ABSTRACT
Capsule: In Egyptian Vultures Neophron percnopterus, both sexes invested similar parental effort throughout the breeding period. However, there was variation in the degree of intensity of parental care during some stages of the breeding period, suggesting that sex-role specialization exists for some activities.
Aims: To quantify parental care behaviour of Egyptian Vultures for the first time and to examine the role of sex, weather conditions, and stage of nesting cycle on breeding ecology.
Methods: We monitored 15 nests of Egyptian Vultures to analyse parental care investment. We collected data on nine different behavioural parameters/activities per sex, which were recorded throughout the entire breeding period. Variation in parental investment was analysed using generalized linear mixed models.
Results: Females invested more effort in incubation/brooding (61.45% for females and 31.54% for males) and egg turning (0.45 events/h for females and 0.37 events/h for males) while males contributed more to nest material delivery to the nest (0.67 deliveries/h for males and 0.14 deliveries/h for females). Conversely, both sexes invested the same effort in nestling attendance (21.89% for females and 21.21% for males) and food provisioning (0.28 items/h for females and 0.25 items/h for males). Furthermore, parental investment was not affected by weather, especially during critical moments such as incubation/brooding, however, changeover rate was positively related to temperature.
Conclusion: Our results suggest that, in the Egyptian Vulture, one sex is not entirely responsible for a particular task and the compensatory effort of the other mate is required. Finally, our findings indicate that major events such as incubation onset and hatching caused important shifts in the patterns of parental investment.
Reproduction is one of the most critical stages of the annual cycle for most animals (Barta Citation2016) and attributes linked to physiology (e.g. hormone levels), morphology (e.g. body size) and behaviour (e.g. parental care) are the most likely to have an impact during this phase of the life history. Of these, parental care is closely related to successful offspring rearing (Kokko & Jennions Citation2008, Hoeck et al. Citation2015), with important effects on individual fitness (Clutton-Brock Citation1991) and population viability (Cruz-López et al. Citation2017). There is considerable variation in parental care strategies across animal taxa. Birds exhibit huge diversity in parental care behavioural strategies across the altricial-precocial spectrum with regard to the amount of care provided, for example in tasks such as nest building, incubation and offspring attendance (Balshine Citation2012). There are two well-distinguished patterns in parental care behaviour (Royle et al. Citation2012, Remeš et al. Citation2015): (1) both male and female are engaged in rearing their brood (perhaps with different degrees of involvement), namely bi-parental care and (2) total involvement by one of the parents during the entire breeding period, namely uni-parental care.
Among birds providing care for their offspring, birds of prey (Accipitriformes, Falconiformes and Strigiformes) are well known for their asymmetric parental care roles. In fact, in most birds of prey, females usually incubate, brood and provision nestlings, whereas males normally hunt, assisted by females only during the latter part of the nestling period, if at all (Newton Citation1979, Citation1986, Cramp & Simmons Citation1980, Cramp Citation1985, Wiehn & Korpimäki Citation1997, Eldegard et al. Citation2003, Eldegard & Sonerud Citation2012). The effects of diet in sex-role asymmetry (Sonerud et al. Citation2014a, Citation2014b), and factors affecting the duration of the post-fledging period, are well known (Arroyo et al. Citation2002, Tarwater & Brawn 2010, Muriel et al. Citation2015). However, other aspects linked to the influence of the stage of the nesting cycle, the parents’ sex and environmental conditions on the level of parental investment remain poorly understood (but see Lens & Dhondt Citation1994, Sánz et al. Citation2003). In fact, although the trade-offs between components of parental care are tightly linked to breeding performance (Monaghan & Nager Citation1997, Byholm et al. Citation2011, Mariette & Griffith Citation2015), other operating factors also affect breeding strategies in many ways. In particular, abiotic factors, such as temperature and precipitation, affect egg turning rates and nest attentiveness (Cresswell et al. Citation2003, Kovarik et al. Citation2009). Furthermore, environmental conditions fluctuate throughout the day and during the course of the breeding season. Hence, the costs of parental care and self-maintenance are also likely to vary (e.g. costs of thermoregulation, Piersma & van Gils Citation2010). On the other hand, biotic factors such as predation risk, food availability, the stage of incubation, adult and nestling age, and individual behavioural differences also affect incubation routines and nest attentiveness (Smith et al. Citation2012, Cole & Quinn Citation2014, Bulla et al. Citation2016, Zuberogoitia et al. Citation2018). These factors remain poorly understood in determinate groups, particularly in old-world vultures (but see Margalida & Bertran Citation2000, Xirouchakkis & Mylonas Citation2007, Bassi et al. Citation2017, Holland et al. Citation2017).
Parental care investment and factors determining why, how and by whom care is provided are of crucial importance in long-lived monogamous, monomorphic species with slow life-history strategies. In fact, these species usually exhibit low fecundity rates (commonly one fledgling per year) and extended breeding periods. This has been demonstrated for large avian scavengers (vultures and condors, Accipitridae and Cathartidae; De Magalhaes & Costa Citation2009). Vultures are typically long-lived, monomorphic monogamous species that provide biparental care for their offspring and in which pair-bonds are maintained from one year to another (Newton Citation1979, Cramp & Simmons Citation1980). Furthermore, the peculiar foraging habits of this group (i.e. exploitation of scarce and unpredictable resources) obligate parents to spend long periods away from the nest (Jackson et al. Citation2008, Deygout et al. Citation2010). This is the case for the Egyptian Vulture Neophron percnopterus, a globally endangered scavenger. Many aspects of the breeding ecology of this species remain unknown (but see Donázar & Ceballos Citation1989, Donázar et al. Citation1994). Our understanding of essential aspects of the breeding biology of this long-lived species is therefore of importance. Moreover, such data could be used as a conservation tool to promote effective management actions (Brooker et al. Citation2016, Merrick & Koprowski Citation2017), thus benefiting wildlife managers concerned with reducing vulture-related conflicts (Zuberogoitia et al. Citation2008, Avery et al. Citation2011), particularly in those populations subjected to high levels of human pressure.
In this study, we use data from a breeding population of Egyptian Vultures that has been the subject of a long-term monitoring programme (2000–2018) in Northern Spain, in order to: (1) describe behavioural patterns during the breeding period; and (2) assess the effects of sex, breeding stage and weather on parental investment in all activities. Given the lack of size dimorphism and the foraging ecology of the Egyptian Vulture, we expect that parental expenditure would be equally divided between both breeding adults and, furthermore, that both sexes would be equally involved in the various breeding activities. Therefore, we do not predict any sex-specific role specialization. We also analyse the effect of weather conditions on the amount of care provided during certain critical phases of the breeding cycle such as incubation onset and offspring hatching.
Methods
Study area
The study was conducted in the administrative region of Biscay (northern Spain; surface area 2384 km2; coordinates from 43°11′00″ to 43°12′70″N and from 3°12′70″ to 2°13′10″W). Barely 50 km separate sea level from the highest altitude (1480 m above sea level). The relief of the study area is abrupt and characterized by the presence of extensive urban and industrialized areas. More than 50% of the area is dedicated to forestry, at the expense of traditional, small-scale farming. A wet and warm Atlantic climate strongly influences the weather conditions. The average annual temperature is around 14°C and the mean annual precipitation fluctuates between 1200 and more than 2000 mm/m2 (Euskalmet Citation2017), making this one of the highest rainfall areas in Europe (NOAA Citation2016).
Study species
The Egyptian Vulture is a medium-sized, long-lived, monogamous, trans-Saharan migratory raptor (Ferguson-Lees & Christie Citation2001). Continental Western European populations of Egyptian Vultures spend the wintering season (and usually their first year of life) in the sub-Saharan Sahel region (García-Ripollés et al. Citation2010, López-López et al. Citation2014a). The European population is estimated at between 3300 and 5050 breeding pairs (BirdLife International Citation2018), of which 1270–1300 pairs are found in the Iberian Peninsula (Iñigo et al. Citation2008). The European population has experienced a severe decline in the past few decades, mainly due to non-natural mortality caused primarily by poisoning (BirdLife International Citation2018). As with other long-lived scavengers, Egyptian Vultures are highly philopatric to their breeding territories (Donázar Citation1993, Carrete et al. Citation2007). They breed in cavities and on cliff ledges located in open landscapes, usually in rugged, arid regions (Cramp & Simmons Citation1980, Donázar Citation1993). In our study area, the species inhabits mountainous areas, far from towns and villages, where extensive cattle farming and timber extraction are the main economic activities. The diet is based mainly on sheep and goat carcasses, and small or medium-sized animals, mostly road-killed mammals and passerines (Hidalgo et al. Citation2005). The main threat to the species in our study area is human disturbance associated with leisure activities and forestry during the breeding season (Zuberogoitia et al. Citation2008). However, these activities have been partially banned during this period thanks to the proposed effective mitigation measures outlined by Zuberogoitia et al. (Citation2014).
Field procedure and data collection
We developed an intensive monitoring programme to study the breeding pairs from 1 February to 30 September 2017, thus covering the whole breeding period. During this time, we monitored five nesting sites intensively. The remaining Egyptian Vulture breeding pairs (n = 15) were monitored intermittently, due to financial and logistic constraints. The five intensively monitored nests were observed weekly, with visits to each of the five nests at least once a week from the arrival of the adults at the breeding grounds until the departure of the offspring, while the remainder were monitored less frequently than the previous five (from 1 to 29 visits/nest), in order to assess breeding performance. Following Zuberogoitia et al. (Citation2008), we monitored nest sites from vantage points that were situated far enough away to avoid disturbance, using 20–60x telescopes. We monitored the nests in all weather conditions. Overall, we spent 583.94 h monitoring the nests (n = 20) on 141 different days during the study period, with an average of 4.30 h/day (sd = 2.02, range = 1–9.42 h) being spent at each nest. One researcher (JM) carried out the intensive monitoring from sunrise to dusk, randomly visiting the five nests during mornings and afternoons throughout the study period. The non-intensive monitoring of the remaining 15 nests was performed from sunrise to dusk by four additional researchers who spent on average 3.91 h/day (sd = 1.75, range = 1–7.35 h) at each nest.
At each viewpoint, we conducted intensive monitoring of nesting sites and their surroundings, both to detect individuals and to record their behaviour. We noted the location of every individual with regard to the nest. We also recorded the time (starting time and duration) of the arrivals and departures of each member of the pair to the nest; the behaviour of each target individual, as described below; and the location and behaviour of its mate.
Gender identification was determined by using facial marks and facial cere colours, which are usually orange in males and yellowish in females (Negro et al. Citation2002, Margalida et al. Citation2012b). This feature was more evident when both members of a pair were together. We took photographs of each bird during the initial monitoring visits, recording facial marks, individual variation in the colour of the greater coverts and flight feathers, including their moult pattern, and colour rings from our long-term ringing project (Zuberogoitia et al. Citation2018). Gender identification was confirmed later by observing the position of partners during copulatory behaviour.
Five of the 20 territorial pairs did not lay eggs. From the 15 pairs that started breeding, only 1 of them raised 2 fledglings and 10 raised 1 fledgling. Two of the intensively monitored pairs failed at an earlier incubation stage, whereas nestlings of another two intensively monitoring pairs died at an advanced stage of growth.
To analyse parental care investment, we collected data on nine different behavioural variables/activities per sex, which were recorded throughout the entire breeding period. We calculated the ratio of each activity as the number of times or percentage of time that the event was observed and the total hours of observation per day. The recorded activities were: (1) material deliveries: the number of times that material was carried to the nest; (2) nest arrangement: the number of times that adults arranged newly added or existing material in the nest; (3) incubation and nestling brooding: the percentage of time invested by adults in incubation and nestling brooding; (4) nestling attendance: the percentage of time invested by adults in offspring attendance; (5) egg turning: the number of times that eggs were turned; (6) changeovers: the number of times each parent was replaced by the other at the nest; (7) food provisioning: the number of times that food was provided to the nest; and (8) nestling feeding: the number of nestling feeding events (feeding events are particular behaviours, not necessarily occurring on every event of food provisioning).
The breeding season was divided into four different stages (see Zuberogoitia et al. Citation2008 for more details): (1) courtship period (February–March): including nest repair/construction, copulation and egg laying; (2) incubation period (April–May): in our study area incubation started on average on 17 April 2017 (sd = 11.44 days, range = 1 April–14 May, n = 15) and spanned 42 days from incubation onset to hatching date; (3) nestling period (May–August): from the hatching date to the first fledging; and (4) fledging period (August–September): from the first fledging until departure from the breeding site.
We considered the week relative to (1) incubation onset and (2) nestling hatching as independent variables in order to test the effects of time on parental care. Likewise, this latter unit represents the breeding stage (i.e. from the hatching week until the end of the fledging period). We also recorded the mean temperature (°C), precipitation (mm), insolation (w/m2) and humidity (%) relative to the surveyed hours in each day from the nearest meteorological stations of the Basque Meteorological Agency (n = 17 stations) (www.euskalmet.euskadi.net) (Zuberogoitia et al. Citation2014).
Statistical analysis
To analyse parental behaviour, we ran generalized linear mixed models (GLMM) with a Gaussian distribution, using the nine behavioural parameters as response variables. We considered the gender, period (week), and the four weather variables as independent variables. Gender was entered as a factor in the models. Territory was included as a random factor, to account for multiple measurements of the same breeding pairs. To avoid collinearity, we preliminarily calculated the Spearman’s correlation coefficients (rs) for all pairs of variables using the ‘rcorr’ function implemented in the R package ‘Hmisc’ (Harrell Citation2013). When two variables were highly correlated (rs > 0.5), we dropped collinear covariates, and the less biologically significant variable was consequently excluded from further analyses (Dormann et al. Citation2013). Thereby, we removed from the models insolation (w/m2), given its high correlation with temperature.
We computed all models, fitted by maximum likelihood methods, using the Laplace approximation, using the ‘lmer’ function as implemented in the ‘lme4’ package (Bates et al. Citation2015) for R (R Core Team 2016). We used the Akaike Information Criterion corrected for small sample size (AICc) for model comparison (Burnham & Anderson Citation2002). We used standard model selection procedures to interpret ΔAICc and AICc ω (weight) among competing models and considered models within 2 AICc units as having substantial empirical support (Burnham & Anderson Citation2002).
Results
Copulatory behaviour
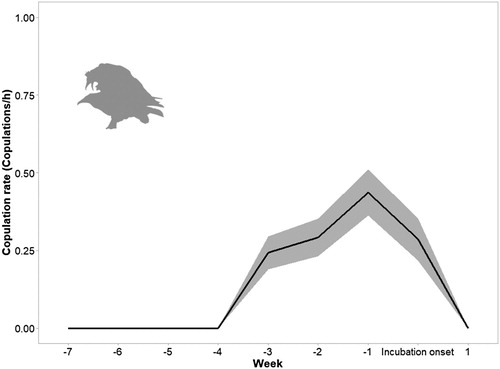
During the breeding season, we observed a total of 24 copulations, including both the intensively and intermittently observed pairs (n = 20). The mean distance of copulation attempts to the nest was 146.62 m (sd = 155.19 m, range = 0–530 m). The average copulation duration was 13.09 s (sd = 6.05 s, range = 0.5–25 s). After each copulation attempt, most pairs remained together (n = 18) and performed mutual preening. Copulations reached their maximum level one week before the onset of incubation and sharply decreased after this date (Spearman correlation test; rho = 0.545, P = 0.005, ).
Nest building and maintenance
Egyptian Vultures started to deliver material to the nest three weeks before incubation onset, reaching peak activity just one week before incubation started ((A)). Material selected for nest construction was transported using the talons or the beak. During the nest-building period, males showed higher material delivery effort (0.67 deliveries/h, sd = 0.78, range = 0–2.75) than females (0.14 deliveries/h, sd = 0.07, range = 0–0.37; ; (A)). However, results for the material delivery models showed a weak support for ‘sex’, which was included in the best model, but only at 1.5 ΔAICc to the null model.
Figure 2. Mean rate of delivery of material to the nest per week relative to incubation onset (week 0) (A). Mean nest arrangement rate (B) per week in relation to hatching week (week 0). The shaded areas in both graphs represent ± se.
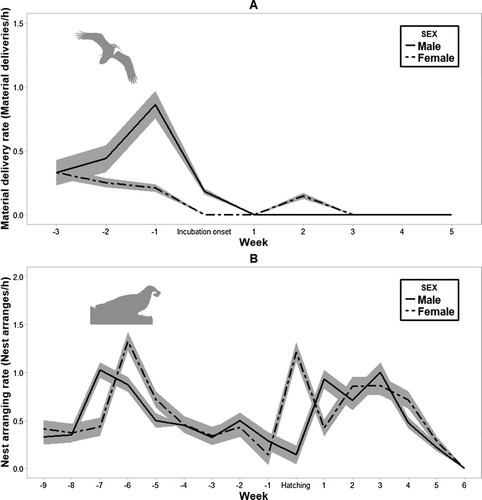
Table 1. Results of the GLMMs for each behaviour recorded in Egyptian Vulture pairs (n = 20) in northern Spain.
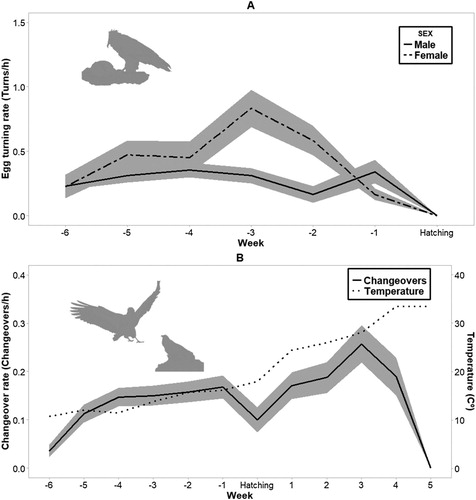
Both sexes invested a similar effort in nest arrangement (0.58 events/h, sd = 0.54, range = 0–2.5, for males; and 0.63 events/h, sd = 0.51, range = 0–2.14, for females; Wilcoxon test, W = 521, P = 0.54), not only during the nest-building phase, but also during incubation and the first weeks of nestling attendance ( and , (B)). We observed that nest arrangement consisted mainly of moving and resettling items that were carried to the nest during nest building or were left over from the previous breeding season. This activity peaked before the onset of incubation, and then decreased gradually before reaching a second peak around hatching time.
Incubation/brooding and nestling attendance
During incubation both males and females covered the clutch continuously, except for a few interruptions that occurred during mate changeovers and egg turning. Females invested significantly more time in egg incubation (61.45%, sd = 35.78, range = 13.7–93.9%) than males (38.54%, sd = 32.68, range = 0–100%, , (A)). Brooding effort dropped after the third week of life of the nestlings, although adults continued brooding nestlings until the fifth week. Both sexes invested almost equal time in nestling attendance (females: 21.89%, sd = 27.40, range = 0–97.2%; males: 21.21%, sd = 26.97, range = 0–89.42%; , (A)), although it decreased over time ( and , (B)).
Figure 3. Incubation and brooding investment (A) and nest attendance investment (B) per week in relation to hatching date (week 0). Values are expressed as a percentage of time. Shaded areas represent ± se.
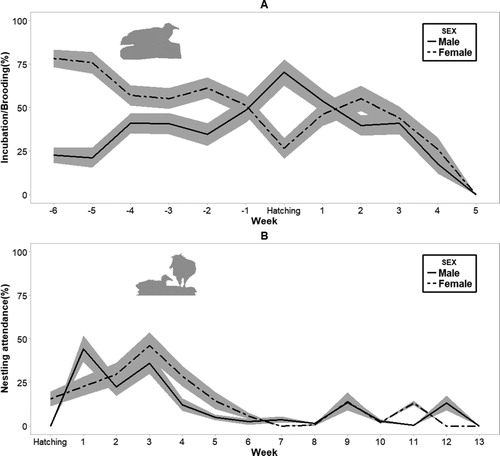
Table 2. Results of GLMMs for the most parsimonious model for each breeding behaviour of Egyptian Vultures.
Egg turning
We observed a total of 61 egg turning events during incubation. Eggs were turned using the beak and the talons. Females invested more effort (0.45 events/h, sd = 0.27, range = 0–0.83) than males (0.37 events/h, sd = 0.33, range = 0–1.16) in egg turning. This differential rate was consistent over time ( and , (A)).
Changeovers
Egyptian Vultures carried out 0.16 changeovers/h (sd = 0.12, range = 0–0.375) during incubation and 0.17 changeovers/h (sd = 0.14, range = 0–0.5) during the brooding period. The changeover rate was related to weather and stage of the nesting cycle, although models showed a weak support (1.2 ΔAICc to the null model; ). Changeover rates were low during the first stages of incubation, when temperatures were lower than those recorded later in the spring. Changeover rates increased from the hatching date onwards, until the nestlings were in their third week, but dropped afterwards when continuous brooding was unnecessary ((B)).
Food provision and nestling feeding
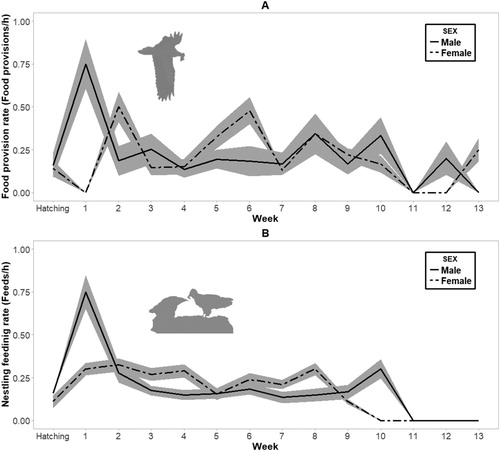
Overall, we observed 56 food provisions and 42 feeding events. Adults always carried the food in the beak and there was no regurgitation of food to feed offspring. Once in the nest, adults prepared the food items, breaking them up to facilitate ingestion. The average food provision rate was 0.25 items per hour for males (sd = 0.15, range = 0–0.75) and 0.28 items for females (sd = 0.16, range = 0–0.67), and there were no significant sex differences (). There were no sex differences in feeding rate (0.24 events/h for males; sd = 0.16, range = 0–0.75 and 0.26 events/h for females; sd = 0.11, range = 0–0.5, ). However, both provisioning and feeding rates decreased over time ( and ), being higher during the first weeks after hatching and slightly decreasing as nestlings grew ((A,B)). Adults still fed juveniles occasionally even when they were ready to fly.
Discussion
Our results showed that the Egyptian Vulture exhibits biparental care throughout its extended breeding period of approximately 24 weeks. However, we observed behavioural asymmetries in the parental investment of each sex depending on specific activities. Furthermore, we found that parental investment type changed over the course of the breeding period in both sexes with regard to almost all activities. Surprisingly, although weather conditions might constrain optimal embryo development and thus increase parental care by mates (Bulla et al. Citation2015), we found that weather did not influence parental investment during critical stages, for example, during incubation and brooding. Normally, Egyptian Vultures use cavities and holes for nesting and hence nests are protected from meteorological events (i.e. rain and storms). This could help to maintain an adequate environment for eggs and nestlings by reducing temperature variation.
The breeding cycle of the Egyptian Vulture starts with nest repair (in the case of reusing a nest site, Donázar Citation1993) or new nest building, and courtship. We observed that both activities took place simultaneously, which suggests a common stimulus (i.e. sperm viability and the fertile female period; Donázar et al. Citation1994). Egyptian Vultures started copulating 25 days before the onset of incubation, showing a peak one week before. This suggests that copulations outside the fertile period could be related to pair bonding, mate assessment and territorial behaviour (Newton Citation1979, Negro & Grande Citation2001). The maximum peak in copulation rate occurred a few days before the laying of the first egg and continued after the laying of the second egg and the onset of incubation (Egyptian Vultures usually lay two eggs, with an interval of 3–4 days; Donázar Citation1993, Margalida et al. Citation2012a). Copulation activity and nest material delivery took place at the same time and followed similar trends during the weeks before incubation onset.
After the onset of incubation, both sexes shared the incubation effort. From incubation to the early stages of the post-hatching period, both sexes continuously covered the clutch or nestlings, except for the time spent in changeovers, egg turning and nest repairing activities. The regular presence of one of the adults at the nest during incubation and brooding may be necessary to protect the eggs or nestlings from low temperatures, insolation and predation (Al-Rashidi et al. Citation2010, Bulla et al. Citation2014, Deeming & Reynolds Citation2016 and references therein). However, our results showed sex asymmetry in incubation behaviour, with females spending significantly more time incubating and egg turning, although there was compensation by each mate in nest attendance ((A)). Given that incubation and brooding are energy-demanding activities (Bulla et al. Citation2014), compensation during incubation may be necessary for Egyptian Vultures to deal with: (1) the difficulty in searching for carrion, which is a spatially and temporally unpredictable resource (Deygout et al. Citation2010, Monsarrat et al. Citation2013, López-López et al. Citation2014b) and (2) the excessive costs of continuous incubation by only one parent (Brunton Citation1988). In fact, our results showed that males progressively invested more effort in incubation, from 25% of the time during the first week to 70% during the hatching week. Nonetheless, the low variability explained by sex in our model (only 4%; ), suggests that other factors such as individual traits (e.g. age, experience and personality; Sanz-Aguilar et al. Citation2017, Zuberogoitia et al. Citation2018) and those related to nest structure and specific micro-environmental characteristics of nest placement could also affect behavioural differences during incubation (Deeming & Reynolds Citation2016). Similarly, we also observed differences between sexes in other activities associated with incubation, like egg turning. Egg turning is crucial for maintaining embryo development and presenting the chick in the correct position for successful hatching (Deeming Citation2002, Wilson et al. Citation2003). Given the importance of this activity, the higher investment of females and the absence of partial or total compensation by males might suggest sex-role specialization ((A)).
All activities related to the rearing of nestlings until the fledging stage were carried out by both parents and decreased in parallel with the growth of the nestlings, as described in other species of the same guild (Donázar Citation1993, Margalida & Bertran Citation2000). During the first month of life, nestlings were continuously accompanied by one parent because of their limited thermoregulatory capacity and to reduce predation risk (Hohtola & Visser Citation1998, Deeming Citation2002, Margalida et al. Citation2007). A reduction in the time spent in nestling attendance occurred from the third week onwards, corresponding with the moment at which nestling energy requirements and thermoregulation ability increases (Newton Citation1979, Komen Citation1991). This pattern is also related to the increase in foraging time and to the rise in the number of prey delivered to the nest (Margalida & Bertran Citation2000, Holland et al. Citation2017). Other factors, such as lower predation risk and better meteorological conditions as the breeding season advances, could also explain our results (Dodge et al. Citation2014). During the final days of nest attendance, parental care was reduced to only food provisioning and some flights in front of the nest.
Species-specific life-history traits, in addition to ecological and environmental conditions, also influence parental care (Klug et al. Citation2013). In this context, our results showed that changeovers were not only dependent on the breeding stage but also on particular environmental conditions, mainly temperature. During incubation and offspring rearing, changeovers are necessary to share breeding costs (Marasco & Spencer Citation2015), and to fit parental effort to the nestlings’ development requirements. This explains observed differences in changeovers during the pre-and post-hatching period. During the nestling stage, the changeover rate increased in parallel with temperature over time ((B)). In fact, the poikilothermic-homeothermic transition of nestlings elicits changes in the amount of care provided. Moreover, changeovers are related to the rate of successful foraging (Newton Citation1979, Cresswell et al. Citation2003, Rollack et al. 2013), which is also related to food availability (Donázar Citation1993, Monsarrat et al. Citation2013, Cortés-Avizanda et al. Citation2016).
According to the pattern observed in similar species with biparental care (Margalida & Bertran Citation2000), food provisioning and feeding rates were equally divided between both mates throughout the nestling period. During the first weeks after hatching, we observed that the feeding rate by adults was probably a response to the increasing food requirements of nestlings (Newton Citation1979) and also to other processes affecting food availability in the surrounding area (Bruun & Smith Citation2003). In subsequent weeks the provisioning rate decreased since the increasing demand of nestlings obliges adults to increase the quantity of food supplied on each visit to the nest. This suggests that adults were forced to expend more time foraging. Furthermore, this could explain the lower contribution of both mates to brooding and nestling attendance. Similarly, the nestling feeding rate decreased with time because the young birds became more skilled at dealing with prey items, and most of the food was self-consumed (Watson Citation2010, Sonerud et al. Citation2014a, Bassi et al. Citation2017). During the final weeks before fledging, food provisioning was maintained but no feeding occurred. This coincided with less time expenditure in food preparation by adults, which suggests an increase in the ability of nestlings to manipulate prey remains. This also compensated for the increased parental effort in searching for food, since the adults spent less time feeding their offspring (Deeming & Reynolds Citation2016). Likewise, this fact could also prevent any conflict between offspring and parent requirements as other authors have previously assessed (Royle et al. Citation2012, Iserbyt et al. Citation2015)
Overall, our findings suggest that Egyptian Vultures invested similar parental effort during the breeding period, although with different degrees of intensity depending on the stage of the cycle were observed. This suggests that biologically relevant events, such as incubation and hatching date, could drive parental investment (Royle et al. Citation2012). This is of key importance in explaining observed behavioural patterns in this species. Our results showed that parental care was similar with regard to certain activities, such as nest arrangement, nestling attendance, food provisioning and nestling feeding. However, sex-specific roles were observed for some activities. Females contributed more to incubation, brooding and egg turning activities, while males participated more actively in other tasks such as the delivery of material to the nest. In fact, similar results were found in the Bearded Vulture Gypaetus barbatus (Margalida & Bertran Citation2000, Bassi et al. Citation2017) and Griffon Vulture Gyps fulvus (Xirouchakkis & Mylonas Citation2007). The absence of apparent differences in both sex roles suggests a balanced distribution of parental care effort, which implies that neither sex could meet nestling requirements alone without help from the mate. Finally, the number of changeovers observed over time suggests that particular environmental conditions and the breeding stage could also explain variation in parental care in long-lived species.
Ethical approval (statement on the welfare of animals)
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Further, all procedures performed in the present study were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Acknowledgements
We would like to thank L. Astorkia, I. Castillo, G. Burgos, C. Cinos, Julio Ruiz, Agurtzane Iraeta, Ainara Azkona, J. Zuberogoitia, M. Pecoraro, Aitor Galarza, Igor Aginako, Iñaki Garmendia, Fran Martínez and Eneko Diaz for their field assistance. In memory of Mikel Larrea. The ‘Servicio Patrimonio Natural-Departamento de Sostenibilidad y Medio Natural de la Diputación Foral de Bizkaia’ partially supported the fieldwork and issued the licenses to work with this species.
ORCID
Jon Morant Etxebarria http://orcid.org/0000-0001-5702-2348
Pascual López-López http://orcid.org/0000-0001-5269-652X
Iñigo Zuberogoitia Arroyo http://orcid.org/0000-0002-8945-7386
Additional information
Funding
References
- Al-Rashidi, M., Kosztolanyi, A., Kupper, C., Cuthill, I.C., Javed, S. & Szekely T. 2010. The influence of a hot environment on the parental cooperation of a ground-nesting shorebird, the Kentish plover Charadrius alexandrinus. Front. Zool. 7: 1–10. doi: 10.1186/1742-9994-7-1
- Arroyo, B.E., De Cornulier, T. & Bretagnolle, V. 2002. Parental investment and parent-offspring conflicts during the post-fledging period in Montagu’s Harrier. Anim. Behav. 63: 235–244. doi: 10.1006/anbe.2001.1899
- Avery, M.L., Humphrey, J.S., Daughtery, T.S., Fischer, J.W., Milleson, M.P & Tillman, E.A. 2011. Vulture flight behavior and implications for aircraft safety. J. Wildlife Manage. 75: 1581–1587. doi: 10.1002/jwmg.205
- Balshine, S. 2012. Patterns of parental care in vertebrates. In Royle, N.J., Smiseth, P.T., Kolliker, M. (eds) The Evolution of Parental Care, 62–75. Oxford University Press, Oxford.
- Barta, Z. 2016. Behavioural change over the annual cycle: optimal annual routines. Curr. Opin. Behav. Sci. 12: 138–141. doi: 10.1016/j.cobeha.2016.11.007
- Bassi, E., Trotti, P., Brambilla, M., Diana, F., Sartirana, F., Galli, L. & Pedrotti, L. 2017. Parental investment in two large raptors breeding in a high prey density area. J. Ornithol. 158: 549–559. doi: 10.1007/s10336-016-1407-6
- Bates, D., Maechler, M., Bolker, B. & Walker, S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. doi: 10.18637/jss.v067.i01
- BirdLife International. 2018. Neophron percnopterus. The IUCN Red List of Threatened Species 2015: e.T22695180A85062680. Downloaded on 12 May 2018.
- Brooker, R.M., Feeney, W.E., White, J.R., Manassa, R.P., Johansen, J.L. & Dixson, D.L. 2016. Using insights from animal behaviour and behavioural ecology to inform marine conservation initiatives. Anim. Behav. 120: 211–221. doi: 10.1016/j.anbehav.2016.03.012
- Brunton, D.H. 1988. Sexual differences in reproductive effort: time-activity budgets of monogamous killdeer, Charadrius vociferous. Anim. Behav. 36: 705–717. doi: 10.1016/S0003-3472(88)80153-2
- Bruun, M. & Smith, H.G. 2003. Landscape composition affects habitat use and foraging flight distances in breeding European starlings. Biol. Conserv. 114: 179–187. doi: 10.1016/S0006-3207(03)00021-1
- Bulla, M., Valcu, M., Rutten, A.L. & Kempenaers, B. 2014. Biparental incubation patterns in a high-Arctic breeding shorebird: how do pairs divide their duties? Behav. Ecol. 25: 152–164. doi: 10.1093/beheco/art098
- Bulla, M., Stich, E., Valcu, M. & Kempenaers, B. 2015. Off-nest behaviour in a biparentally incubating shorebird varies with sex, time of day and weather. Ibis 157: 575–589. doi: 10.1111/ibi.12276
- Bulla, M., Valcu, M., Dokter, A., Kosztolanyi, A., Rutten, A., Helm, B., Sandercock, B., Casler, B., Ens, B.J., Spiegel, C.S., Hassell, C., Kupper, C., Minton, C., Riera, D.B., Lank, D.B., Payer, D., Loktionov, E.Y., Nol, E., Kwon, E., Smith, F., Hillig, F., Vitnerova, H., Preuter, H., Clair, J.S., Rausch, J., Reneerkens, J., Conklin, J.R., Lamarre, J., Johnson, J., Burger, J., Liebzeit, J., Bety, J., Coleman, J., Figuerola, J., Hooijmeijer, J., Alves, J.A., Weidinger, K., Koivula, K., Gosbell, K., Niles, L., Koloski, L., McKinnon, L., Klaassen, M., Giroux, M.A., Sladecek, M., Boldenow, M., Exo, M., Goldstein, M.I., Salek, M., Senner, N., Reonkea, N., Lecomte, N., Gilg, O., Vincze, O., Johnson, O., Smith, PA., Tomkovich, P., Battley, P., Bentzen, R., Lanctot, R., Gates, R., Porter, R., Saalfeld, S., Freeman, S., Brown, S., Yezerinac, S., Haig, S.M., Szekely, T., Piersma, T., Montalvo, T., Loverti, V., Pakanen, V.M., Tijsen, W. & Kempenaers, B. 2016. Unexpected diversity in socially synchronized rhythms of shorebirds. Nature 540: 109–113. doi: 10.1038/nature20563
- Burnham, K.P. & Anderson, D.R. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. Springer, New York.
- Byholm, P., Rousi, H., & Sole, I. 2011. Parental care in nesting hawks: breeding experience and food availability influence the outcome. Behav. Ecol. 22: 609–615. doi: 10.1093/beheco/arr019
- Carrete, M., Grande, J.M., Tella, J.L., Sánchez-Zapata, J.A., Donázar, J.A., Díaz-Delgado, R. & Romo, A. 2007. Habitat, human pressure, and social behavior: partialling out factors affecting large-scale territory extinction in an endangered vulture. Biol. Conserv. 136: 143–154. doi: 10.1016/j.biocon.2006.11.025
- Clutton-Brock, T.H. 1991. The Evolution of Parental Care. Princeton University Press, Princeton, NJ.
- Cole, E.F. & Quinn, J.L. 2014. Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild. Biol Letters 10: 20140178. doi: 10.1098/rsbl.2014.0178
- Cortés-Avizanda, A., Blanco, G., DeVault, T.L., Markayanda, A., Virani, M.Z., Brandt, J. & Donázar, J.A. 2016. Supplementary feeding and endangered avian scavengers: benefits, caveats, and controversies. Front. Ecol. Environ. 14: 191–199. doi: 10.1002/fee.1257
- Cramp, S. 1985. The Birds of the Western Palearctic. Oxford University Press, London.
- Cramp, S. & Simmons, K.E.L. 1980. The Birds of the Western Palearctic. Oxford University Press, London.
- Cresswell, W., Holt, S., Reid, J.M., Whitfield, D.P. & Mellanby, R.J. 2003. Do energetic demands constrain incubation scheduling in a biparental species? Behav. Ecol. 14: 97–102. doi: 10.1093/beheco/14.1.97
- Cruz-López, M., Eberhart-Phillips, L.J., Fernández, G., Beamonte-Barrientos, R., Székely, T., Serrano-Meneses, M.A. & Küpper, C. 2017. The plight of a plover: viability of an important snowy plover population with flexible brood care in Mexico. Biol. Conserv. 209: 440–448. doi: 10.1016/j.biocon.2017.03.009
- Deeming, D.C. 2002. Avian Incubation: behaviour, environment, and evolution. Oxford University Press, Oxford.
- Deeming, D.C. & Reynolds, S.J. 2016. Nests, Eggs and Incubation: new ideas about avian reproduction. Oxford University Press, Oxford.
- De Magalhaes, J.P. & Costa, J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22: 1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x
- Deygout, C., Gault, A., Duriez, O., Sarrazin, F. & Bessa-Gomes, C. 2010. Impact of food predictability on social facilitation by foraging scavengers. Behav. Ecol. 21: 1131–1139. doi: 10.1093/beheco/arq120
- Dodge, S., Bohrer, G., Bildstein, K., Davidson, S.C., Weinzierl, R., Bechard, M.J., Barber, D., Kays, R., Brandes, D., Han, J. & Wikelski, M. 2014. Environmental drivers of variability in the movement ecology of Turkey vultures (Cathartes aura) in North and South America. Philos. Trans. Roy. Soc. B. 369: 1–17. doi: 10.1098/rstb.2013.0195
- Donázar, J.A. 1993. Los Buitres Ibéricos: Biología y Conservación. JM Reyero, Madrid.
- Donázar, J.A. & Ceballos, O. 1989. Growth rates of nestling Egyptian Vultures. Ardea 77: 217–226.
- Donázar, J.A., Ceballos, O. & Tella, J.L. 1994. Copulation behaviour in the Egyptian Vulture Neophron percnopterus. Bird Study 41: 37–41. doi: 10.1080/00063659409477195
- Dormann, C., Elith, J. & Bacher, S. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
- Eldegard, K. & Sonerud, G.A. 2012. Sex roles during post-fledging care in birds: female Tengmalm’s owls contribute little to food provisioning. J. Ornithol. 153: 385–398. doi: 10.1007/s10336-011-0753-7
- Eldegard, K., Selås, V., Sonerud, G.A., Steel, C. & Rafoss, T. 2003. The effect of parent sex on prey deliveries to fledgling Eurasian sparrowhawks Accipiter nisus. Ibis 145: 667–672. doi: 10.1046/j.1474-919X.2003.00229.x
- Euskalmet 2017. http://www.euskalmet.euskadi.eus/s07-5853x/es/meteorologia/home.apl?e=5 [accessed on 20 December 2017 ].
- Ferguson-Lees, J. & Christie, D.A. 2001. Raptors of the World. Christopher Helm, London.
- García-Ripollés, C., López-López, P. & Urios, V. 2010. First description of migration and wintering of adult Egyptian Vultures Neophron percnopterus tracked by GPS satellite telemetry. Bird Study 57: 261–265 doi: 10.1080/00063650903505762
- Harrell, F.E., Jr. 2013. Hmisc: Harrell miscellaneous. R package version 3.12-2. Computer software. http://cran.r-project.org/web/packages/Hmisc.
- Hidalgo, S., Zabala, J., Zuberogoitia, I., Azkona, A. & Castillo, I. 2005. Food of the Egyptian Vulture (Neophron percnopterus) in Biscay. Buteo 14: 23–29.
- Hoeck, P.E., Wolak, M.E., Switzer, R.A., Kuehler, C.M. & Lieberman, A.A. 2015. Effects of inbreeding and parental incubation on captive breeding success in Hawaiian crows. Biol. Conserv. 184: 357–364. doi: 10.1016/j.biocon.2015.02.011
- Hohtola, E. & Visser, G. 1998. Development of locomotion and endothermy in altricial and precocial birds. In: Starck, J.M. & Ricklefs, R.E. (eds) Avian Growth and Development: evolution within the altricial-precocial spectrum, 157–173. Oxford University Press, Oxford.
- Holland, A.E., Byrne, M.E., Bryan, A.L., DeVault, T.L., Rhodes, O.E. & Beasley, J.C. 2017. Fine-scale assessment of home ranges and activity patterns for resident black vultures (Coragyps atratus) and Turkey vultures (Cathartes aura). PloS One. 12. e0179819. doi: 10.1371/journal.pone.0179819
- Iñigo, A., Barov, B., Orhun, C. & Gallo-Orsi, U. 2008. Action plan for the Egyptian Vulture Neophron percnopterus in the European Union. BirdLife International for the European Commission, Brussels.
- Iserbyt, A., Eens, M. & Müller, W. 2015. Sexually antagonistic selection during parental care is not generated by a testosterone-related intralocus sexual conflict – insights from full-sib comparisons. Sci. Rep. 5: 17715. doi: 10.1038/srep17715
- Jackson, A.L., Ruxton, G.D. & Houston, D.C. 2008. The effect of social facilitation on foraging success in vultures: a modelling study. Biol Letters 4: 311–313. doi: 10.1098/rsbl.2008.0038
- Klug, H., Bonsall, M.B. & Alonzo, S.H. 2013. Sex differences in life history drive evolutionary transitions among maternal, paternal, and bi-parental care. Ecol. Evol. 3: 792–806. doi: 10.1002/ece3.494
- Kokko, H. & Jennions, M.D. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21: 919–948. doi: 10.1111/j.1420-9101.2008.01540.x
- Komen, J. 1991. Energy requirements of nestling Cape Vultures. The Condor. 93: 153–158. doi: 10.2307/1368617
- Kovařík, P., Pavel, V. & Chutný, B. 2009. Incubation behaviour of the Meadow Pipit (Anthus pratensis) in an alpine ecosystem of Central Europe. J. Ornithol. 150: 549. doi: 10.1007/s10336-009-0380-8
- Lens, L. & Dhondt, A.A. 1994. Effects of habitat fragmentation on the timing of crested tit Parus cristatus natal dispersal. Ibis 136: 147–152. doi: 10.1111/j.1474-919X.1994.tb01078.x
- López-López, P., García-Ripollés, C. & Urios, V. 2014a. Individual repeatability in timing and spatial flexibility of migration routes of trans-Saharan migratory raptors. Curr. Zool. 60: 642–652. doi: 10.1093/czoolo/60.5.642
- López-López, P., García-Ripollés, C. & Urios, V. 2014b. Food predictability determines space use of endangered vultures: implications for management of supplementary feeding. Ecol. Appl. 24: 938–949. doi: 10.1890/13-2000.1
- Marasco, V. & Spencer, K.A. 2015. Improvements in our understanding of behaviour during incubation. In Deeming, D.C. & Reynolds, J. (eds) Nests, Eggs, and Incubation: new ideas about avian reproduction. Oxford University Press, Oxford.
- Margalida, A. & Bertran, J. 2000. Breeding behaviour of the Bearded Vulture Gypaetus barbatus: minimal sexual differences in parental activities. Ibis 142: 225–234. doi: 10.1111/j.1474-919X.2000.tb04862.x
- Margalida, A., González, L.M., Sánchez, R., Oria, J. & Prada, L. 2007. Parental behaviour of Spanish Imperial Eagles Aquila adalberti: sexual differences in a moderately dimorphic raptor. Bird Study 54: 112–119. doi: 10.1080/00063650709461462
- Margalida, A., Benitez, J.R., Sánchez-Zapata, J.A., Avila, E., Arenas, R. & Donazar, J.A. 2012a. Long-term relationship between diet breadth and breeding success in a declining population of Egyptian Vultures Neophron percnopterus. Ibis 154: 184–188. doi: 10.1111/j.1474-919X.2011.01189.x
- Margalida, A., García, D. & Bertran J. 2012b. Els Voltors a Catalunya: biologia, conservació i síntesi. Grup d’Estudi i Protecció de Trencalos, Barcelona, El Pont de Suert.
- Mariette, M.M. & Griffith, S.C. 2015. The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185: 270–280. doi: 10.1086/679441
- Merrick, M.J. & Koprowski, J.L. 2017. Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 209: 34–44. doi: 10.1016/j.biocon.2017.01.021
- Monaghan, P. & Nager, R.G. 1997. Why don’t birds lay more eggs? Trends Ecol. Evol. 12: 270–274. doi: 10.1016/S0169-5347(97)01094-X
- Monsarrat, S., Benhamou, S., Sarrazin, F., Bessa-Gomes, C., Bouten, W. & Duriez, O. 2013. How predictability of feeding patches affects home range and foraging habitat selection in avian social scavengers? Plos One 8: 1–11. doi: 10.1371/journal.pone.0053077
- Muriel, R., Ferrer, M., Balbontín, J., Cabrera, L. & Calabuig, C.P. 2015. Disentangling the effect of parental care, food supply, and offspring decisions on the duration of the post-fledging period. Behav. Ecol. 26: 1587–1596. doi: 10.1093/beheco/arv114
- Negro, J.J. & Grande, J.M. 2001. Territorial signalling: a new hypothesis to explain frequent copulation in raptorial birds. Anim. Behav. 62: 803–809. doi: 10.1006/anbe.2001.1811
- Negro, J.J., Grande, J.M., Tella, J.L., Garrido, J., Hornero, D., Donázar, J.A., Sánchez-Zapata, J.A., Benítez, J.R. & Barcell, M. 2002. Coprophagy: an unusual source of essential carotenoids. Nature 416: 807–808. doi: 10.1038/416807a
- Newton, I. 1979. Population Ecology of Raptors. Poyser, London.
- Newton, I. 1986. The Sparrowhawk. Poyser, London.
- NOAA. 2016. World climate maps. https://www.ncdc.noaa.gov/temp-and-precip/globalmaps/201613?products[]=map-prcp-percent#global-maps-select [accessed on 5 January 2017].
- Piersma, T. & van Gils, J.A. 2010. The Flexible Phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford University Press, Oxford.
- R Development Core Team 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
- Remeš, V., Freckleton, R.P., Tökölyi, J., Liker, A., & Székely, T. 2015. The evolution of parental cooperation in birds. Proc. Natl. Acad. Sci. USA 112: 13603–13608. doi: 10.1073/pnas.1512599112
- Rollack, C.E., Wiebe, K., Stoffel, M.J. & Houston, C.S. 2013. Turkey vulture breeding behavior studied with trail cameras. J. Raptor Res. 47: 153–160. doi: 10.3356/JRR-12-40.1
- Royle, N.J., Smiseth, P.T. & Kölliker, M. 2012. The Evolution of Parental Care. Oxford University Press, Oxford.
- Sanz, J.J., Potti, J., Moreno, J., Merino, S. & Frías, O. 2003. Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Glob. Change Biol. 9: 461–472. doi: 10.1046/j.1365-2486.2003.00575.x
- Sanz-Aguilar, A., Cortés-Avizanda, A., Serrano, D., Blanco, G., Ceballos, O., Grande, J.M., Tella, J.L. & Donázar, J.A. 2017. Sex- and age-dependent patterns of survival and breeding success in a long-lived endangered avian scavenger. Sci. Rep. 7: 40204. doi: 10.1038/srep40204
- Smith, P.A., Tulp, I., Schekkerman, H., Gilchrist, H.G. & Forbes, M.R. 2012. Shorebird incubation behaviour and its influence on the risk of nest predation. Anim. Behav. 84: 835–842. doi: 10.1016/j.anbehav.2012.07.004
- Sonerud, G.A., Steen, R., Løw, L.M., Røed, L.T., Skar, K., Selås, V. & Slagsvold, T. 2014a. Evolution of parental roles in raptors: prey type determines role asymmetry in the Eurasian kestrel. Anim. Behav. 96: 31–38. doi: 10.1016/j.anbehav.2014.07.011
- Sonerud, G.A., Steen, R., Selås, V., Aanonsen, O.M., Aasen, G.H., Fagerland, K.L., Fosså, A., Kristiansen, L., Løw, L.M., Rønning, M.E., Skouen, S.K., Asakskogen, E., Johansen, H.M., Johnsen, J.T., Karlsen, L.I., Nyhus, G.C., Røed, L.T., Skar, K., Sveen, B.A., Tveiten, R. & Slagsvold, T. 2014b. Evolution of parental roles in provisioning birds: diet determines role asymmetry in raptors. Behav. Ecol. 25: 762–772. doi: 10.1093/beheco/aru053
- Tarwater, C.E. & Brawn, J.D. 2010. The post-fledging period in a tropical bird: patterns of parental care and survival. J. Avian Biol. 41: 479–487. doi: 10.1111/j.1600-048X.2010.05006.x
- Watson, J. 2010. The Golden Eagle. Bloomsbury, London.
- Wiehn, J. & Korpimäki, E. 1997. Food limitation on brood size: experimental evidence in the Eurasian kestrel. Ecology 78: 2043–2050. doi: 10.1890/0012-9658(1997)078[2043:FLOBSE]2.0.CO;2
- Wilson, H.R., Neuman, S.L., Eldred, A.R. & Mather, F.B. 2003. Embryonic malpositions in broiler chickens and bobwhite quail. J. Appl. Poultry Res. 12: 14–23. doi: 10.1093/japr/12.1.14
- Xirouchakkis, S.M. & Mylonas, M. 2007. Breeding behaviour and parental care in the Griffon Vulture Gyps fulvus on the island of Crete (Greece). Ethol. Ecol. Evol. 19: 1–26. doi: 10.1080/08927014.2007.9522578
- Zuberogoitia, I., Zabala, J., Martínez, J.A., Martínez, J.E. & Azkona, A. 2008. Effect of human activities on Egyptian Vulture breeding success. Anim. Conserv. 11: 313–320. doi: 10.1111/j.1469-1795.2008.00184.x
- Zuberogoitia, I., Zabala, J., Martínez, J.E., González-Oreja, J.A. & López-López, P. 2014. Effective conservation measures to mitigate the impact of human disturbances on the endangered Egyptian Vulture. Anim. Conserv. 17: 410–418. doi: 10.1111/acv.12107
- Zuberogoitia, I., Martínez, J.E., Larrea, M. & Zabala, J. 2018. Parental investment of male Peregrine Falcons during incubation: influence of experience and weather. J. Ornithol. 159: 275–282. doi: 10.1007/s10336-017-1503-2