ABSTRACT
Capsule: For declining migratory birds, including many aerial insectivores, such as swallows, there is evidence that adult survival is a demographic process with strong effects on population trends.
Aims: The aim was to identify and quantify the effect of threats affecting adult survival and potentially driving population declines for five well-studied swallow species: Barn Swallow Hirundo rustica, Cliff Swallow Petrochelidon pyrrhonota, Tree Swallow Tachycineta bicolor, Sand Martin Riparia riparia, and Purple Martin Progne subis.
Methods: We reviewed the literature to identify the threats to adult survival, quantified the magnitude of the effect and identified whether threats had a direct or indirect effect on survival.
Results: We identified habitat change, weather, competition, incidental loss, contaminants, insect availability, disease, and predation as threats to adult survival in swallows, although for many of these threats there was limited information to quantify their impact. However, weather, particularly cold snaps and precipitation, had negative effects on survival for many populations of four species, either directly or indirectly through effects on insect availability. When there was a relationship, weather was associated with a 13–53% decrease in survival.
Conclusion: Based on the available research, weather conditions throughout the annual cycle is a key threat to adult survival for several swallow species. However, future research on the threats to these species should consider examining the effect of insect availability and the effect of threats during the non-breeding period on survival. Finally, we suggest that new research should be devoted to understanding the importance of adult survival for declining bird populations.
Population declines across many species are often driven by a diversity of threats (Dulvy et al. Citation2014, Purcell et al. Citation2014, Paleczny et al. Citation2015). Identifying and addressing the threats with the greatest effect on each species is challenging. One approach to understanding the effect of threats on populations is to examine the relationships between threats and demographic processes, such as fecundity, recruitment, and survival, which provide insights into how threats drive declines (Rappole & McDonald Citation1994, Rushing et al. Citation2016, Selwood et al. Citation2015). In particular, by focusing on the threats to demographic processes that have the greatest effect on population dynamics, it is possible to direct conservation efforts toward mitigating the threats with the greatest effect on populations (Green Citation1999).
Life history theory predicts that population trends in species with a short life span and high reproductive output should be driven by fecundity (Wilson & MacArthur Citation1967, Sæther & Bakke Citation2000). This prediction has been upheld for some migratory passerines (Ambrosini et al. Citation2011, Gardali et al. Citation2000), a group of birds that typifies this pace of life. However, for other species of migratory passerines, particularly those experiencing population declines, population dynamics are driven by adult survival (Baillie & Peach Citation1992, Murphy Citation2001, Fletcher et al. Citation2006, Buehler et al. Citation2008, Perlut et al. Citation2008, Ambrosini et al. Citation2011). Specifically, for these species, decreased adult survival is the demographic process driving population declines. For example, using a population projection model with sensitivity and elasticity analyses, population trends for Cerulean Warblers Dendroica cerulea were found to be most strongly related to changes in adult survival, rather than breeding success or juvenile survival (Buehler et al. Citation2008). Additionally, this model showed that conservation efforts aimed at increasing breeding success to offset reductions in adult survival could not fully compensate for the loss of adults in the population (Buehler et al. Citation2008). Despite the importance of adult survival on population trends, research on declining bird populations is often focussed on the effects of threats and other regulating factors (collectively referred to as threats hereafter) on fecundity.
One group of birds presently experiencing steep declines in parts of North America and Europe are aerial insectivores (Inger et al. Citation2015, Michel et al. Citation2016, Nebel et al. Citation2010, Smith et al. Citation2015). While the cause(s) of the declines are still unknown, considerable research has examined the effects of threats on fecundity in swallows (Fassbinder-Orth et al. Citation2013, Møller Citation2013, Winkler et al. Citation2013, Rioux Paquette et al. Citation2014). Indeed, a Biological Abstracts (Thomson Reuters) search for published papers on fecundity for swallows and martins returned 1840 results, whereas a similar search for adult survival returned 132 results. While not all of those results may be directly pertinent to the role of threats on population declines, it does suggest a bias in the types of studies that are conducted. More importantly, it affects the inferences we can make about the role of different demographic rates on population dynamics.
Despite the lack of research on adult survival, several recent papers suggest that local population declines in some swallow populations may be driven by reductions in survival rates, particularly for adults (Cox et al. Citation2018, Imlay et al. Citation2018b, Norman & Peach Citation2013, Schaub et al. Citation2015, Taylor et al. Citation2018). Four of these studies examined the vital rates that drive population dynamics (often with an integrated population model or demographic model) in Palearctic-African populations of Barn Swallow Hirundo rustica and Sand Martin Riparia riparia, and two Nearctic-Neotropical populations of Tree Swallow Tachycineta bicolor (Cox et al. Citation2018, Norman & Peach Citation2013, Schaub et al. Citation2015, Taylor et al. Citation2018). Their results demonstrate that breeding population size was strongly related to adult survival, with large populations when survival was higher. In some of these studies, population dynamics were also influenced by juvenile recruitment (Cox et al. Citation2018) or immigration (Schaub et al. Citation2015, Taylor et al. Citation2018). However, Taylor et al. (Citation2018) noted that the effects of immigration on population size may also be due to lower adult survival rates in source populations which reduce the availability of potential immigrants. Finally, reduced breeding success in recent years has only been observed for one of four Nearctic-Neotropical swallow species, the Sand Martin, whereas breeding success was either unchanged or higher for the other three species (Imlay et al. Citation2018b). This suggests that population declines for at least three species must be due to reductions in other demographic rates (Imlay et al. Citation2018b). While the most influential demographic rate for population trends can vary across a species range (Weegman et al. Citation2017), collectively, the findings from the above studies, along with the large spatial scales covered by this work, provide strong support for the importance of understanding and addressing the threats to adult survival in swallow populations.
Therefore, our broad goal was to identify threats to adult survival throughout the annual cycle for five species of swallows: Barn Swallow, Cliff Swallow Petrochelidon pyrrhonota, Tree Swallow, Sand Martin, and Purple Martin Progne subis. These species are all declining in at least part of their geographic range () and adult survival could be a key demographic process in the population declines (Cox et al. Citation2018, Imlay et al. Citation2018b, Norman & Peach Citation2013, Schaub et al. Citation2015, Taylor et al. Citation2018). We conduct a literature review and compare threats based on magnitude of effects on adult survival and determine whether specific threats have direct or indirect effects on survival. We used a multi-species approach to determine if there was evidence of common threats among species and to identify knowledge gaps that, if addressed, may help to understand and address population declines.
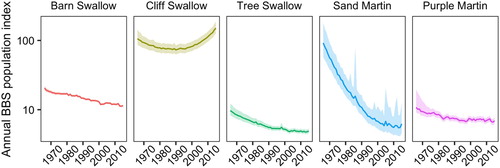
Figure 1. Annual population trends using the hierarchical models described by Link and Sauer (Citation2002) with the 95% credible intervals from the North American Breeding Bird Survey for Barn Swallow, Cliff Swallow, Tree Swallow, Sand Martin, and Purple Martin populations from 1966 to 2015. The annual population trend is represented with a log scale and data is from Sauer et al. (Citation2017).
Methods
Focal species
Our five focal species, Barn Swallow, Cliff Swallow, Tree Swallow, Sand Martin, and Purple Martin, are all New World passerines; the Barn Swallow and Sand Martin also have Old World populations. All five species typically breed in their second year and live for a maximum of 8–13 years, depending on the species and population (Brown & Bomberger Brown Citation1999, Brown et al. Citation2017, Brown & Tarof Citation2013, Garrison Citation1999, Winkler et al. Citation2011). While most swallows raise one brood each year, Barn Swallows and Sand Martins often raise two successful broods (Brown & Bomberger Brown Citation1999, Cowley Citation1983). Throughout their annual cycle, these swallows are often associated with human infrastructure and working landscapes for breeding, foraging, and roosting (Brown & Bomberger Brown Citation1999, Brown et al. Citation2017, Brown & Tarof Citation2013, Garrison Citation1999, Winkler et al. Citation2011). Apart from the consumption of some berries (e.g. bayberry and myrtle Morella spp.) by Tree Swallows (Piland & Winkler Citation2015), swallows solely forage on aerial insects throughout the annual cycle.
Review of threats to adult survival
To determine the documented threats to adult survival in swallows, we searched Biological Abstracts (Thomson Reuters) using keywords associated with the common and scientific names of the focal species (Barn Swallow, Cliff Swallow, Tree Swallow, Sand Martin, Bank Swallow, Purple Martin, Hirundo rustica, Petrochelidon pyrrhonota, Tachycineta bicolor, Riparia riparia, and Progne subis), adult survival (survival, return rate, mortal*, fatal*, lethal*, and impact), and potential threats (habitat, loss, degradation, weather, climate, temperature, precipitation, wind, compet*, density-depend*, road, collision, aggregat*, quarr*, pit, pesticide*, pollut*, insect, food, diseas*, parasit*, predat*, harvest*, and human). The search included studies conducted throughout the species’ geographic range, including New and Old World populations, from 1990 to 2018. We selected this time period because all species experienced population declines across at least part of their geographic range during this time (Heldbjerg & Fox Citation2008, Sauer et al. Citation2017) () and because most studies on adult survival for these species were conducted during this time. The literature search was completed on 30 October 2018.
Calculating maximum effects of threat on adult survival
To determine the threats with the greatest effect on adult survival, we calculated the maximum effect of each threat. Given the differences in how effects on adult survival were reported in the literature, we used three different approaches to assess impacts on survival. The first approach used the per cent of the population negatively affected by the threat (sometimes accounting for detectability, e.g. Zimmerling et al. Citation2013), such as the proportion of the population that died (8 of 25 studies or 32%). In these cases, we expressed the maximum effect as the per cent of carcasses compared to the total population size. The second approach used differences in recapture rates determined with a (generalized) linear (mixed) model or Chi-square test (4 studies or 16%). The third approach used differences in annual or daily survival rates determined with a capture–mark–recapture model (13 studies or 52%). For these latter two approaches, we expressed the maximum effect as the difference in survival between highest (when the threat had no or little effect) and lowest values (when the threat had the greatest effect) for each study.
For all approaches, if the paper provided differences in survival for different groups (e.g. sexes or populations), then we provided a range of values for the maximum effect of the threat on survival. Also, if reported values showed no or negligible effects on survival (defined by authors without data provided) we indicate a value of 0%. Finally, when results were presented as figures, we used ImageJ 1.52a (Schneider et al. Citation2012) with the Figure Calibration plugin (http://www.astro.physik.uni-goettingen.de/~hessman/ImageJ/Figure_Calibration/ [accessed 19 October 2018]) to obtain values that were then used in our calculations above.
Within each section of the Results below, we provide a summary of the research on each threat, quantify the maximum effect of the threat, and discuss whether the threats have direct and/or indirect effects. Also, where possible, we provide an indication about whether effects of these threats may change in the future.
Results
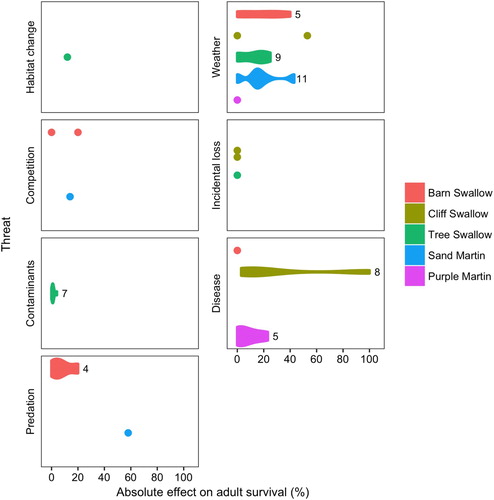
Our literature review revealed eight broad categories of threats ().
Table 1. Research documenting the maximum effects of different threats on adult survival or population for five swallow species.
Habitat change
We were only able to find one study relating habitat change to adult survival. Lower pond abundance during the breeding season was associated with a 12% decrease in adult survival for Tree Swallows ( and ) and was attributed to lower insect availability when ponds were less abundant (Clark et al. Citation2018). In general, while familiarity with habitats can confer higher survival for swallows (Brown et al. Citation2008), ultimately, habitat changes have indirect effects on adult survival.
Figure 2. The effects of seven different threats on adult survival for Barn Swallow, Cliff Swallow, Tree Swallow, Sand Martin, and Purple Martin. The effect of each threat on adult survival was measured as the proportion of the population affected, or the difference in highest and lowest return or survival rates. For species where we could only determine the effect on adult survival from one or two studies or subgroups within a study (e.g. sexes, populations), single data points represent the results. Otherwise the effect of each threat for each species is represented as a violin plot with the sample size indicated (i.e. number of separate survival estimates across all studies).
Weather
Many studies have examined relationships between adult survival in swallows and weather throughout the annual cycle, especially in Old World populations. Extended periods of cold temperatures and heavy rain or snowfall during the pre-breeding and migratory periods are associated with mass mortality events for Barn, Cliff, and Tree Swallows (Brown & Bomberger Brown Citation1998, Hess et al. Citation2008, Møller Citation2011), which may directly or indirectly kill as many as 53% of adults in the population ( and ). Precipitation in the pre-breeding period is also associated with up to a 15% decrease in survival for Tree Swallows (Clark et al. Citation2018), but hot-dry conditions during breeding can decrease survival up to 25% (Weegman et al. Citation2017). In contrast, higher levels of precipitation during breeding are associated with lower survival (up to 18%) for some populations of Sand Martins (Cowley & Siriwardena Citation2005, but see Norman & Peach Citation2013), but not Barn Swallows (Robinson et al. Citation2008). Higher levels of winter rainfall are related to increased survival for Barn Swallows and Sand Martins (Cowley & Siriwardena Citation2005, Norman & Peach Citation2013, Robinson et al. Citation2008). In general, when rainfall has an effect on survival, the maximum effect is a decrease in survival of 13–43% ().
Conditions associated with large-scale climate indices have mixed effects on adult survival. Two commonly studied indices, the El Niño Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO), measure climatic conditions along a warm/dry – cool/wet or warm/wet – cool/dry continuum, respectively. While there was no relationship between ENSO and Purple Martin survival in one region (Stutchbury et al. Citation2009), Barn and Tree Swallow survival is often correlated with ENSO and/or NAO with a potential decrease in survival of up to 29% (Balbontín et al. Citation2009, Clark et al. Citation2018, García-Pérez et al. Citation2014). For some populations, cool–dry conditions resulted in lower survival; however there was considerable variation in the relationship between ENSO or NAO and survival across the range of the species in these studies. For example, adult survival for Barn Swallows was related to ENSO and NAO in one population, but not in another population over 3000 km away (García-Pérez et al. Citation2014). The variability in these relationships may be due to differences in the effect of these indices on weather at different breeding and wintering locations or during migration (Balbontín et al. Citation2009, García-Pérez et al. Citation2014) and/or low migratory connectivity between breeding and wintering populations (e.g. breeding populations wintering in a broad geographic area) (García-Pérez et al. Citation2014, Stutchbury et al. Citation2009).
Since weather conditions can have large effects on adult survival (13–53%), it is possible that it is contributing to population declines. These effects may be direct and/or indirect as a result of both local and large-scale climatic conditions, for example, the direct effects due to mortality from exposure (Brown & Bomberger Brown Citation1998, Hess et al. Citation2008, Møller Citation2011) and the indirect effects often due to changes in insect availability (Clark et al. Citation2018, García-Pérez et al. Citation2014, Norman & Peach Citation2013). Clearly there is substantial species and geographic variability in how weather affects populations.
Competition
There is limited research on the effect of intraspecific competition on adult survival for swallows and none on interspecific competition. Density dependence can result in up to a 20% decrease in adult survival ( and ) for some Barn Swallow and Sand Martin populations (Norman & Peach Citation2013, Balbontín & Møller Citation2015, but see Paradis et al. Citation2002). These density-dependent effects are likely driven by competition between conspecifics for food (Norman & Peach Citation2013), suggesting that this is an indirect relationship between competition and adult survival. However, other threats may affect this relationship. For example, in Barn Swallows, when predation pressure was low, adult survival was lower at larger breeding colonies, but when predation pressure was high, survival was higher at larger breeding colonies (Balbontín & Møller Citation2015). Together, this suggests that density-dependent effects vary not only with population size but could interact with other threats.
Incidental loss
Several studies have examined the effects on incidental loss on swallow populations, although only one study quantifies the effects on adult survival. Swallows are frequently observed during road mortality surveys during the breeding season (Erritzoe et al. Citation2003, Bishop & Brogan Citation2013), particularly on roads with tree belts or hedgerows as these areas, presumably, provide good foraging sites (Grüebler et al. Citation2008, Orlowski Citation2005). Unlike predation, road mortality is more likely to affect individuals in good condition (Bujoczek et al. Citation2011), resulting in the loss of high quality individuals. In Europe, it is estimated that road mortality affects 1 million Barn Swallows annually, of which roughly one-third are adult (Orlowski Citation2005). In North America, however, selection on Cliff Swallow wing morphology has reduced road mortality (Brown & Bomberger Brown Citation2013), with less than 0.1% of adult Cliff Swallows in a population being hit by vehicles. There are other potential sources of incidental losses. A wind turbine study estimated that less than 0.01% of adult Tree Swallows were killed (Zimmerling et al. Citation2013). Collectively, though, the estimated impacts of incidental losses on adult survival or populations ( and ) are relatively small.
Contaminants
Numerous studies have documented high levels of organophosphates, organochlorines, polychlorinated biphenyls (PCBs), and metals with potentially lethal or sub-lethal effects in adult swallows (e.g. Baron et al. Citation1999, Burgess et al. Citation1999, Mora et al. Citation2002, Citation2012, Custer et al. Citation2007, Hawley et al. Citation2009). However, only three studies have examined effects of contaminants on adult survival. Two studies compared survival of female Tree Swallows breeding in areas with high levels of PCBs compared to the control areas. One reported a decrease in survival of up to 3.9% for swallows exposed to higher PCB levels (Custer et al. Citation2007), but there was no difference in the second study, possibly due to the lower environmental levels of PCBs (Custer et al. Citation2012). In the third study, there was an up to 1.1% decrease in survival for Tree Swallows exposed to high levels of mercury compared to control groups (Hallinger et al. Citation2011). Together these results suggest that the contaminants studied thus far have a much lower effect than other threats (), at least at levels recorded in these studies. The transfer of contaminants to swallows may be directly from their environment or indirectly through insect prey (Fernie et al. Citation2018, Haroune et al. Citation2015, Mora et al. Citation2002). These studies suggest that the effects of the examined contaminants on swallow populations are small.
Insect availability
Despite the importance of aerial insects as the only or primary food source, we were unable to find any research directly relating insect availability to adult survival. However, lower adult survival as a result of habitat change or weather was often attributed to those threats reducing insect availability (Norman & Peach Citation2013, García-Pérez et al. Citation2014, Weegman et al. Citation2017, Clark et al. Citation2018). The effects of habitat change and weather on insect abundance are well studied. For example, habitat changes from low landscape-level homogeneity (often associated with low intensity agricultural land use, e.g. pastures and hayfields separated by hedgerows) to high landscape-level homogeneity (often associated with intensive agricultural practices, e.g. corn and soy production) reduces insect abundance (Benton et al. Citation2002, Grüebler et al. Citation2008, Paquette et al. Citation2013) and alters diet composition (Orłowski & Karg Citation2013). These intensive agricultural practices often entail increased pesticide use, which affects the abundance of aerial insects (Morrissey et al. Citation2015, Pisa et al. Citation2015, Van Dijk et al. Citation2013) and possibly leads to changes in diet shown in other aerial insectivores (English et al. Citation2017). Also, neonicotinoids are implicated in the decline of one Barn Swallow population (Hallmann et al. Citation2014). Additionally, reduced insect availability during breeding occurs during periods of cold temperatures, rainfall, and high winds (Nooker et al. Citation2005, Møller Citation2013, Winkler et al. Citation2013). Since declines in aerial insect populations have been severe in some regions (Hallmann et al. Citation2017), it is likely that reduced food availability is contributing to population declines for swallows, but the magnitude of these effects is unknown.
Disease
Many studies have documented the presence of various bacteria, viruses, and parasites (i.e. arthropods, flatworms, nematodes, and protozoa) in adult swallows (Brown et al. Citation2007, Caron et al. Citation2014, Davidar & Morton Citation2006, Heneberg et al. Citation2011, Stenkat et al. Citation2014, Von Ronn et al. Citation2015). These infections often had either beneficial or negligible effects on adult survival for swallows. Feather mites increase Cliff Swallow survival during breeding, presumably through the removal of old preening oil and/or removal or competition with other infectious agents on feathers (Brown et al. Citation2006), but have no effect on Barn Swallows (Pap et al. Citation2005). Neither the increased prevalence of West Nile Virus nor chronic infection with a protozoan Haemoptroteus progenei has an effect on Purple Martin survival (Davidar & Morton Citation1993, Stutchbury et al. Citation2009), however, the initial protozoan infection may reduce survival (Davidar & Morton Citation1993). Infection with an unidentified filarial nematode is associated with lower survival in Purple Martins, and, although uncommon, most adults with both infections died (Davidar & Morton Citation2006). Collectively, these findings suggest that disease has a wide range of direct effects on adult survival () and when they are negative, the effects can result in up to a 5–23% decrease in survival, depending on the specific infection ().
Predation
Many studies have identified a diverse group of predators (Ash Citation1995, Bijlsma & van den Brink Citation2005, Møller & Nielsen Citation1997, O’Brien et al. Citation2014, Rebecca Citation2004), that, in some cases, can directly result in large losses (up to 58%, and ) of adult swallows (Young Citation1994). Mortality from predation may also be female biased (Møller & Nielsen Citation1997) which further reduces fecundity and contributes to population declines. The effects of predation on adult survival are, however, generally localized to areas where predators learn to exploit an abundant food source (Ash Citation1995, Rebecca Citation2004, Young Citation1994) and are unlikely to represent broader-scale population-level effects, nor contribute in a substantial manner to population declines.
Discussion
Our literature review suggests that the effects of weather conditions on adult survival in swallows are well studied, resulting in the most conclusive information of all the threats considered. Adverse or extreme weather conditions can result in a 13–53% decrease in adult survival for swallows, depending on the specific weather conditions. Of these conditions, cold snaps or precipitation during the spring pre-breeding period and autumn migration (Brown & Bomberger Brown Citation1996, Citation1998, Hess et al. Citation2008, Møller Citation2011, Clark et al. Citation2018) and reduced rainfall during the winter (Cowley & Siriwardena Citation2005, García-Pérez et al. Citation2014, Norman & Peach Citation2013, Robinson et al. Citation2008, Szép Citation1995a, Citation1995b) had the most consistent and negative effects on survival. The mechanism behind lower survival for the latter two relationships is likely indirect, with these weather conditions negatively affecting insect abundance, which, in turn, affects survival.
Although there is strong support for the effects of weather on adult survival in swallows, perhaps, in part, due to the relative ease through which this threat can be studied, there are two important caveats to consider in relation to population declines. First, much of this research, especially during the winter, is on Palearctic-African populations, rather than Nearctic-Neotropical populations. Given the differences in precipitation patterns in Africa, compared to the Neotropics, it is possible that the relationships between winter rainfall and adult survival may not be the same for wintering Nearctic-Neotropical swallow populations. Second, although weather patterns do affect adult survival, this threat can only result in population declines if the frequency of these extreme weather events is increasing. Climate change is associated with changing weather patterns, including more extreme weather events (Hayhoe et al. Citation2007, Djomou et al. Citation2015, Screen et al. Citation2015, Ummenhofer & Meehl Citation2017), however, no studies have examined long-term data on adult survival for swallows and population trends in relation to the frequency of severe weather conditions. Therefore, it is unknown if declining population trends, in relation to adult survival, are driven by changes in weather patterns.
We also observed that for some Barn Swallow, Sand Martin, and Purple Martin populations, competition, disease, and predation can have large negative effects on survival, e.g. maximum 20%, 22.8%, and 58%, respectively (). These large effects are often localized to different populations and/or under specific environmental conditions. Furthermore, determining whether these effects are driven by natural or anthropogenic sources is difficult. Therefore, it is important to be careful not to generalize these results across a broader region. Despite these limitations in our findings, we consider that competition, disease, and predation, especially if these factors are increasing with habitat and climate change, could have additive effects on other threats and could be important contributors to lower survival in some regions.
Clearly our findings are limited by several factors. First, we lacked information for some threats, and for different species, particularly throughout their geographic ranges. Second, the approach to measuring survival and threats varied across studies. Survival was measured in terms of the per cent of the population found dead, and in differences in recapture or apparent survival rates. The former two approaches rarely account for detectability, and the latter two cannot distinguish between true survival and permanent emigration. Threats were also not measured on a consistent scale, and many studies, particularly on weather, used different indices to examine weather conditions. Finally, the geographic scale and sample sizes all varied across different studies. Therefore, while our findings provide insight into the known relationships between threats and adult survival, we recognize that these factors limit the scope of our conclusions, particularly for poorly studied threats. However, our review provides important directions for future research to address knowledge gaps and these limitations.
Future research
We were unable to find any papers relating adult survival to insect availability and very little information on the effects of habitat change, incidental loss, and contaminants on adult survival. Three of these threats (i.e. habitat change, contaminants, and insect availability) have received extensive attention in the literature for their effects on fecundity in Barn and Tree Swallows (McCarty & Winkler Citation1999, Bishop et al. Citation2000, Ambrosini et al. Citation2002, Nooker et al. Citation2005, Fredricks et al. Citation2012, Winkler et al. Citation2013, Rioux Paquette et al. Citation2014, Schifferli et al. Citation2014, Imlay et al. Citation2017), but very little is known about their relationships with adult survival. This lack of information is particularly concerning as reductions in insect availability (possibly as a result of habitat change, weather, and/or contaminants) is considered to be the most likely driver of population declines for aerial insectivores (Nebel et al. Citation2010). Clearly, this represents a key knowledge gap for aerial insectivore declines as a whole. Since demands on food are highest during the breeding season and autumn migration (Kelly et al. Citation2013), reductions in food availability during these times could have a disproportionate effect on populations and should be investigated.
We also found that for most threats, except weather, there was very little information on their effects during the non-breeding period. This represents another key knowledge gap as non-breeding conditions are strongly related to population trends for several species (Ambrosini et al. Citation2011, Ockendon et al. Citation2014, Sicurella et al. Citation2016). Additionally, for other migratory birds, adult survival rates are lowest during migration (Klaassen et al. Citation2014, Sillett & Holmes Citation2002), suggesting that adults may be more vulnerable to threats during this stage of the annual cycle than other stages. The lack of information on threats throughout this period may be partly due to knowledge gaps on migration routes and winter locations for some non-breeding populations of Nearctic-Neotropical swallows. However, this knowledge gap is quickly being addressed with tracking technologies and stable isotopes (Fraser et al. Citation2012, Hobson et al. Citation2015, Szép et al. Citation2017, Imlay et al. Citation2018a, Knight et al. Citation2018), and, in the future, it may be possible to estimate survival during different periods of the annual cycle. With an improved understanding of these movements, threats during these times can be identified and related to survival.
Conclusion
Recent research shows that adult survival is a key factor in population trends for some populations of swallows. In our literature review, we identified many threats to adult survival for several declining swallow species and compared the effects of these threats. This review highlights that the effects of threats may across the geographic range of a species and among species. Therefore, caution is clearly warranted when trying to relate environmental conditions during one stage of the annual cycle for a population to broad-scale population declines across a much larger geographic area. Especially as Michel et al. (Citation2016) suggests that populations are affected by multiple threats that vary throughout their range. However, our findings do suggest that at least one well-studied threat, adverse weather, often has similar effects across most swallow populations and species.
As a result of human activities, the effects of at least seven threats (all except competition) are predicted to increase in both their occurrence and severity in the future (Bommarco et al. Citation2013, Farmer et al. Citation2008, Hansen et al. Citation2006, Martinez & Merino Citation2011, Tilman et al. Citation2001, Dulac Citation2013). As threats increase over time and space, they will have a greater effect on adult survival. Also, because many threats are inter-related, their effects on survival are likely to be synergistic rather than additive. For example, adverse weather conditions due to climate change may lead to further habitat loss (beyond that as a result of other human activities) (Mantyka-Pringle et al. Citation2012) and increased contaminent and disease loads (Hallinger & Cristol Citation2011, Møller Citation2011). These synergistic effects could result in an even greater effect on adult survival rates than if the effects were additive, possibly leading to lower adult survival rates in the future.
Developing effective conservation strategies requires understanding when and where species experience threats during the annual cycle and determining how these threats affect demographic processes. However, most research on threats focuses on the breeding grounds (Faaborg et al. Citation2010, Marra et al. Citation2015) and on fecundity rather than adult survival. In doing so, conservation efforts may focus on addressing threats that only affect populations during one stage of the annual cycle. Furthermore, for migratory birds, this could result in conservation efforts that do not target the demographic processes that have the greatest effect on population trends.
Acknowledgements
We thank K. Hobson, P. Marra, J. Nocera, L. Philmore, R. Ronconi, and two anonymous reviewers for feedback on an earlier draft of this manuscript.
Additional information
Funding
References
- Ambrosini, R., Bolzern, A.M., Canova, L., Arieni, S., Møller, A.P. & Saino, N. 2002. The distribution and colony size of Barn Swallows in relation to agricultural land use. J. Appl. Ecol. 39: 524–534. doi: 10.1046/j.1365-2664.2002.00721.x
- Ambrosini, R., Orioli, V., Massimino, D. & Bani, L. 2011. Identification of putative wintering areas and ecological determinants of population dynamics of common House-Martin (Delichon urbicum) and Common Swift (Apus apus) breeding in northern Italy. Avian Conserv. Ecol. 6: 3.
- Ash, J. 1995. An immense swallow roost in Nigeria. BTO News 200: 8–9.
- Baillie, S.R. & Peach, W.J. 1992. Population limitation in Palearctic-African migrant passerines. Ibis 134: 120–132. doi: 10.1111/j.1474-919X.1992.tb04742.x
- Balbontín, J. & Møller, A.P. 2015. Environmental conditions during early life accelerate the rate of senescence in a short-lived passerine bird. Ecology 96: 948–959. doi: 10.1890/14-1274.1
- Balbontín, J., Møller, A.P., Hermosell, I.G., Marzal, A., Reviriego, M. & De Lope, F. 2009. Divergent patterns of impact of environmental conditions on life history traits in two populations of a long-distance migratory bird. Oecologia. 159: 859–872. doi: 10.1007/s00442-008-1267-8
- Baron, L.A., Sample, B.E. & Suter IIG.W. 1999. Ecological risk assessment in a large river-reservoir: 5. Aerial insectivorous wildlife. Environ. Toxicol. Chem. 18: 621–627.
- Benton, T.G., Bryant, D.M., Cole, L. & Crick, H.Q.P. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 39: 673–687. doi: 10.1046/j.1365-2664.2002.00745.x
- Bijlsma, R.G. & van den Brink, B. 2005. A Barn Swallow Hirundo rustica roost under attack: timing and risks in the presence of African Hobbies. Falco Cuvieri. 93: 37–48.
- Bishop, C.A. & Brogan, J.M. 2013. Estimates of avian mortality attributed to vehicle collisions in Canada. Avian Conserv. Ecol. 8: 2.
- Bishop, C.A., Collins, B., Mineau, P., Burgess, N.M., Read, W.F. & Risley, C. 2000. Reproduction of cavity-nesting birds in pesticide-sprayed apple orchards in southern Ontario, Canada, 1988–1994. Environ. Toxicol. Chem. 19: 588–599.
- Bommarco, R., Kleijn, D. & Potts, S.G. 2013. Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28: 230–238. doi: 10.1016/j.tree.2012.10.012
- Brown, C.R. & Bomberger Brown, M. 1996. Coloniality in the Cliff Swallow: the effect of group size on social behavior. University of Chicago Press, Chicago, IL.
- Brown, C.R. & Bomberger Brown, M. 1998. Intense natural selection on body size and wing and tail asymmetry in Cliff Swallows during severe weather. Evolution (NY) 52: 1461–1475. doi: 10.1111/j.1558-5646.1998.tb02027.x
- Brown, C.R. & Bomberger Brown, M. 1999. Barn Swallow (Hirundo rustica), version 2.0, The Birds of North America (P. G. Rodewald, Ed.).
- Brown, C.R. & Bomberger Brown, M. 2013. Where has all the road kill gone? Curr. Biol. 23: R233–R234. doi: 10.1016/j.cub.2013.02.023
- Brown, C.R., Bomberger Brown, M. & Brazeal, K.R. 2008. Familiarity with breeding habitat improves daily survival in colonial Cliff Swallows. Anim. Behav. 76: 1201–1210. doi: 10.1016/j.anbehav.2008.03.028
- Brown, C.R., Bomberger Brown, M., Moore, A.T. & Komar, N. 2007. Bird movement predicts Buggy Creek virus infection in insect vectors. Vector Borne Zoonotic Dis. 7: 304–314. doi: 10.1089/vbz.2006.0646
- Brown, C.R., Bomberger Brown, M., Pyle, P. & Patten, M.A. 2017. Cliff Swallow (Petrochelidon pyrrhonata), version 3.0, The Birds of North America (P. G. Rodewald, Ed.).
- Brown, C.R., Brazeal, K.R., Strickler, S.A. & Bomberger Brown, M. 2006. Feather mites are positively associated with daily survival in Cliff Swallows. Can. J. Zool. 84: 1307–1314. doi: 10.1139/z06-110
- Brown, C.R. & Tarof, S. 2013. Purple Martin (Progne subis), The Birds of North America (P. G. Rodewald, Ed.).
- Buehler, D.A., Giocomo, J.J., Jones, J., Hamel, P.B., Rogers, C.M., Beachy, T.A., Varble, D.W., Nicholson, C.P., Roth, K.L., Barg, J., Robertson, R.J., Robb, J.R. & Islam, K. 2008. Cerulean Warbler reproduction, survival, and models of population decline. J. Wildl. Manage. 72: 646–653. doi: 10.2193/2006-339
- Bujoczek, M., Ciach, M. & Yosef, R. 2011. Road-kills affect avian population quality. Biol. Conserv. 144: 1036–1039. doi: 10.1016/j.biocon.2010.12.022
- Burgess, N., Hunt, K., Bishop, C. & Weseloh, D. 1999. Cholinesterase inhibition in Tree Swallows (Tachycineta bicolor) and Eastern Bluebirds (Sialia sialis) exposed to organophosphorus insecticides in apple orchards in Ontario, Canada. Environ. Toxicol. Chem. 18: 708–716. doi: 10.1002/etc.5620180417
- Caron, A., Grosbois, V., Etter, E., Gaidet, N. & de Garine-Wichatitsky, M. 2014. Bridge hosts for avian influenza viruses at the wildlife/domestic interface: an eco-epidemiological framework implemented in Southern Africa. Prev. Vet. Med. 117: 590–600. doi: 10.1016/j.prevetmed.2014.09.014
- Clark, R.G., Winkler, D.W., Dawson, R.D., Shutler, D., Hussell, D.J.T., Lombardo, M.P., Thorpe, P.A., Dunn, P.O. & Whittingham, L.A. 2018. Geographic variation and environmental correlates of apparent survival rates in adult tree swallows Tachycineta bicolor. J. Avian Biol. e01659. doi: 10.1111/jav.01659.
- Cowley, E. 1983. Multi-brooding and mate infidelity in the Sand Martin. Bird Study 30: 1–7. doi: 10.1080/00063658309476768
- Cowley, E. & Siriwardena, G.M. 2005. Long-term variation in survival rates of Sand Martins Riparia riparia: dependence on breeding and wintering ground weather, age and sex, and their population consequences. Bird Study 52: 237–251. doi: 10.1080/00063650509461397
- Cox, A.R., Robertson, R.J., Fedy, B.C., Rendell, W.B. & Bonier, F. 2018. Demographic drivers of local population decline in Tree Swallows (Tachycineta bicolor) in Ontario, Canada. Condor. 120: 842–851. doi: 10.1650/CONDOR-18-42.1
- Custer, C.M., Custer, T.W. & Hines, J.E. 2012. Adult tree swallow survival on the polychlorinated biphenyl-contaminated Hudson River, New York, USA, between 2006 and 2010. Environ. Toxicol. Chem. 31: 1788–1792. doi: 10.1002/etc.1894
- Custer, C.M., Custer, T.W., Hines, J.E., Nichols, J.D. & Dummer, P.M. 2007. Adult Tree Swallow (Tachycineta bicolor) survival on the polychlorinated biphenyl-contaminated Housatonic River, Massachusetts, USA. Environ. Toxicol. Chem. 26: 1056–1065. doi: 10.1897/06-337R.1
- Davidar, P. & Morton, E.S. 1993. Living with parasites: prevalence of a blood parasite and its effect on survivorship in the Purple Martin. Auk 110: 109–116.
- Davidar, P. & Morton, E.S. 2006. Are multiple infections more severe for Purple Martins (Progne subis) than single infections? Auk 123: 141–147. doi: 10.1093/auk/123.1.141
- Djomou, Z.Y., Monkam, D. & Chamani, R. 2015. Characterization of climatic zones, variability and trend in Northern Africa. Clim. Dyn. 44: 3481–3491. doi: 10.1007/s00382-015-2485-5
- Dulac, J. 2013. Global land transport infrastructure requirements: estimating road and railway infrastructure capacity and costs to 2050. International Energy Agency. https://doi.org/10.1007/s12469-014-0089-9.
- Dulvy, N.K., Fowler, S.L., Musick, J.A., Cavanagh, R.D., Kyne, P.M., Harrison, L.R., Carlson, J.K., Davidson, L.N.K., Fordham, S.V., Francis, M.P., Pollock, C.M., Simpfendorfer, C.A., Burgess, G.H., Carpenter, K.E., Compagno, L.J.V., Ebert, D.A., Gibson, C., Heupel, M.R., Livingstone, S.R., Sanciangco, J.C., Stevens, J.D., Valenti, S. & White, W.T. 2014. Extinction risk and conservation of the world’s sharks and rays. Elife 3: e00590. doi: 10.7554/eLife.00590
- English, P.A., Nocera, J.J., Pond, B.A. & Green, D.J. 2017. Habitat and food supply across multiple spatial scales influence the distribution and abundance of a nocturnal aerial insectivore. Landsc. Ecol. 32: 343–359. doi: 10.1007/s10980-016-0454-y
- Erritzoe, J., Mazgajski, T.D. & Rejt, Ł. 2003. Bird casualties on European roads – a review. Acta Ornithol. 38: 77–93. doi: 10.3161/068.038.0204
- Faaborg, J., Holmes, R.T., Anders, A.D., Bildstein, K.L., Dugger, K.M., GauthreauxJr.S.A., Heglund, P., Hobson, K.A., Jahn, A.E., Johnson, D.H., Latta, S.C., Levey, D.J., Marra, P.P., Merkord, C.L., Nol, E., Rothstein, S.I., Sherry, T.W., Sillett, T.S., Thompson III, F.R. & Warnock, N. 2010. Conserving migratory land birds in the New World: Do we know enough? Ecol. Appl. 20: 398–418. doi: 10.1890/09-0397.1
- Farmer, C.J., Bell, R.J., Drolet, B., Goodrich, L.J., Greenstone, E., Grove, D., Hussell, D.J.T., Mizrahi, D., Nicoletti, F.J. & Sodergren, J. 2008. Trends in autumn counts of migratory raptors in northeastern North America, 1974–2004. State North Am. Birds Prey. Ser. Ornithol. 3: 179–215.
- Fassbinder-Orth, C.A., Barak, V.A. & Brown, C.R. 2013. Immune responses of a native and an invasive bird to Buggy Creek Virus (Togaviridae: Alphavirus) and its arthropod vector, the swallow bug (Oeciacus vicarius). PLoS One 8: e58045. doi: 10.1371/journal.pone.0058045
- Fernie, K.J., Marteinson, S.C., Chen, D., Eng, A., Harner, T., Smits, J.E.G. & Soos, C. 2018. Elevated exposure, uptake and accumulation of polycyclic aromatic hydrocarbons by nestling tree swallows (Tachycineta bicolor) through multiple exposure routes in active mining-related areas of the Athabasca oil sands region. Sci. Total Environ. 624: 250–261. doi: 10.1016/j.scitotenv.2017.12.123
- Fletcher, R.J., Koford, R.R. & Seaman, D.A. 2006. Critical demographic parameters for declining songbirds breeding in restored grasslands. J. Wildl. Manage. 70: 145–157. doi: 10.2193/0022-541X(2006)70[145:CDPFDS]2.0.CO;2
- Fraser, K.C., Stutchbury, B.J.M., Silverio, C., Kramer, P.M., Barrow, J., Newstead, D., Mickle, N., Cousens, B.F., Lee, C.J., Morrison, D.M., Shaheen, T., Mammenga, P., Applegate, K. & Tautin, J. 2012. Continent-wide tracking to determine migratory connectivity and tropical habitat associations of a declining aerial insectivore. Proc. R. Soc. B Biol. Sci. 279: 4901–4906. doi: 10.1098/rspb.2012.2207
- Fredricks, T.B., Zwiernik, M.J., Seston, R.M., Coefield, S.J., Glaspie, C.N., Tazelaar, D.L., Kay, D.P., Newsted, J.L. & Giesy, J.P. 2012. Reproductive success of three passerine species exposed to dioxin-like compounds near Midland, Michigan, USA. Ecotoxicology 21: 1145–1154. doi: 10.1007/s10646-012-0869-4
- García-Pérez, B., Hobson, K.A., Albrecht, G., Cadman, M.D. & Salvadori, A. 2014. Influence of climate on annual survival of Barn Swallows (Hirundo rustica) breeding in North America. Auk 131: 351–362. doi: 10.1642/AUK-13-145.1
- Gardali, T., Ballard, G., Nur, N. & Geupel, G.R. 2000. Demography of a declining population of Warbling Vireos in coastal California. Condor 102: 601–609. doi: 10.1093/condor/102.3.601
- Garrison, B.A. 1999. Bank Swallow (Riparia riparia), version 2.0, The Birds of North America (P. G. Rodewald, Ed.).
- Green, R.E. 1999. Applications of large-scale studies of demographic rates to bird conservation. Bird Study 46: S279–S288. doi: 10.1080/00063659909477255
- Grüebler, M.U., Morand, M. & Naef-Daenzer, B. 2008. A predictive model of the density of airborne insects in agricultural environments. Agric. Ecosyst. Environ. 123: 75–80. doi: 10.1016/j.agee.2007.05.001
- Hallinger, K.K., Cornell, K.L., Brasso, R.L. & Cristol, D.A. 2011. Mercury exposure and survival in free-living Tree Swallows (Tachycineta bicolor). Ecotoxicology 20: 39–46. doi: 10.1007/s10646-010-0554-4
- Hallinger, K.K. & Cristol, D.A. 2011. The role of weather in mediating the effect of mercury exposure on reproductive success in Tree Swallows. Ecotoxicology 20: 1368–1377. doi: 10.1007/s10646-011-0694-1
- Hallmann, C.A., Foppen, R.P.B., van Turnhout, C.A.M., de Kroon, H. & Jongejans, E. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511: 341–343. doi: 10.1038/nature13531
- Hallmann, C.A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., Stenmans, W., Muller, A., Sumser, H., Horren, T., Goulson, D. & de Kroon, H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12: e0185809. doi: 10.1371/journal.pone.0185809
- Hansen, J.E., Sato, M., Ruedy, R., Lo, K., Lea, D.W. & Medina-Elizade, M. 2006. Global temperature change. Proc. Natl. Acad. Sci. 103: 14288–14293. doi: 10.1073/pnas.0606291103
- Haroune, L., Cassoulet, R., Lafontaine, M.P., Bélisle, M., Garant, D., Pelletier, F., Cabana, H. & Bellenger, J.P. 2015. Liquid chromatography-tandem mass spectrometry determination for multiclass pesticides from insect samples by microwave-assisted solvent extraction followed by a salt-out effect and micro-dispersion purification. Anal. Chim. Acta. 891: 160–170. doi: 10.1016/j.aca.2015.07.031
- Hayhoe, K., Wake, C.P., Huntington, T.G., Luo, L., Schwartz, M.D., Sheffield, J., Wood, E., Anderson, B., Bradbury, J., DeGaetano, A., Troy, T.J. & Wolfe, D. 2007. Past and future changes in climate and hydrological indicators in the US Northeast. Clim. Dyn. 28: 381–407.
- Hawley, D.M., Hallinger, K.K. & Cristol, D.A. 2009. Compromised immune competence in free-living Tree swallows exposed to mercury. Ecotoxicology 18: 499–503. doi: 10.1007/s10646-009-0307-4
- Heldbjerg, H. & Fox, T. 2008. Long-term population declines in Danish trans-Saharan migrant birds. Bird Study 55: 267–279. doi: 10.1080/00063650809461532
- Heneberg, P., Szép, T., Iciek, T. & Literák, I. 2011. Collyriclosis in central European hirundines. Parasitol. Res. 109: 699–706. doi: 10.1007/s00436-011-2301-z
- Hess, P.J., Zenger, C.G. & Schmidt, R.A. 2008. Weather-related Tree Swallow mortality and reduced nesting effort. Northeast. Nat. 15: 630–631. doi: 10.1656/1092-6194-15.4.630
- Hobson, K.A., Kardynal, K.J., Van Wilgenburg, S.L., Albrecht, G., Salvadori, A., Cadman, M.D., Liechti, F. & Fox, J.W. 2015. A continent-wide migratory divide in North American breeding Barn Swallows (Hirundo rustica). PLoS One 10: e0129340. doi: 10.1371/journal.pone.0129340
- Imlay, T.L., Hobson, K.A., Roberto-Charron, A. & Leonard, M.L. 2018a. Wintering areas, migratory connectivity and habitat fidelity of three declining Nearctic-Neotropical migrant swallows. Anim. Migr. 5: 1–16. doi: 10.1515/ami-2018-0001
- Imlay, T.L., Mann, H.A.R. & Leonard, M.L. 2017. No effect of insect abundance on nestling survival and mass in Barn, Cliff and Tree swallows. Avian Conserv. Ecol. 12: 19. doi: 10.5751/ACE-01092-120219
- Imlay, T.L., Mills Flemming, J., Saldanha, S., Wheelwright, N.T. & Leonard, M.L. 2018b. Breeding phenology and performance for four swallows over 57 years: relationships with temperature and precipitation. Ecosphere. 9: e02166. doi: 10.1002/ecs2.2166
- Inger, R., Gregory, R., Duffy, J.P., Stott, I., Voříšek, P. & Gaston, K.J. 2015. Common European birds are declining rapidly while less abundant species’ numbers are rising. Ecol. Lett. 18: 28–36. doi: 10.1111/ele.12387
- Kelly, J.F., Bridge, E.S., Frick, W.F. & Chilson, P.B. 2013. Ecological energetics of an abundant aerial insectivore, the Purple Martin. PLoS One 8: e76616. doi: 10.1371/journal.pone.0076616
- Klaassen, R.H.G., Hake, M., Strandberg, R., Koks, B.J., Trierweiler, C., Exo, K.M., Bairlein, F. & Alerstam, T. 2014. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 83: 176–184. doi: 10.1111/1365-2656.12135
- Knight, S.M., Bradley, D.W., Clark, R.G., Gow, E.A., Bélisle, M., Berzins, L.L., Blake, T., Bridge, E.S., Burke, L., Dawson, R.D., Dunn, P.O., Garant, D., Holroyd, G.L., Hussell, D.J.T., Lansdorp, O., Laughlin, A.J., Leonard, M.L., Pelletier, F., Shutler, D., Siefferman, L., Taylor, C.M., Trefry, H.E., Vleck, C.M., Vleck, D., Winkler, D.W., Whittingham, L.A. & Norris, D.R. 2018. Constructing and evaluating a continent-wide migratory songbird network across the annual cycle. Ecol. Monogr. 88: 445–460. doi: 10.1002/ecm.1298
- Link, W.A. & Sauer, J.R. 2002. A hierarchical model of population change with application to Cerulean Warblers. Ecology. 83: 2832–2840. doi: 10.1890/0012-9658(2002)083[2832:AHAOPC]2.0.CO;2
- Mantyka-Pringle, C.S., Martin, T.G. & Rhodes, J.R. 2012. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob. Chang. Biol. 18: 1239–1252. doi: 10.1111/j.1365-2486.2011.02593.x
- Marra, P., Cohen, E.B., Loss, S.R., Rutter, J.E. & Tonra, C.M. 2015. A call for full annual cycle research in animal ecology. Biol. Lett. 11: 20150552. doi: 10.1098/rsbl.2015.0552
- Martinez, J. & Merino, S. 2011. Host–parasite interactions under extreme climatic conditions. Curr. Zool. 57: 390–405. doi: 10.1093/czoolo/57.3.390
- McCarty, J.P. & Winkler, D.W. 1999. Relative importance of environmental variables in determining the growth of nestling Tree Swallows Tachycineta bicolor. Ibis 141: 286–296. doi: 10.1111/j.1474-919X.1999.tb07551.x
- Michel, N.L., Smith, A.C., Clark, R.G., Morrissey, C.A. & Hobson, K.A. 2016. Differences in spatial synchrony and interspecific concordance inform guild-level population trends for aerial insectivorous birds. Ecography 39: 774–786. doi: 10.1111/ecog.01798
- Møller, A.P. 2011. Behavioral and life history responses to extreme climatic conditions: studies on a migratory songbird. Curr. Zool. 57: 351–362. doi: 10.1093/czoolo/57.3.351
- Møller, A.P. 2013. Long-term trends in wind speed, insect abundance and ecology of an insectivorous bird. Ecosphere. 4: 1–11. doi: 10.1890/ES12-00310.1
- Møller, A.P. & Nielsen, J. 1997. Differential predation cost of a secondary sexual character: Sparrowhawk predation on Barn Swallows. Anim. Behav. 54: 1545–1551. doi: 10.1006/anbe.1997.9998
- Mora, M., Sericano, J. & Baxter, C. 2012. Swallows as indicators of environmental pollution of the Rio Grande/Rio Bravo basin: are persistent organic pollutants a concern? Arch. Environ. Contam. Toxicol. 62: 512–518. doi: 10.1007/s00244-011-9718-3
- Mora, M., Skiles, R., McKinney, B., Paredes, M., Buckler, D., Papoulias, D. & Klein, D. 2002. Environmental contaminants in prey and tissues of the Peregrine Falcon in the Big Bend Region, Texas, USA. Environ. Pollut. 116: 169–176. doi: 10.1016/S0269-7491(01)00207-X
- Morrissey, C.A., Mineau, P., Devries, J.H., Sanchez-Bayo, F., Liess, M., Cavallaro, M.C. & Liber, K. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ. Int. 74: 291–303. doi: 10.1016/j.envint.2014.10.024
- Murphy, M.T. 2001. Source-sink dynamics of a declining Eastern Kingbird population and the value of sink habitats. Conserv. Biol. 15: 737–748. doi: 10.1046/j.1523-1739.2001.015003737.x
- Nebel, S., Mills, A., McCracken, J.D. & Taylor, P.D. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 5: 1. doi: 10.5751/ACE-00358-050101
- Nooker, J.K., Dunn, P.O. & Whittingham, L.A. 2005. Effects of food abundance, weather, and female condition on reproduction in Tree Swallows (Tachycineta bicolor). Auk 122: 1225–1238. doi: 10.1093/auk/122.4.1225
- Norman, D. & Peach, W.J. 2013. Density-dependent survival and recruitment in a long-distance Palaearctic migrant, the Sand Martin Riparia riparia. Ibis 155: 284–296. doi: 10.1111/ibi.12036
- O’Brien, G.C., Jacobs, F., Evans, S.W. & Smit, N.J. 2014. First observation of African tigerfish Hydrocynus vittatus predating on Barn Swallows Hirundo rustica in flight. J. Fish Biol. 84: 263–266. doi: 10.1111/jfb.12278
- Ockendon, N., Johnston, A. & Baillie, S.R. 2014. Rainfall on wintering grounds affects population change in many species of Afro-Palaearctic migrants. J. Ornithol. 155: 905–917. doi: 10.1007/s10336-014-1073-5
- Orlowski, G. 2005. Factors affecting road mortality of the Barn Swallows Hirundo rustica in farmland. Acta Ornithol. 40: 117–125. doi: 10.3161/068.040.0207
- Orłowski, G. & Karg, J. 2013. Partitioning the effects of livestock farming on the diet of an aerial insectivorous passerine, the Barn Swallow Hirundo rustica. Bird Study 60: 111–123. doi: 10.1080/00063657.2012.748717
- Paleczny, M., Hammill, E., Karpouzi, V. & Pauly, D. 2015. Population trend of the world’s monitored seabirds, 1950–2010. PLoS One 10: e0129342. doi: 10.1371/journal.pone.0129342
- Pap, P., Tokolyi, J. & Szep, T. 2005. Host-symbiont relationship and abundance of feather mites in relation to age and body condition of the Barn Swallow (Hirundo rustica): an experimental study. Can. J. Zool. 83: 1059–1066. doi: 10.1139/z05-100
- Paradis, E., Baillie, S.R., Sutherland, W.J. & Gregory, R.D. 2002. Exploring density-dependent relationships in demographic parameters in populations of birds at a large spatial scale. Oikos 97: 293–307. doi: 10.1034/j.1600-0706.2002.970215.x
- Perlut, N.G., Strong, A.M., Donovan, T.M. & Buckley, N.J. 2008. Regional population viability of grassland songbirds: effects of agricultural management. Biol. Conserv. 141: 3139–3151. doi: 10.1016/j.biocon.2008.09.011
- Piland, N.C. & Winkler, D.W. 2015. Tree Swallow frugivory in winter. Southeast. Nat. 14: 123–136. doi: 10.1656/058.014.0117
- Pisa, L.W., Amaral-Rogers, V., Belzunces, L.P., Bonmatin, J.M., Downs, C.A., Goulson, D., Kreutzweiser, D.P., Krupke, C., Liess, M., McField, M., Morrissey, C.A., Noome, D.A., Settele, J., Simon-Delso, J., Stark, J.D., Van der Sluijs, J.P., Van Dyck, M. & Wiemers, M. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 22: 68–102. doi: 10.1007/s11356-014-3471-x
- Purcell, S.W., Polidoro, B.A., Hamel, J.-F., Gamboa, R.U. & Mercier, A. 2014. The cost of being valuable: predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proc. R. Soc. B. 281: 20133296. doi: 10.1098/rspb.2013.3296
- Rappole, J.H. & McDonald, M.V. 1994. Cause and effect in population declines of migratory birds. Auk 111: 652–660.
- Rebecca, G.W. 2004. Forest nesting Merlin apparently specialising on Barn Swallows. Scott. Birds 24: 46–48.
- Rioux Paquette, S., Garant, D., Pelletier, F. & Bélisle, M. 2013. Seasonal patterns in Tree Swallow prey (Diptera) abundance are affected by agricultural intensification. Ecol. Appl. 123: 122–133. doi: 10.1890/12-0068.1
- Rioux Paquette, S., Pelletier, F., Garant, D. & Bélisle, M. 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proc. R. Soc. B. 281: 20140649. doi: 10.1098/rspb.2014.0649
- Robinson, R.A., Balmer, D.E. & Marchant, J.H. 2008. Survival rates of hirundines in relation to British and African rainfall. Ringing Migr. 24: 1–6. doi: 10.1080/03078698.2008.9674375
- Rushing, C.S., Ryder, T.B. & Marra, P.P. 2016. Quantifying drivers of population dynamics for a migratory bird throughout the annual cycle. Proc. R. Soc. B. 283: 20152846. doi: 10.1098/rspb.2015.2846
- Sæther, B.-E. & Bakke, Ø. 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology. 81: 642–653. doi: 10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2
- Sauer, J.R., Niven, D.K., Hines, J.E., Ziolkowski, D.J., Jr., Pardieck, K.L., Fallon, J.E. & Link, W.A. 2017. The North American Breeding Bird Survey, results and analysis 1966–2015. Version 02.07.2017. Retrieved June 13, 2019, from https://www.mbr-pwrc.usgs.gov/bbs/.
- Schaub, M., von Hirschheydt, J. & Grüebler, M.U. 2015. Differential contribution of demographic rate synchrony to population synchrony in Barn Swallows. J. Anim. Ecol. 84: 1530–1541. doi: 10.1111/1365-2656.12423
- Schifferli, L., Grüebler, M.U., Meijer, H.A.J., Visser, G.H. & Naef-Daenzer, B. 2014. Barn Swallow Hirundo rustica parents work harder when foraging conditions are good. Ibis 156: 777–787. doi: 10.1111/ibi.12186
- Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9: 671–675. doi: 10.1038/nmeth.2089
- Screen, J.A., Deser, C. & Sun, L. 2015. Reduced risk of North American cold extremes due to continued Arctic sea ice loss. Bull. Am. Meteorol. Soc. 96: 1489–1503. doi: 10.1175/BAMS-D-14-00185.1
- Selwood, K.E., McGeoch, M.A. & Mac Nally, R. 2015. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 90: 837–853. doi: 10.1111/brv.12136
- Sicurella, B., Musitelli, F., Rubolini, D., Saino, N. & Ambrosini, R. 2016. Environmental conditions at arrival to the wintering grounds and during spring migration affect population dynamics of Barn Swallows Hirundo rustica breeding in Northern Italy. Popul. Ecol. 58: 135–145. doi: 10.1007/s10144-015-0529-7
- Sillett, T.S. & Holmes, R.T. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71: 296–308. doi: 10.1046/j.1365-2656.2002.00599.x
- Smith, A.C., Hudson, M.-A.R., Downes, C.M. & Francis, C.M. 2015. Change points in the population trends of aerial-insectivorous birds in North America: synchronized in time across species and regions. PLoS One 10: e0130768. doi: 10.1371/journal.pone.0130768
- Stenkat, J., Krautwald-Junghanns, M.-E., Schmitz Ornés, A., Eilers, A. & Schmidt, V. 2014. Aerobic cloacal and pharyngeal bacterial flora in six species of free-living birds. J. Appl. Microbiol. 117: 1564–1571. doi: 10.1111/jam.12636
- Stutchbury, B.J., Hill IIIJ.R., Kramer, P.M., Rush, S.A. & Tarof, S.A. 2009. Sex and age-specific annual survival in a neotropical migratory songbird, the Purple Martin (Progne subis). Auk 126: 278–287. doi: 10.1525/auk.2009.08038
- Szép, T. 1995a. Relationship between west African rainfall and the survival of central European Sand Martins. Ibis 137: 162–168. doi: 10.1111/j.1474-919X.1995.tb03235.x
- Szép, T. 1995b. Survival rates of Hungarian Sand Martins and their relationship with Sahel rainfall. J. Appl. Stat. 22: 891–904. doi: 10.1080/02664769524694
- Szép, T., Liechti, F., Nagy, K., Nagy, Z. & Hahn, S. 2017. Discovering the migration and non-breeding areas of Sand Martins and House Martins breeding in the Pannonian basin (central-Eastern Europe). J. Avian Biol. 48: 114–122. doi: 10.1111/jav.01339
- Taylor, L.U., Woodworth, B.K., Sandercock, B.K. & Wheelwright, N.T. 2018. Demographic drivers of collapse in an island population of Tree Swallows. Condor 120: 828–841. doi: 10.1650/CONDOR-18-75.1
- Tilman, D., Fargione, J., Wolff, B., D’Antonio, C., Dobson, A., Howarth, R., Schindler, D., Schlesinger, W.H., Simberloff, D. & Swackhamer, D. 2001. Forecasting agriculturally driven global environmental change. Science 292: 281–284. doi: 10.1126/science.1057544
- Ummenhofer, C.C. & Meehl, G.A. 2017. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B Biol. Sci. 372: 20160135. doi: 10.1098/rstb.2016.0135
- Van Dijk, T.C., Van Staalduinen, M.A. & Van der Sluijs, J.P. 2013. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One 8: e62374. doi: 10.1371/journal.pone.0062374
- Von Ronn, J.A.C., Harrod, C., Bensch, S. & Wolf, J.B.W. 2015. Transcontinental migratory connectivity predicts parasite prevalence in breeding populations of the European Barn Swallow. J. Evol. Biol. 28: 535–546. doi: 10.1111/jeb.12585
- Weegman, M.D., Arnold, T.W., Dawson, R.D., Winkler, D.W. & Clark, R.G. 2017. Integrated population models reveal local weather conditions are the key drivers of population dynamics in an aerial insectivore. Oecologia 185: 119–130. doi: 10.1007/s00442-017-3890-8
- Wilson, E.O. & MacArthur, R.H. 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, NJ.
- Winkler, D.W., Hallinger, K.K., Ardia, D.R., Robertson, R.J., Stutchbury, B.J. & Cohen, R.R. 2011. Tree Swallow (Tachycineta bicolor), version 2.0, The Birds of North America (P. G. Rodewald, Ed.).
- Winkler, D.W., Luo, M.K. & Rakhimberdiev, E. 2013. Temperature effects on food supply and chick mortality in Tree Swallows (Tachycineta bicolor). Oecologia 173: 129–138. doi: 10.1007/s00442-013-2605-z
- Young, J.G. 1994. Sparrowhawk exploiting a Sand Martin colony. Scott. Birds 17: 236–237.
- Zimmerling, J.R., Pomeroy, A.C., D’Entremont, M.V. & Francis, C.M. 2013. Canadian estimate of bird mortality due to collisions and direct habitat loss associated with wind turbine developments. Avian Conserv. Ecol. 8: 10.
