grebes Podiceps cristatus to human disturbance - A sign of adaptation? Biol. Conserv. 49: 31–45. doi: 10.1016/0006-3207(89)90111-0
- Keller, V. 1992. Die Bedeutung des Nestbauverhaltens während der Brutphase bei Haubentauchern Podiceps cristatus. Ornithol. Beob. 89: 171–176.
- Keller, V. 2000. Winter distribution and population change of Red-crested Pochard Netta rufina in southwestern and central Europe. Bird Study 47: 176–185. doi: 10.1080/00063650009461173
- Kerekes, J.J. 1990. Possible correlation of summer common loon (Gavia immer) population with the trophic state of a water body. SIL Proceedings, 1922–2010 24: 349–353. doi: 10.1080/03680770.1989.11898757
- Krosigk, E. von & Köhler, P. 2000. Langfristige Änderungen von Abundanz und räumlicher Verteilung mausernder Wasservogelarten nach Änderungen von Trophiestatus, Fischbesatz und Wasserstand im Ramsar-Gebiet “Ismaninger Speichersee mit Fischteichen”. Ornithol. Anz. 39: 135–158.
- Liechti, P. 1994. Der Zustand der Seen in der Schweiz. Bundesamt für Umwelt, Wald und Landschaft, Bern.
- Møller, A.P. & Laursen, K. 2015. Reversible effects of fertilizer use on population trends of waterbirds in Europe. Biol. Conserv. 184: 389–395. doi: 10.1016/j.biocon.2015.02.022
- Müller, R. 1992. Trophic state and its implications for natural reproduction of salmonid fish. Hydrobiologia 243–244: 261–268. doi: 10.1007/BF00007041
- Müller, R., Ventling-Schwank, A., Meng, H.J. & Bia, M.M. 1993. Fische. In: Sempachersee. Mitteilungen der Naturforschenden Gesellschaft Luzern 33: 129–142.
- Nilsson, L. 1978. Breeding waterfowl in eutrophicated lakes in south Sweden. Wildfowl 29: 101–110.
- Nilsson, S.G. & Nilsson, I.N. 1978. Breeding bird community densities and species richness in lakes. Oikos 31: 214–221. doi: 10.2307/3543565
- Noordhuis, R., van der Molen, D.T. & van den Berg, M.S. 2002. Response of herbivorous water-birds to the return of Chara in lake Veluwemeer, The Netherlands. Aquat. Bot. 72: 349–367. doi: 10.1016/S0304-3770(01)00210-8
- Ostendorp, W. 1989. ‘Die-back’ of reeds in Europe — a critical review of literature. Aquat. Bot. 35: 5–26. doi: 10.1016/0304-3770(89)90063-6
- Paszkowski, C.A. & Tonn, W.M. 2006. Foraging guilds of aquatic birds on productive boreal lakes: environmental relations and concordance patterns. Hydrobiologia 567: 19–30. doi: 10.1007/s10750-006-0053-z
- R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
- Rönkä, M.T.H., Saari, C.L.V., Lehikoinen, E.A., Suomela, J. & Häkkilä, K. 2005. Environmental changes and population trends of breeding waterfowl in northern Baltic Sea. Ann. Zool. Fennici 42: 587–602.
- Smith, V.H., Tilman, G.D. & Nekola, J.C. 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100: 179–196. doi: 10.1016/S0269-7491(99)00091-3
- Stadelmann, P. 1988. Zustand des Sempachersees vor und nach der Inbetriebnahme der see-internen Massnahmen: künstlicher Sauerstoffeintrag und Zwangszirkulation 1980–1987. Wasser, Energie, Luft 80: 81–96.
- Suter, W. 1994. Overwintering waterfowl on Swiss lakes: how are abundance and species richness influenced by trophic status and lake morphology? In Kerekes, J.J. (ed.) Aquatic Birds in the Trophic Web of Lakes, 279/280, 1–14. Kluwer Academic Publishers, Dordrecht.
- Thomas, E.A. 1953. Empirische und experimentelle Untersuchungen zur Kenntnis der Minimumsstoffe in 46 Seen der Schweiz und angrenzender Gebiete. Schweiz. Ver. Gas- & Wasserfachmänner, Monatlbull. 9/10: 1–11.
- Ulfvens, J. 1988. Nest characteristics and nest survival in the horned grebe Podiceps auritus and great crested grebe Podiceps cristatus in a Finnish archipelago. Ann. Zool. Fennici 25: 293–298.
- Utschick, H. 1976. Die Wasservögel als Indikatoren für den ökologischen Zustand von Seen. Verh. orn. Ges. Bayern 22: 395–438.
- van Eerden, M. & Gregersen, J. 1995. Long-term changes in the northwest European population of cormorants Phalacrocorax carbo sinensis. Ardea 83: 61–79.
- van Eerden, M.R., Piersma, T. & Lindeboom, R. 1993. Competitive food exploitation of smelt Osmerus eperlanus by great crested grebes Podiceps cristatus and perch Perca fluviatilis at lake IJsselmeer, The Netherlands. Oecologia 93: 463–474. doi: 10.1007/BF00328953
- Virkkala, R. 2016. Variation in population trends and spatial dynamics of waterbirds in a boreal lake complex. Ornis Fenn. 93: 197–211.
- Woollhead, J. 1987. A method for estimating the number of breeding pairs of Great Crested Grebes Podiceps cristatus on lakes. Bird Study 34: 82–86. doi: 10.1080/00063658709476939