ABSTRACT
We compared habitat characteristics between territories of paired and unpaired males of the long-distance migratory Common Redstart Phoenicurus phoenicurus. Nesting possibilities and reachable sparse vegetation were more abundant in territories of paired males, clearly highlighting the importance of both parameters when implementing habitat enhancements for the species.
Farmland birds that migrate over long distances are one of the strongest declining groups all over Europe (Lemoine et al. Citation2007). Additional to the threats on migration and in the winter quarters, agricultural intensification has decreased habitat quality in the breeding range (Donald et al. Citation2001). In central Europe, orchards with high-stem trees are a very important habitat for the Common Redstart Phoenicurus phoenicurus (Martinez & Roth Citation2017). The severe loss of orchards with high-stem trees in recent decades has led to further declines and prevented the recovery to former population densities in central Europe (Felix & Felix Citation2004, Zwarts et al. Citation2009).
Vital habitat parameters for Common Redstarts are trees, breeding cavities and insect-rich habitats (Glutz von Blotzheim & Bauer Citation1988). Common Redstarts mainly hunt using a sit-and-wait strategy from vantage points (Menzel Citation1971). Prey accessibility is improved in areas with sparse vegetation (Martinez et al. Citation2010), and clutch size increases with the availability of sparse vegetation in a territory (Martinez Citation2012). Common Redstarts in our study area showed a skewed sex ratio of breeding birds (adult sex ratio) consistent over multiple years with approximately 25 percent of males failing to attract a breeding partner (Martinez & Roth Citation2017). In this study, we compared territory quality between paired and unpaired male Common Redstarts. The aim was to investigate whether paired males held territories with higher habitat quality, i.e. with more cavities and more sparse vegetation reachable from vantage points, which would be an indication of the importance of these factors when implementing habitat management for conservation.
In 2013 and 2018 we mapped territories of male Common Redstarts during at least three early morning visits in April and May in several areas of northwestern Switzerland around Basel (47°33′N 7°35′E). We mapped territories in the municipalities of Allschwil, Biel-Benken, Dornach, Ettingen, Reinach and Therwil. Only territories with three independent observations of a singing male between 25th April and 25th May were retained for further analyses, resulting in a total of 47 territories. We searched for the presence of a female in each territory during several visits for 30 min at most. This procedure has been used successfully for Common Redstarts by Martinez & Roth (Citation2017) where 95% of the females were found after less than 15 min (mean ± se = 7.3 ± 1.4 min), strongly suggesting that males were unpaired when no female was found after 30 min.
The territories were all in similar habitats, mostly dominated by high-stem fruit orchards with meadows and often a mixture of crops and small gardens, as well as sometimes forest. Most fruit trees are mature and planted with a density of up to 100 trees per hectare. Territories were distributed all over the study area for paired and unpaired males. In June, the habitat was mapped in a circular area around the middle of the territory with a radius of 50 m (7850 m2), which corresponds to the size of a Common Redstart territory (1400–5000 m2, Menzel Citation1971, up to 10,000 m2, Glutz von Blotzheim & Bauer Citation1988). The middle of territory was defined as the midpoint of the first three independent sightings of a singing male. Within each circle, vegetation was assigned to a predefined list of sparse (e.g. pasture) and dense vegetation (e.g. dense meadow) following Martinez et al. (Citation2010). In forest patches, sparse vegetation was only considered if the ground was largely unvegetated. Using a grid with 2500 evenly distributed points, we assigned each point to a vegetation type. Then we recorded the percentage by classifying each vegetation type as dense or sparse. Furthermore, we recorded all visibly available nesting possibilities (natural cavities and predominantly nest boxes) as well as all vantage points, defined as structures between 0.30 and 1.50 m high that could support a Redstart’s weight, e.g. posts, hedges and trees. The amount of sparse vegetation that could be reached from vantage points was then determined, assuming that Common Redstarts mainly catch prey in a radius of 7 m around a vantage point (Glutz von Blotzheim & Bauer Citation1988).
To analyse the relationship between pairing status of males and habitat quality we used logistic regression with the pairing status as the dependent variable. Explanatory variables were ‘amount of reachable sparse vegetation’, ‘number of nesting possibilities’ and year. The number of nesting possibilities was categorized into four groups (0, 1, 2–3, >3) because it was skewed, and because it seems likely that different biological processes work at the qualitative level of absence versus presence (0 vs. >0), and at the quantitative level of the number of nesting opportunities given presence. Hence, we do not necessarily assume a logit-linear relationship between a number of nesting opportunities (including 0) and the paired status. The amount of reachable sparse vegetation was centred at zero and scaled to one standard deviation. The year was removed from the model as it neither improved model fit nor lowered the Akaike information criterion for small sample sizes (AICc). The second order polynomials of the habitat variables as well as the interaction between the variables ‘nesting possibilities’ and ‘reachable sparse vegetation’ did not improve the model fit or lower the AICc value, hence they were not included in the final model. Model fit was assessed graphically by plotting observed versus fitted values. Model predictions and 95% credible intervals were calculated using Bayesian methods (Gelman et al. Citation2004). We used non-informative priors and 5000 values were simulated from the joint posterior distribution of the model parameters to describe uncertainty (credible intervals) and calculate model predictions.
We collected data in 47 territories, of which 32 were occupied by paired males, reflecting the consistent skewed sex ratio in the study area (Martinez & Roth Citation2017). The amount of reachable sparse vegetation covered between 0.2 and 95.0% of the territory area (median 30.4%). The number of nest site possibilities ranged from 0 to 22 (median 2). The explained variance (deviance of the model divided by the deviance of the null model) equalled 66%. The amount of reachable sparse vegetation showed a positive relationship with paired status (, (a)). All territories, but one, with more than 50% cover of ‘reachable sparse vegetation’ were occupied by paired males.
Figure 1. Probability of a male Common Redstart being paired in relation to territory attributes: (a) the amount of reachable sparse vegetation from vantage points and (b) the number of nest site possibilities. (a) The value of the other explanatory variable (amount of reachable sparse vegetation) was set to its mean. Dashed lines show the 95% credible intervals. (b) The number of nesting possibilities was set to 2–3. Open circles = paired (1) or unpaired male (0), jittered in a vertical direction for better visibility of single points.
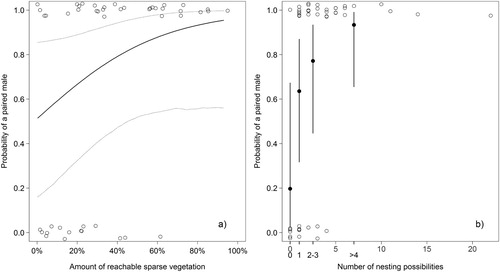
Table 1. Parameter estimates and their 95% credible intervals of the logistic model estimating the relationship between the paired status of Common Redstarts (on the logit scale) and explanatory variables.
The number of nesting possibilities had a positive effect on male pairing status (). This result was affected strongly by whether a possible nest site was found at all. Without a nesting possibility, all but one male remained unpaired, hence the pairing probability was much lower than when available nesting possibilities were present ((b)). If there were nest sites available, the number of sites also appeared to have a positive effect on the pairing status of males, though there was quite some overlap between the estimates and the credible intervals among the groups ((b)).
The amount of reachable sparse vegetation as well as the number of nesting possibilities seemed to positively influence the pairing status of territory holding male Common Redstarts. It is possible that males use these factors to assess habitat quality when establishing their territories after their arrival from the wintering grounds. Possibly females use habitat characteristics for their choice of a mate, too, as do females of other passerine birds (Buchanan & Catchpole et al. Citation1997, Siitari et al. Citation2002). However, since more experienced males, and males in a better body condition, are likely to occupy the best territories (Kokko Citation1999), we cannot tell exactly which factors influence mate choice of females. The home ranges for the individual birds were not assessed in our study, however, based on our own observations, birds mainly foraged inside the 50 m radius and circular territories are supported by the central place foraging theory (Stephens & Krebs Citation1986).
Our result concerning sparse vegetation is consistent with findings of Martinez et al. (Citation2010) that territories of Common Redstarts contained more sparse vegetation than neighbouring habitats. A major part of the sparse vegetation in the territories in our study was accessible from vantage points (median = 90.1%). However, the two factors ‘amount of reachable sparse vegetation’ and ‘total amount of sparse vegetation’ were highly correlated (r = 0.83), and so it is not possible to reveal the relative importance of the two factors in relation to male paired status. The Common Redstart is a sit-and-wait-predator, thus we assume that small-scale arrangements of such sparse vegetation patches are likely to be crucial, since they need to be a reachable distance from suitable vantage points to be of any benefit. Sparse vegetation must be considered when implementing land management for the conservation of Common Redstarts and other sit-and-wait predators of open landscapes, such as Red-backed Shrikes Lanius collurio and Woodchat Shrikes Lanius senator. In Switzerland, measures for Common Redstarts include the creation of ecologically valuable, small and sparsely vegetated structures (Spaar et al. Citation2012).
Our results concerning nesting possibilities confirm the importance of installing suitable nesting boxes when performing species action plans for the Common Redstart (Martinez & Roth Citation2017). When doing this, we strongly recommend placing several nesting boxes in suitable habitat in groups to enhance attractiveness and quality of potential territories, rather than to distribute nesting boxes evenly.
Acknowledgements
We would like to thank Stefan Häring and Urban Willi for assistance in the field and answering questions.
ORCID
Valentin Moser http://orcid.org/0000-0002-9627-1934
Kim Meichtry-Stier http://orcid.org/0000-0001-6455-9442
References
- Buchanan, K. & Catchpole, C. 1997. Female choice in the sedge warbler Acrocephalus schoenobaenus multiple cues from song and territory quality. Proc. R. Soc. Lond. [Biol] 264: 521–526. doi: 10.1098/rspb.1997.0074
- Donald, P., Green, R. & Heath, M. 2001. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. [Biol] 268: 25–29. doi: 10.1098/rspb.2000.1325
- Felix, K. & Felix, L. 2004. Bestandsentwicklung des Gartenrotschwanzes Phoenicurus phoenicurus in der Gemeinde Horgen 1965–2003. Ornithol. Beob. 101: 109–114.
- Gelman, A., Charlin, J., Stern, H. & Ruben, D. 2004. Bayesian Data Analysis. Chapman & Hall/CRC, Boca Raton, Fla.
- Glutz von Blotzheim, U. & and Bauer, K. 1988. Handbuch der Vögel Mitteleuropas. Bd. 11, Passeriformes (2. Teil). Akademische Verlagsgesellschaft, Aula, Wiesbaden.
- Kokko, H. 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68: 940–950. doi: 10.1046/j.1365-2656.1999.00343.x
- Lemoine, N., Bauer, H., Peintinger, M. & Böhning-Gaese, K. 2007. Effects of climate and land-use change on species abundance in a central European bird community. Conserv. Biol. 21: 495–503. doi: 10.1111/j.1523-1739.2006.00633.x
- Martinez, N. 2012. Sparse vegetation predicts clutch size in Common Redstarts Phoenicurus phoenicurus. Bird Study 59: 315–319. doi: 10.1080/00063657.2012.672949
- Martinez, N., Jenni, L., Wyss, E. & Zbinden, N. 2010. Habitat structure versus food abundance: The importance of sparse vegetation for the Common Redstart Phoenicurus phoenicurus. J Ornithol. 151: 297–307. doi: 10.1007/s10336-009-0455-6
- Martinez, N. & Roth, T. 2017. Bestandsentwicklung und Brutbiologie des Gartenrotschwanzes Phoenicurus phoenicurus in der Nordwestschweiz. Ornithol. Beob. 114: 179–200.
- Menzel, H. 1971. Der Gartenrotschwanz: Phoenicurus phoenicurus. Ziemsen, Wittenberg Lutherstadt: Neue Brehm Bücherei Nr. 438.
- Siitari, H., Honkavaara, J., Huhta, E. & Viitala, J. 2002. Ultraviolet reflection and female mate choice in the pied flycatcher, Ficedula hypoleuca. Anim. Behav. 63: 97–102. doi: 10.1006/anbe.2001.1870
- Spaar, R., Ayé, R., Zbinden, N. & Rehsteiner, U. 2012. Elemente für Artenförderungsprogramme Vögel Schweiz – Update 2011. Koordinationsstelle des Rahmenprogramms «Artenförderung Vögel Schweiz».
- Stephens, D. & Krebs, J. 1986. Foraging Theory. Monographs in Behavior and Ecology. Princeton University Press, Princeton, N.J.
- Zwarts, L., Bijlsma, R., van der Kamp, J. & Wymenga, E. 2009. Living on the Edge: Wetlands and Birds in a Changing Sahel. KNNV Publishing, Zeist, The Netherlands.
