ABSTRACT
Capsule: Our findings regarding Hen Harrier Circus cyaneus territory site selection and breeding success in Ireland offer an opportunity for the development of initiatives and conservation actions aimed at enhancing the suitability of upland areas for breeding Hen Harriers and ensuring the long-term persistence of the species.
Aims: To investigate landscape-scale associations between habitat composition and Hen Harrier territory site selection, and to explore the influence of habitat and climate on breeding success.
Methods: We used multi-model inference from generalized linear models and Euclidean distance analyses to explore the influence of habitat, topographic, anthropogenic and climatic factors on Hen Harrier territory selection and breeding success in Ireland, based on data from national breeding surveys in 2010 and 2015.
Results: Hen Harrier territories were associated with heath/shrub, bog and pre-thicket coniferous forests. Comparisons between territories and randomly generated pseudo-absences (upland and lowland) showed that breeding pairs preferentially select for these habitats. Breeding success was negatively influenced by rainfall early in the breeding season and by climatic instability, and was positively influenced by the presence of heath/shrub and bog.
Conclusions: The results suggest that Hen Harrier breeding success is compromised by the synergistic effects of climate, landscape composition and management. Effective conservation of Hen Harriers in Ireland will therefore rely on landscape-scale initiatives.
Upland areas, typically found at higher elevation than nearby areas of enclosed farmland (O’Rourke & Kramm Citation2009), are of high conservation importance and support a diverse and characteristic assemblage of habitats and species (Thompson et al. Citation1995, Roche et al. Citation2014). However, uplands are also subject to a suite of pressures that result in the degradation and fragmentation of habitats (Douglas et al. Citation2008, O’Rourke & Kramm Citation2009, Ratcliffe Citation2010, Renou-Wilson et al. Citation2011, O’Riordan et al. Citation2015). This has led to the decline of many upland bird populations (Marquiss et al. Citation1985, Brawn et al. Citation2001, Julliard et al. Citation2004).
Hen Harriers Circus cyaneus are medium sized, ground-nesting birds of prey that are widely distributed throughout Eurasia, including the UK and Ireland (Millon et al. Citation2002, Redpath et al. Citation2002, Amar et al. Citation2008, Ruddock et al. Citation2016, Sachslehner et al. Citation2016). Populations have declined across the species’ range and they are now a Species of European Conservation Concern (SPEC; Staneva & Burfield Citation2017). They are listed under Annex I of the European Union (EU) Birds Directive (European Council Directive 79⁄409⁄EEC) which requires that EU member states protect them where they occur within national boundaries. This includes the designation of Natura 2000 sites, or Special Protected Areas (SPAs), as directed in Article 4 (Directive 2009/147/EC), and the implementation of ongoing monitoring initiatives such as the regular national surveys of breeding Hen Harriers in Ireland (Norriss et al. Citation2002, Barton et al. Citation2006, Ruddock et al. Citation2012, Ruddock et al. Citation2016).
Hen Harriers typically use upland habitats during the breeding season, often nesting in heather moorland (Redpath et al. Citation1998, Amar et al. Citation2008, Watson Citation2017). Elsewhere, Hen Harriers are known to use other habitats, such as cereal fields and young forest plantations (Millon et al. Citation2002, Wilson et al. Citation2009, Citation2012, Ruddock et al. Citation2016, Sachslehner et al. Citation2016) where the dense understory provides nesting habitat and foraging opportunities (Redpath et al. Citation1998, Madders Citation2000). The breeding success of Hen Harriers can be affected by many factors, including food availability (Amar & Redpath Citation2002, Amar et al. Citation2003), predation (Irwin et al. Citation2012, Ruddock et al. Citation2016), habitat (Amar et al. Citation2008, Wilson et al. Citation2012), proximity to wind farms (Fernandez-Bellon et al. Citation2015) and climate (García & Arroyo Citation2001, Redpath et al. Citation2002). Breeding success varies considerably between different areas and the average number of chicks raised to fledging in Ireland is lower than observed in the UK (Fielding et al. Citation2011, Irwin et al. Citation2012). The subsequent survival of juveniles, and the proportion recruited into the Irish breeding population, are largely unknown at present.
Hen Harriers were once widespread in Ireland until historic habitat loss resulted in substantial reductions in both range and abundance (O’Flynn Citation1983, Whilde Citation1993). The population showed some signs of recovery during the mid-twentieth century, peaking at a reported 200–300 pairs in the 1970s (Watson Citation2017) though the decline resumed thereafter (Norriss et al. Citation2002, Barton et al. Citation2006, Ruddock et al. Citation2012, Citation2016). The current Hen Harrier population in Ireland is moderately small, with 108–157 breeding pairs recorded in 2015 (Ruddock et al. Citation2016). Thus, the species is of considerable conservation concern in Ireland (Colhoun & Cummins Citation2013). In 2007, six SPAs were established for Hen Harrier conservation in the Republic of Ireland. Afforestation, forest management, development (e.g. windfarms) and recreational activities are regulated in these areas and they include important breeding habitats such as heather moorland, bogs, rough grassland and young conifer plantations (Wilson et al. Citation2009). However, all SPAs contain considerable forest cover, primarily in the form of non-native conifer plantations (Moran & Wilson-Parr Citation2015). This is typical of upland areas in Ireland where large tracts of upland habitats have been afforested in recent decades (O’Leary et al. Citation2000).
Afforestation (the planting of forest in an area where there was little or no previous tree cover) has resulted in significant declines of some upland bird populations (Thompson et al. Citation1988, Ratcliffe Citation2010) including the Hen Harrier (O’Flynn Citation1983, Wilson et al. Citation2009). Ireland’s afforestation goals are ambitious, with forest estate coverage expected to expand from the current 11% of total land cover to 18% by 2046 (National Parks & Wildlife Service Citation2015). This represents a considerable change in land-use with implications for Hen Harrier conservation, particularly as forest plantations mature and become unusable for nesting and foraging (Picozzi Citation1978, Wilson et al. Citation2012). Furthermore, afforestation has negative implications for upland species beyond the immediate transformation of open habitats. For example, forest fragments act as reservoirs for generalist predators (Small & Hunter Citation1988, Andren Citation1992, Kurki et al. Citation1998), increasing the risk of nest depredation, particularly near forest edges, and/or driving avoidance of habitat patches associated with forest edges (Douglas et al. Citation2011, Wilson et al. Citation2014). Thus, appreciating the links between habitat abundance, quality and/or connectivity and the persistence of a species requires a nuanced understanding of the focal species’ ecology.
Bird populations can also be negatively affected by changes in temperature (Wingfield Citation1984) and rainfall (Elkins Citation1984), mediated by effects on reproductive success related to the thermoregulatory inefficiencies of young chicks (Nye Citation1964, Elkins Citation1984) and associated adult brooding behaviour. In cold environments, both chicks and adults may expend more energy counteracting heat loss, leading to greater food demands (Weathers Citation1979). This can result in adults spending more time foraging (Redpath et al. Citation2002), thus increasing chick vulnerability via exposure or, conversely, substantially increase brooding time which can result in chick mortality via starvation (Beintema & Visser Citation1989). The effects of cold temperatures may be exacerbated by rainfall as the downy feathers of young chicks are not fully water-repellent; wet chicks lose heat more rapidly than dry chicks (Nye Citation1964). However, while both temperature and rainfall have been shown to affect Hen Harrier breeding success (Schipper Citation1979, García & Arroyo Citation2001, Redpath et al. Citation2002), their impacts vary across the species’ range, likely due to regional differences in climate. For example, Hen Harrier brood size was positively related to temperature in Scotland (Redpath et al. Citation2002) while the opposite was true in Spain (García & Arroyo Citation2001). Thus, understanding the relationship between climate and breeding success in this species requires discrete, region-specific studies.
Here we used data derived from national breeding Hen Harrier surveys in Ireland, together with data on landscape, climate and man-made features to explore local factors affecting the location of breeding-pair territories and landscape-scale factors affecting breeding success. We hypothesize that: (i) Hen Harrier territories will be strongly associated with pre-thicket coniferous forests; (ii) breeding success will be negatively affected by the amount of coniferous forest in the landscape; and (iii) there will be no discernible effect of SPAs status on patterns of Hen Harrier settlement or breeding success. We discuss our findings in the context of previous work on the habitat associations of Hen Harriers in Ireland and Hen Harrier conservation. Consequently, we provide recommendations regarding habitat management and investigative avenues for future research which would provide a basis for the development of ecologically appropriate conservation and management measures.
Methods
Data sources and preparation
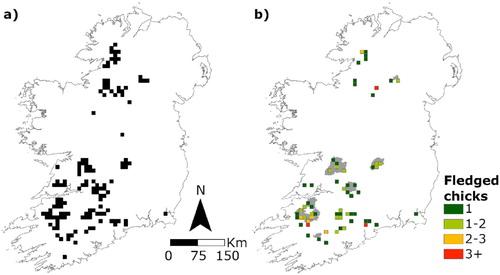
A total of 668 records of potential Hen Harrier territories collected during national breeding Hen Harrier surveys in Ireland in 2010 and 2015 were provided by the National Parks and Wildlife Service (NPWS). These data were collected by an extensive network of staff, members and volunteers from the NPWS, Irish Raptor Study Group (IRSG), BirdWatch Ireland (BWI) and Golden Eagle Trust (GET), university researchers, as well as independent commercial and voluntary ornithological surveyors working across Ireland (Ruddock et al. Citation2012, Citation2016). Two discrete datasets were derived from the raw data. The first comprised of all confirmed territories (n = 236; 2010 = 128, 2015 = 108; (a). The second was restricted to records with known breeding outcomes (i.e. success or failure; n = 191; 2010 = 94, 2015 = 97; (b).
Figure 1. (a) Confirmed territory locations and (b) mean productivity (number of chicks fledged) of Hen Harriers in Ireland in 2010 and 2015, combined. Special Protection Areas (SPAs) are represented by grey polygons in (b).
Pseudoabsences (pa1) were randomly generated within the altitudinal range of confirmed Hen Harrier territories (n = 500; 36 m–570 m). Each point (i.e. territory or pseudoabsence) was buffered to three distances (Graf et al. Citation2005): 1, 2 and 5 km, chosen to represent variable foraging distances from the nest and to enable comparisons with previous studies (Schipper Citation1977, Wilson et al. Citation2009, Arroyo et al. Citation2014). Breeding Hen Harriers in Ireland have been reported to travel over 11 km from an active nest, via GPS tracking (Irwin et al. Citation2012), and males in Scotland have been observed travelling up to 9 km from nests (Arroyo et al. Citation2014). However, typical foraging ranges are reported to be much smaller and, in most centrally placed foragers, the intensity with which suitable foraging areas are used declines with distance from the nest or roost site to which individuals return (Arroyo et al. Citation2014). Hence, conservative distances were used.
To account for spatial autocorrelation, i.e. clustering of presence records, Moran’s I Index scores were calculated for each point using the cluster and outlier analysis (Anselin Local Moran’s I) function in the ArcGIS toolbox, that calculates a Local Moran’s I value for each point data in the dataset, allowing the identification of spatially autocorrelated data (e.g. hot-spots, outliers).
We investigated the effect of several, ecologically relevant variables on Hen Harrier territory location and breeding success, including: forest composition (broadleaved or coniferous); coniferous forest age; land class; temperature; rainfall; hilliness; elevation; SPA (inside/outside site boundary); proximity to windfarms; proximity to post-thicket coniferous forest; and proximal road density (see for variable-specific references). Data temporally relevant to the 2010 and 2015 Hen Harrier surveys (i.e. nest site/success, climate, weather, forest age) were grouped accordingly. Non-forest land class variables were assumed to be temporally consistent between surveys.
Table 1. Variables used in Hen Harrier territory site selection and breeding performance models. ‘Raw’ variables were not manipulated prior to analyses. Variables are listed according to the order in which they occur in the main text. CORINE class details are given in parentheses where appropriate. References are given to support the inclusion of each variable.
Forest data were extracted from the CORINE 2012 land cover dataset (European Environment Agency Citation2016; see for CORINE class details) and were augmented with data from Coillte (public forests in Ireland), NPWS (private forests in Ireland) and the Forest Service Northern Ireland (public and private forests in Northern Ireland). Forest data were classified by type (broadleaved or coniferous); mixed forest where conifers accounted for ≤50% of the total area were classified as broadleaved and mixed forest with >50% conifers were classified as coniferous. Coniferous forests were further divided into three age categories, according to known Hen Harrier nest site selection preferences (Irwin et al. Citation2012, Wilson et al. Citation2012): (i) early (0–2 years, post-planting); (ii) pre-thicket (3–12 years, post-planting); and (iii) post-thicket (≥13 years, post-planting). Post-thicket forest data were merged with CORINE coniferous data, which represent mature forests. Early and pre-thicket forest data were then erased from the composite CORINE-post-thicket shapefile. The accuracy of derived forest shapefiles in describing total forest coverage was visually assessed via comparison with satellite optical imagery. In order to investigate the effects of land-use, additional, non-forest land cover variables were extracted from the CORINE dataset: two composites (arable; heath/shrub) and four raw variables (bog; natural grassland; pasture; urban; see for CORINE class details).
The total area of each land cover variable and forest category and road density were calculated within each point buffer. The effect of spatial scale was explored by constructing GLMMs for individual variables across all buffers. The most suitable buffer distance for each variable was chosen, a priori, based on the size of the regression coefficients from these exploratory models; selected scales had the largest coefficients. Euclidean distances were calculated from each point to the nearest stand (edge) of post-thicket forest.
Weekly temperature (°C) and rainfall (mm) data were obtained from 27 weather stations dispersed across the island of Ireland, from Met Éireann (http://www.met.ie) and the Met Office (https://data.gov.uk). Rainfall data were further split into early-to-mid breeding season (‘early’ hereafter; March–May, inclusive) and mid-to-late breeding season (‘late’ hereafter; June–August, inclusive). Mean weekly rainfall and associated variance were calculated for each period. Temperature measurements – mean of weekly minima and associated variance – were calculated across the entire breeding season. Variance was taken as a proxy for climatic stability. For example, low daily variance in rainfall would suggest that the amount of rain that fell on a daily basis was temporally consistent. In contrast, high variance could suggest irregular patterns of rainfall or a trend in rainfall over time. Interpolated regularized raster surfaces (grid-based data structures; Aggrey Citation2002) were constructed at 1 km resolution for each climate metric using the Spline function in ArcGIS 10.4.1 (ESRI Citation2015), giving 100% coverage to the island of Ireland. Climate measurements for each nest were taken as the interpolated value for the 1 km square within which the point was located.
We used a 30 arc-second Digital Elevation Model (DEM) from NASA’s Shuttle Radar Topography Mission (SRTM; https://eros.usgs.gov/) to derive elevation data for each point (‘elevation’). Shapefiles describing SPA boundaries and the locations of windfarms – given as centroids – across Ireland, correct to 2016, were provided by the NPWS. Euclidean distances were calculated from each point to the nearest windfarm. Road data were downloaded from OpenStreetMap.org (https://www.openstreetmap.org). Only roads, link roads and tracks were included in our analyses (see https://wiki.openstreetmap.org/wiki/Key:highway for more on OpenStreetMap highway categories), all of which included road types which were present in areas used by Hen Harriers. Road density was calculated as a function of the total length of roads divided by total polygon area. Shapefile and raster processing and manipulation were carried out using the statistical program R (R Core Team Citation2017), particularly the packages raster (Hijmans Citation2017), rgeos (Bivand & Rundel Citation2017), rgdal (Bivand et al. Citation2017) and maptools (Bivand & Lewin-Koh Citation2017) and ArcGIS 10.4.1 (ESRI Citation2015).
Territory selection models
The centres of putative Hen Harrier territories were estimated as nest locations, where these were known, or as the approximate midpoint of observations involving behaviours and activities consistent with breeding, for other breeding territories identified during the survey (Ruddock et al. Citation2016). Hen Harrier territory sites were compared to hypothetical territory sites (i.e. pseudoabsences) in the wider landscape to establish the ecological distinctiveness of territories relative to other habitat mosaics.
Territory selection was examined using binomial, log-linked generalized linear mixed models (GLMMs) and model weighting using the R packages lme4 (Bates et al. Citation2015) and MuMIn (Bates et al. Citation2015). The presence or pseudoabsence of a territory was fitted as the dependent variable; Moran’s I scores were fitted as a random factor. Predictor variables were tested for multicollinearity, ensuring that Tolerance values were >0.2, variance inflation factor (VIF) values were <10.0 and bivariate correlations had r < 0.5 (Quinn & Keogh Citation2002). Variables were standardized to have a mean of zero and a standard deviation of 1 prior to analysis, thus permitting the direct comparison of regression coefficients. We used the Akaike Information Criterion (AIC) to rank all possible model permutations. The top subset of models was defined by the threshold ΔAIC ≤ 2 units (Burnham & Anderson Citation2002). The model with the lowest Akaike weight (ωi) was identified as being the best approximating model within the top subset of N models. To determine the relative importance of each variable, the Σωi of all models containing the focal variable within the top subset was calculated (McAlpine et al. Citation2006), where the Σωi of omnipresent variables = 1. The effect size (β coefficient) of each variable was determined via multi-model inference and model averaging (Burnham & Anderson Citation2002). Variables were ranked, first by Σωi, and, secondarily where variables had equal Σωi values, by the magnitude of their regression coefficients. The performance of the best approximating model was assessed using a 60% training set and a 40% test set with 10-fold cross-validation (R package caret; Kuhn Citation2017).
Territory records and pa1 were augmented by an additional set of pseudoabsences (pa2) to facilitate inferential exploration of habitat choice via ecological distance analysis. To create pa2, we generated 500 randomly placed points across the remaining Irish landscape, beyond elevational constraints described above. These additional locations provided a broader context for interpretation of ecological distances between territory locations and pa1. Principal Component Analysis was used to reduce climate and habitat variables associated with all locations to five hypothetical axes with eigenvalues >1. We calculated a single measure of ecological, Euclidean distance between groups (territories, pa1, pa2) in nth-dimensional space across all Principal Components simultaneously. Euclidean distances were calculated using the R package pdist (Wong Citation2013) and the base function dist.
Breeding success models
Breeding success models were constructed to explore factors affecting Hen Harriers at mixed landscape scales using the methods described for territory models (see Territory selection models, above) but on the subset of territories with known nest success outcomes (i.e. success/failure). Territory centroids were assumed to be nest locations based on the best available data. Additional point data for each centroid were extracted for SPA (inside or outside the boundary); minimum temperature; the variance of minimum temperature across the breeding season; mean weekly rainfall in the early breeding season; and mean weekly rainfall in the late breeding season. Eighty six centroids were located inside SPAs with 112 occurring outside SPA boundaries (2010 = 36:65; 2015 = 50:47).
Breeding success was examined using a Poisson GLMM; the number of chicks successfully fledged (b) was fitted as the dependent variable and Moran’s I was fitted as a random factor. Model construction, selection and evaluation followed the same methods described for territory selection models (see Territory selection models, above). Data used in this project were subject to confidentiality and data sharing agreements. However, code used to manipulate data and shapefiles and construct models and plots can be found at http://doi.org/10.5281/zenodo.3549584.
Results
Hen Harrier territory locations exhibited significant spatial autocorrelation (I = −0.003 ± 0.005, P < 0.0001; ). Hen Harrier nest sites are typically used in successive years, though not necessarily by the same breeding pair (Korpimaki Citation1984, Picozzi Citation1978, Citation1984, Watson Citation2017). Here, 2010 territories were located at least 141 m (mean ± sd = 3.80 ± 7.61 km) from the nearest territory in 2015. The top subset (ΔAIC ≤ 2) consisted of 18 models (supplementary online Table S1). The best approximating model for territory site selection was positively influenced by heath/shrub, pre-thicket forest and bog at 1 km, indicating that Hen Harrier territories were strongly associated with habitats that ostensibly offer an appropriate nesting environment. There was a negative association with pasture at 2 km and with broadleaved woodland at 5 km, two habitats that are not typically associated with breeding Hen Harriers. Territories were also positively associated with increased elevation, being found at higher altitudes than pa1 (). The predictive accuracy of the best-approximating model, assessed via 10-fold cross-validation, was 0.82 (±0.02).
Figure 2. Relative importance of variables in explaining the locations of confirmed Hen Harrier territories relative to pseudoabsences at multiple spatial scales (1, 2 and 5 km, selected a priori), except for elevation which was extracted at each point location. D_ = distance to. Variables were ranked according to the sum of their Akaike weights within the top set of models (ΔAIC < 2). Black bars indicate variables that were present in the best approximating model; white bars indicate variables otherwise included in the top subset. Standardized coefficients ± se and P values are given to the right, where * = P < 0.05, ** = P < 0.001 and *** = P < 0.0001. The inset plot describes model accuracy as evaluated using randomly split 60:40 training:test datasets with 10-fold cross-validation.
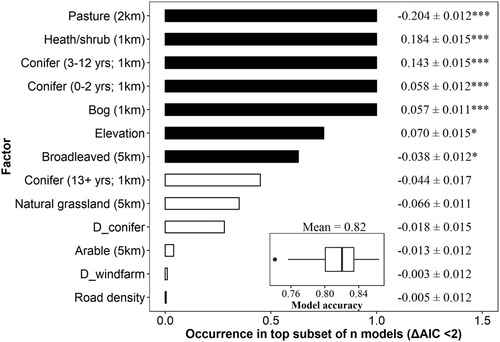
Figure 3. Euclidean distances (±1 sd) across five Principal Component scores for pairwise combinations Hen Harrier territory locations (t), upland pseudoabsences (pa1) and pseudoabsences distributed across the rest of Ireland (pa2).
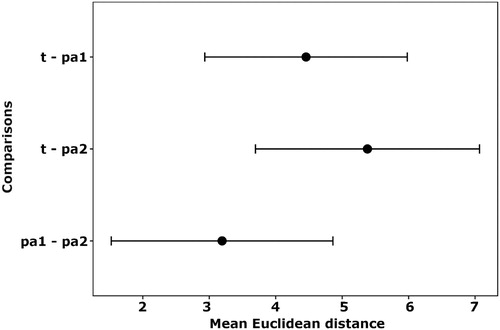
According to single-metric nth-dimensional Euclidean distance analyses, territory locations were on average 17% further away from pa2 than pa1 and 27% further away from pa1 than pa1 and pa2 were from each other (). This indicates that Hen Harriers are not only using upland habitats as territory locations but that they are specifically using the landscape non-randomly with regard to habitat availability.
Figure 4. Relative importance of variables in explaining the breeding success of nesting Hen Harriers at multiple spatial scales (1, 2 and 5 km, selected a priori). Variables were ranked according to the sum of their Akaike weights within the top set of models (ΔAIC < 2). Black bars indicate variables that were present in the best approximating model; white bars indicate variables otherwise included in the top subset. Standardized coefficients ± se and P-values are given to the right, where * = P < 0.05, ** = P < 0.001 and *** = P < 0.0001. The inset plot describes model accuracy as evaluated using randomly split 60:40 training:test datasets with 10-fold cross-validation.
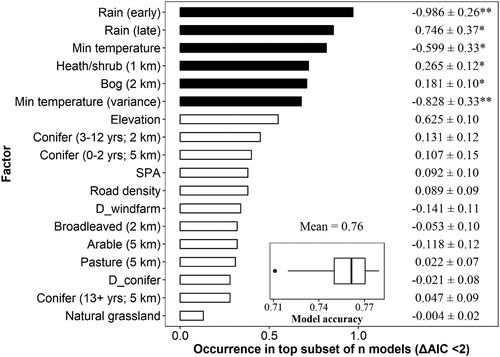
Hen Harrier territory locations with known breeding success outcomes exhibited significant spatial autocorrelation (I = −0.118 ± 0.001, P = 0.002). The top subset (ΔAIC ≤ 2) consisted of 23 models (online Table S2). The best approximating model for breeding success was negatively influenced by mean weekly rainfall early in the breeding season, mean weekly minimum temperatures and the variance in mean weekly minimum temperature. This suggests that chicks are most vulnerable to stochastic changes in minimum temperature, possibly exacerbated by rainfall that could cause prolonged chilling, during the early stages of the breeding season. There were positive associations with mean weekly rainfall late in the breeding season, heath/shrub habitat at the 1 km scale and bog at 2 km. Both habitats are typically associated with breeding Hen Harriers elsewhere in the species’ range. In contrast to territory analyses, coniferous forest age classes did not feature in the best approximating model for breeding success (). The predictive accuracy of the best-approximating model, assessed via 10-fold cross-validation, was 0.76 (±0.01).
Discussion
Across the 2010 and 2015 Hen Harrier national survey data, the influence of land class and associated parameters on the utilization of habitats for territories contrasted with their influence on subsequent breeding success. Hen Harrier territories in Ireland were positively associated with heath/shrub, bog, areas at high elevation, and pre-thicket coniferous forest (i.e. 0–12 years old), confirming our first hypothesis. Breeding success was similarly positively associated with heath/shrub and bog. However, there was a negative association with rain in the early months of the breeding season and minimum temperature metrics. In contrast to territory models, pre-thicket forests were not observed to have an effect on breeding success, rejecting our second hypothesis. SPAs were not included in the best approximating breeding success model but were observed to have a moderate positive effect on breeding success, leading us to reject our third hypothesis.
The strong positive associations between habitats typical of open, upland landscapes in Ireland (i.e. heath/shrub and bog) and both territory location and breeding success models emphasize the importance of these habitats for breeding and foraging Hen Harriers (Redpath et al. Citation1998, Madders Citation2000, Amar et al. Citation2008, Arroyo et al. Citation2009). Optimal nesting habitat should offer a complex vegetation structure for nest concealment and protection, while good foraging habitats will have high prey availability. These factors asynchronously contribute towards determining how prospecting Hen Harriers choose their territories and subsequent foraging behaviour and breeding success. Research has shown that while male Hen Harriers forage independently of nest location, females frequently hunt within 300–500 m of the nest (Arroyo et al. Citation2009). Hen Harrier breeding success in one UK SPA was positively related to a greater abundance of preferred foraging habitat within 2 km of nest sites (Amar et al. Citation2008) and breeding success can be affected by food availability before and during nesting (Amar & Redpath Citation2002, Amar et al. Citation2003, Citation2005). Thus, territory location and the proximity of good quality foraging habitats are strongly linked. It is possible that differences in prey species assemblages, abundance and availability (Wilson et al. Citation2012) between habitats could help explain the observed differences between territory selection and breeding success models in the current study. Given the importance of health/shrub and bog habitats across both models, conservation measures targeted at stabilizing and subsequently increasing the Hen Harrier population in Ireland should aim to improve the quality and abundance of these important habitats.
There was a particular association between Hen Harrier territories and pre-thicket forests 3–12 years post-planting. While previous studies at a number of locations across Ireland and the UK have described similar associations with pre-thicket forest (Madders Citation2000, Barton et al. Citation2006, Wilson et al. Citation2009, O’Donoghue Citation2010, Irwin et al. Citation2012), this is the first to consider this on such a large scale (the whole of Ireland). Pre-thicket forest undergrowth may consist of heather (Ericaceae), gorse (Ulex sp.) and bramble (Rubus fruticosus agg.), providing nest security against potential predators (O’Flynn Citation1983) and making these areas attractive to breeding Hen Harriers. The use of these habitats by Hen Harriers may be indicative of a lack of more suitable nesting and/or foraging habitat in the wider landscape. While there was no apparent impact of pre-thicket forests on breeding success, they were sub-optimal when compared to heath/shrub and bog. Indeed, Hen Harriers breeding in coniferous forest in Scotland exhibit lower breeding success than those that nest in moorlands (Etheridge et al. Citation1997). Furthermore, while Hen Harriers can and do make use of pre-thicket forests for nesting and foraging, maturation of forests beyond the pre-thicket stage to closed canopies results in unsuitable nesting habitat and limits foraging opportunities (Madders Citation2003, though see Wilson et al. Citation2012). Afforestation of heath/shrub and bog habitats would, therefore, result in a net decline in Hen Harrier breeding success in afforested upland areas.
The location of centroids relative to SPA boundaries (i.e. inside or outside) was retained in the top subset of breeding success models (46% of all models), though it was not retained in the best approximating model and SPAs were positively associated with breeding success. Proposed land use changes and industrial activities within SPAs (e.g. road construction, clear-felling, afforestation) are subject to a suite of regulations in Ireland, many of which are aimed at mitigating disturbance of breeding Hen Harriers in high sensitivity areas (i.e. ‘Red Areas’, NPWS Citation2015). The apparent success of SPAs in facilitating breeding success appears to be skewed by increased success in locations where heather and moorland nesting and foraging habitats may be of higher quality and/or less fragmented (). It is important to note, however, that over 50% of the breeding Hen Harrier population was located outside of the six breeding Hen Harrier SPAs during both survey years and that the Hen Harrier population in the SPA network has declined over this time (Ruddock et al. Citation2012, Citation2016). The value of the wider countryside to Hen Harrier conservation is twofold. First, a species with a wider breeding range will be more robust to pressures acting at a site level. Second, it is possible that the breeding population within SPAs could drop below a critical level. A sufficiently large and persistent population outside of the SPA network could improve the recolonization potential for those SPAs that are at risk of local extinctions. We recommend, therefore, that conservation initiatives aimed at bolstering Hen Harrier populations in Ireland embrace a landscape-scale approach and do not focus on SPAs alone.
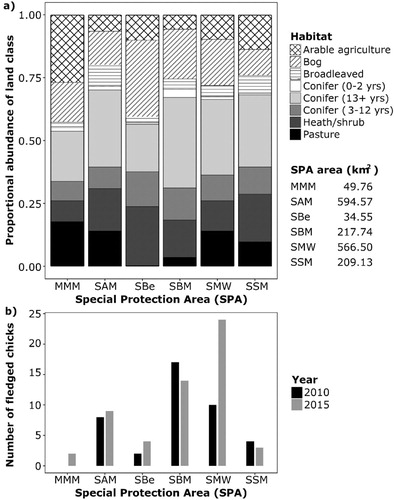
Figure 5. (a) Habitat composition of Special Protection Areas (SPAs) in Ireland that contained (b) successful Hen Harrier nests (produced at least 1 fledged chick) in 2010 and 2015. Natural grassland was omitted as it comprised a small fraction of available habitats across all SPAs. MMM = Mullaghanish to Musheramore Mountains; SAM = Slieve Aughty Mountains SPA; SBe = Slieve Beagh; SBM = Slieve Bloom Mountains; SMW = Stacks to Mullaghareirk Mountains, West Limerick Hills and Mount Eagle; SSM = Slievefelim to Silvermines Mountains. SPA areas were derived from the NPWS SPA shapefile 2017_06.
Hen Harrier breeding success was affected by temperature and climatic instability (i.e. the variation in minimum temperature) throughout the breeding season, and by rainfall in the early breeding season. The mechanisms by which temperature and rainfall influence Hen Harrier breeding success are unclear at present, as studies elsewhere in the species’ range reveal regionally variable effects (Schipper Citation1979, García & Arroyo Citation2001, Redpath et al. Citation2002). This suggests that climate may be masking discrete ecological and behavioural phenomena. For example, poor foraging opportunities in the surrounding landscape may be placing a larger provisioning burden on both parents who consequently must travel greater distances to find food (e.g. see flight distances in Irwin et al. Citation2012). Decreased parental attendance may also result in greater vulnerability of eggs and chicks to predation. Potential predators of Hen Harrier nests in Ireland include Red Foxes Vulpes vulpes, European Badgers Meles meles, Pine Martens Martes martes, American minks Neovison vison, Stoats Mustela erminea, Common Buzzards Buteo buteo, Ravens Corvus corax and Hooded Crows Corvus cornix. Such predators are typically more abundant in fragmented habitats (Andren Citation1992, Kurki et al. Citation1998) and can have substantial negative impacts on ground-nesting birds (Paton Citation1994, Fletcher et al. Citation2010). Foxes and Pine Martens have been observed depredating Hen Harrier chicks in studies using remote-sensing camera traps (Monaghan Citation2015, Ruddock et al. Citation2016, Fernández-Bellon et al. Citation2018). Furthermore, increased rainfall may place an additional thermoregulatory burden on young chicks via increased metabolic costs and greater food demands (Weathers Citation1979, Olsen & Olsen Citation1992, Redpath et al. Citation2002). These impacts could be exacerbated by the stochastic effects of an increasingly unpredictable climate such that young chicks are rendered particularly vulnerable to chilling during the coldest periods. Thus, the synergistic effects of reduced parental attendance, increased predation risk and increased energetic demands of exposed chicks via unsupported thermoregulation could go some way to explaining the observed impacts of climate on Hen Harrier breeding success in the current study.
Our findings have implications for the long-term viability and security of Hen Harrier populations in Ireland under continued land use change and future climate change. The early months of the Hen Harrier breeding season are predicted to become increasingly warmer and wetter under future climate change scenarios, while summer months (i.e. late breeding season) will be drier (Gleeson et al. Citation2013). Many studies have demonstrated that climate change can impact breeding birds via several mechanisms, including egg-laying phenology (Crick et al. Citation1997, Geyer et al. Citation2011), disease (Benning et al. Citation2002) and changes in prey availability (e.g. Pearce-Higgins Citation2010). Climate change impacts may be exacerbated by changes in land management that could simultaneously reduce the proportion of suitable foraging habitat in the landscape (Kleijn et al. Citation2010). It is therefore important that the potential impacts of climate change on Irish Hen Harrier breeding success and distribution are monitored and that appropriate mitigation measures are explored and established.
Hen Harriers in Ireland currently face an uncertain future. Hen Harriers in this study preferentially selected health/shrub and pre-thicket coniferous forests, habitats that provide nesting and foraging opportunities, for territory locations. Rainfall and climatic instability early in the breeding season were found to have strong negative effects on subsequent breeding success, suggesting that the population is at further risk under future climate change. Upland habitats typically used by Hen Harriers elsewhere, i.e. heath/shrub and bog, were positively associated with breeding success of Hen Harriers in this study, emphasizing the importance of such habitats for this threatened species. Continued afforestation of upland areas, moorlands and bog in particular, along with maturation of the existing ‘usable’ forest estate beyond the pre-thicket stage, and the impacts of climate change, will likely negatively impact Hen Harrier populations in Ireland. Effective conservation of Hen Harriers in Ireland is therefore likely to rely on landscape-scale initiatives, including the creation/restoration of suitable nesting and breeding habitat and protection for this species within and beyond the boundaries of the SPA network.
Acknowledgements
The authors would like to thank the many researchers, ornithological surveyors and members of staff and volunteers from the NPWS, IRSG, BWI and GET for their work in collecting data for the 2010 and 2015 national breeding Hen Harrier surveys, without whom this research would not have been possible. Thanks to Coillte and the Forest Service for the use of their databases. We are particularly grateful to Mark Wilson, Niamh Hennessy, Pat Neville, Kevin Collins, Ilse Corkery, John Ballinger and Ryan Wilson-Parr who, along with the authors, contributed to stakeholder and scientific steering groups for the Supporting Hen Harriers in Novel Environments (SHINE) research project at UCC. We offer additional thanks to Darío Fernández-Bellon and Brian Clayton. We thank the Editor and two reviewers whose comments helped to improve the manuscript. AC dedicates this work to his daughter, Indy Rey Caravaggi.
ORCID
Anthony Caravaggi http://orcid.org/0000-0002-1763-8970
John O’Halloran http://orcid.org/0000-0002-8150-7510
Additional information
Funding
References
- Aggrey, S.E. 2002. Comparison of three nonlinear and spline regression models for describing chicken growth curves. Poul. Sci. 81: 1782–1788. doi: 10.1093/ps/81.12.1782
- Amar, A. & Redpath, S.M. 2002. Determining the cause of the Hen Harrier decline on the Orkney Islands: An experimental test of two hypotheses. Anim. Conserv. 5: 21–28. doi: 10.1017/S1367943002001038
- Amar, A. & Redpath, S.M. 2005. Habitat use by Hen Harriers Circus cyaneus on Orkney: Implications of land-use change for this declining population. Ibis 147: 37–47. doi: 10.1111/j.1474-919x.2004.00314.x
- Amar, A., Redpath, S. & Thirgood, S. 2003. Evidence for food limitation in the declining Hen Harrier population on the Orkney Islands, Scotland. Biol. Conserv. 111: 377–384. doi: 10.1016/S0006-3207(02)00306-3
- Amar, A., Picozzi, N., Meek, E.R., Redpath, S.M. & Lambin, X. 2005. Decline of the Orkney Hen Harrier Circus cyaneus population: Do changes to demographic parameters and mating system fit a declining food hypothesis? Bird Study 52: 18–24. doi: 10.1080/00063650509461370
- Amar, A., Arroyo, B., Meek, E., Redpath, S. & Riley, H. 2008. Influence of habitat on breeding performance of Hen Harriers Circus cyaneus in Orkney. Ibis 150: 400–404. doi: 10.1111/j.1474-919X.2007.00765.x
- Andren, H. 1992. Corvid density and nest predation in relation to forest fragmentation: A landscape perspective. Ecology 73: 794–804. doi: 10.2307/1940158
- Arroyo, B., Amar, A., Leckie, F., Buchanan, G.M., Wilson, J.D. & Redpath, S. 2009. Hunting habitat selection by Hen Harriers on moorland: Implications for conservation management. Biol. Conserv. 142: 586–596. doi: 10.1016/j.biocon.2008.11.013
- Arroyo, B., Leckie, F., Amar, A., McCluskie, A. & Redpath, S. 2014. Ranging behaviour of Hen Harriers breeding in special protection areas in Scotland. Bird Study 61: 48–55. doi: 10.1080/00063657.2013.874976
- Barton, C., Pollock, C., Norriss, D.W., Nagle, T., Oliver, G.A. & Newston, S. 2006. The second national survey of breeding Hen Harriers Circus cyaneus in Ireland. Irish Birds 8: 1–20.
- Bates, D., Maechler, M., Bolker, B. & Walker, S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. doi: 10.18637/jss.v067.i01
- Beintema, A.J. & Visser, G.H. 1989. The effect of weather on time budgets and development of chicks of meadow birds. Ardea 77: 181–192.
- Benning, T.L., LaPointe, D., Atkinson, C.T. & Vitousek, P.M. 2002. Interactions of climate change with biological invasions and land use in the Hawaiian Islands: Modeling the fate of endemic birds using a geographic information system. P. Natl. Acad. Sci. 99: 14246–14249. doi: 10.1073/pnas.162372399
- Bivand, R. & Lewin-Koh, N. 2017. maptools: Tools for Reading and Handling Spatial Objects. R package version 0.9-4. Accessed 10th February 2017. Available at: https://CRAN.R-project.org/package=maptools
- Bivand, R. & Rundel, C. 2017. rgeos: Interface to Geometry Engine – Open Source (‘GEOS’). R package version 0.4-2. Accessed 7th February 2017. Available at: https://CRAN.R-project.org/package=rgeos
- Bivand, R., Keitt, T. & Rowlingson, B. 2017. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R package version 1.3-6. Accessed 7th February 2017. Available at: https://CRAN.R-project.org/package=rgdal.
- Brawn, J.D., Robinson, S.K. & Thompson III F.R. 2001. The role of disturbance in the ecology and conservation of birds. Ann. Rev. Ecol. Syst. 32: 251–276. doi: 10.1146/annurev.ecolsys.32.081501.114031
- Burnham, K. & Anderson, D. 2002. Model Selection and Multi-Model Inference: a practical information-theoretic approach. Springer, New York.
- Colhoun, K. & Cummins, S. 2013. Birds of conservation concern in Ireland. Irish Birds 9: 523–544.
- Cormier, J.-P., Fustec, J., Pithon, J. & Choisy, P. 2008. Selection of nesting habitat by Montagu’s Harriers Circus pygargus and Hen Harriers Circus cyaneus in managed heaths. Bird Study 55: 86–93. doi: 10.1080/00063650809461508
- Crick, H.Q.P., Dudley, C., Glue, D.E. & Thomson, D.L. 1997. UK birds are laying eggs earlier. Nature 388: 526–526. doi: 10.1038/41453
- Douglas, C., Fernandez, F. & Ryan, J. 2008. Peatland habitat conservation in Ireland. In Farrell, C. & Feehan, J. (eds) 13th International Peat Congress: after wise-use: the future of Peatlands, 13. International Peat Society, Tullamore, Ireland.
- Douglas, D.J.T., Bellamy, P.E. & Pearce-Higgins, J.W. 2011. Changes in the abundance and distribution of upland breeding birds at an operational wind farm. Bird Study 58: 37–43. doi: 10.1080/00063657.2010.524914
- Elkins, N. 1984. Weather and Bird Behaviour. Bloomsbury Publishing, London.
- ESRI. 2015. ArcGIS Desktop: release 10. Environmental Systems Research Institute, Redlands, CA.
- Etheridge, B., Summers, R.W. & Green, R.E. 1997. The effects of illegal killing and destruction of nests by humans on the population dynamics of the hen harrier Circus cyaneus in Scotland. J. Appl. Ecol. 34: 1081–1105. doi: 10.2307/2405296
- European Environment Agency. 2016. Corine Land Cover (CLC) 2012. Accessed 12th January 2017. Available at: http://land.copernicus.eu/pan-european/corine-land-cover/clc-2012.
- Fernández-Bellon, D., Irwin, S., Wilson, M. & O’Halloran, J. 2015. Reproductive output of Hen Harriers Circus cyaneus in relation to wind turbine proximity. Irish Birds 10: 143–150.
- Fernández-Bellon, D., Wilson, M., Irwin, S., Kelly, T.C., O’Mahony, B. & O’Halloran, J. 2018. Video evidence of siblicide and cannibalism, movement of nestlings by adults, and interactions with predators in nesting hen harriers. J. Raptor Res. 52: 393–399. doi: 10.3356/JRR-17-58.1
- Feys, S., Guelinckx, R., Verdonckt, F. & Louette, G. 2013. Successful reproduction of Hen Harrier Circus cyaneus in intensive arable farmland (central-east Belgium). Belg. J. Zool. 143: 142–147.
- Fielding, A., Haworth, P., Whitfield, P., McLeod, D. & Riley, H. 2011. A Conservation Framework for Hen Harriers in the UK. Joint Nature Conservation Committee, Peterborough.
- Fletcher, K., Aebischer, N.J., Baines, D., Foster, R. & Hoodless, A.N. 2010. Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J. Appl. Ecol. 47: 263–272. doi: 10.1111/j.1365-2664.2010.01793.x
- García, J.T. & Arroyo, B.E. 2001. Effect of abiotic factors on reproduction in the centre and periphery of breeding ranges: A comparative analysis in sympatric harriers. Ecography 24: 393–402. doi: 10.1034/j.1600-0587.2001.d01-195.x
- Geary, M., Haworth, P.F. & Fielding, A.H. 2018. Hen harrier Circus cyaneus nest sites on the Isle of Mull are associated with habitat mosaics and constrained by topography. Bird Study 65: 62–71. doi: 10.1080/00063657.2017.1421611
- Geyer, J., Kiefer, I., Kreft, S., Chavez, V., Salafsky, N., Jeltsch, F. & Ibisch, P.L. 2011. Classification of climate-change-induced stresses on biological diversity: Climate-change-induced stresses. Conserv. Biol. 25: 708–715. doi: 10.1111/j.1523-1739.2011.01676.x
- Gleeson, E., McGrath, R. & Treanor, M. 2013. Ireland’s Climate: the road ahead. Met Éireann, Dublin.
- Graf, R.F., Bollmann, K., Suter, W. & Bugmann, H. 2005. The importance of spatial scale in habitat models: Capercaillie in the Swiss Alps. Landsc. Ecol. 20: 703–717. doi: 10.1007/s10980-005-0063-7
- Hijmans, R. 2017. raster: Geographic Data Analysis and Modeling. R package version 2.8-19. Accessed 10th February 2017. Available at: https://CRAN.R-project.org/package=raster
- Irwin, S., Wilson, M.W., Kelly, T.C., O’Mahony, B., Oliver, G., Troake, P., Ryan, B., Cullen, C., O’Donoghue, B. & O’Halloran, J. 2011. The breeding biology of Hen Harriers Circus cyaneus in Ireland over a five year period. Irish Birds 9: 165–172.
- Irwin, S., Wilson, M., O’Donoghue, B., O’Mahony, B., Kelly, T. & O’Halloran, J. 2012. Optimum Scenarios for Hen Harrier Conservation in Ireland. Report produced for the Department of Agriculture, Food and the Marine by the School of Biological, Earth and Environmental Sciences, University College Cork, Cork, Ireland.
- Julliard, R., Jiguet, F. & Couvet, D. 2004. Common birds facing global changes: What makes a species at risk? Glob. Chang. Biol. 10: 148–154. doi: 10.1111/j.1365-2486.2003.00723.x
- Kleijn, D., Schekkerman, H., Dimmers, W.J., Van Kats, R.J.M., Melman, D. & Teunissen, W.A. 2010. Adverse effects of agricultural intensification and climate change on breeding habitat quality of Black-tailed Godwits Limosa l. limosa in the Netherlands: Farming intensification, climate change and Godwits. Ibis 152: 475–486. doi: 10.1111/j.1474-919X.2010.01025.x
- Korpimaki, E. 1984. Population dynamics of birds of prey in relation to fluctuations in small mammal populations in western Finland. Ann. Zool. Fennici. 21: 287–293.
- Kuhn, M. 2017. caret: Classification and Regression Training. R package version 6.0-81. Accessed 10th February 2017. Available at: https://CRAN.R-project.org/package=caret
- Kurki, S., Nikula, A., Helle, P. & Lindén, H. 1998. Abundances of red fox and pine marten in relation to the composition of boreal forest landscapes. J. Anim. Ecol. 67: 874–886. doi: 10.1046/j.1365-2656.1998.6760874.x
- Madders, M. 2000. Habitat selection and foraging success of Hen Harriers Circus cyaneus in west Scotland. Bird Study 47: 32–40. doi: 10.1080/00063650009461158
- Madders, M. 2003. Hen Harrier Circus cyaneus foraging activity in relation to habitat and prey. Bird Study 50: 55–60. doi: 10.1080/00063650309461290
- Marquiss, M., Ratcliffe, D.A. & Roxburgh, R. 1985. The numbers, breeding success and diet of golden eagles in southern Scotland in relation to changes in land use. Biol. Cons. 34: 121–140. doi: 10.1016/0006-3207(85)90104-1
- McAlpine, C., Rhodes, J., Callaghan, J., Bowen, M., Lunney, D., Mitchell, D., Pullar, D. & Possingham, H. 2006. The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: A case study of koalas in Queensland, Australia. Biol. Cons. 132: 153–165. doi: 10.1016/j.biocon.2006.03.021
- Millon, A., Bourrioux, J.-L., Riols, C. & Bretagnolle, V. 2002. Comparative breeding biology of Hen Harrier and Montagu’s Harrier: An 8-year study in north-eastern France: Comparative breeding biology in harriers. Ibis 144: 94–105. doi: 10.1046/j.0019-1019.2001.00009.x
- Monaghan, J. 2015. Slieve Bloom Hen Harrier Project 2015. PhD thesis. National Parks and Wildlife Service, Ireland.
- Moran, P. & Wilson-Parr, R. 2015. Hen Harrier Special Protection Area (SPA) habitat mapping project 2014. National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Ireland.
- National Parks & Wildlife Service. 2015. Hen Harrier Conservation and the Forestry Sector in Ireland. National Parks & Wildlife Service, Dublin, Ireland.
- Norriss, D.W., Marsh, J., McMahon, D. & Oliver, G.A. 2002. A national survey of breeding hen harriers Circus cyaneus in Ireland. Irish Birds 7: 1–10.
- Nye, P.A. 1964. Heat loss in wet ducklings and chicks. Ibis 106: 189–197. doi: 10.1111/j.1474-919X.1964.tb03695.x
- O’Donoghue, B.G. 2010. The Ecology and Conservation of Hen Harriers (Circus cyaneus) in Ireland. PhD thesis, University College Cork.
- O’Flynn, W.J. 1983. Population changes of the Hen Harrier in Ireland. Irish Birds 2: 337–343.
- O’Leary, T.N., McCormack, A.G. & Clinch, J.P. 2000. Afforestation in Ireland – regional differences in attitude Land Use Policy 17: 39–48. doi: 10.1016/S0264-8377(99)00036-8
- Olsen, P. & Olsen, J. 1992. Does rain hamper hunting by breeding raptors? Emu 92: 184–187. doi: 10.1071/MU9920184
- O’Riordan, M., Mahon, M. & McDonagh, J. 2015. Power, discourse and participation in nature conflicts: The case of turf cutters in the governance of Ireland’s raised bog designations. J. Environ. Pol. Plan. 17: 127–145. doi: 10.1080/1523908X.2014.914895
- O’Rourke, E. & Kramm, N. 2009. Changes in the management of the Irish uplands: A case-study from the Iveragh Peninsula. Eur. Countryside 1: 53–69. doi: 10.2478/v10091-009-0005-5
- Paton, P.W.C. 1994. The effect of edge on avian nest success: How strong is the evidence? Cons. Biol. 8: 17–26. doi: 10.1046/j.1523-1739.1994.08010017.x
- Pearce-Higgins, J. 2010. Using diet to assess the sensitivity of northern and upland birds to climate change. Clim. Res. 45: 119–130. doi: 10.3354/cr00920
- Picozzi, N. 1978. Dispersion, breeding and prey of the Hen Harrier Circus cyaneus in Glen Dye, Kincardineshire. Ibis 120: 498–509. doi: 10.1111/j.1474-919X.1978.tb06814.x
- Picozzi, N. 1984. Breeding biology of polygynous hen harriers Circus c. cyaneus in Orkney. Ornis Scand. 15: 1–10. doi: 10.2307/3675994
- Quinn, G. & Keogh, M. 2002. Experimental Design and Data Analysis for Biologists. University Press, Cambridge.
- Ratcliffe, D.A. 2010. Bird Life of Mountain and Upland. Cambridge University Press, Cambridge.
- R Core Team. 2017. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Redpath, S.M., Madders, M., Donnelly, E., Anderson, B., Thirgood, S., Martin, A. & Mcleod, D. 1998. Nest site selection by Hen Harriers in Scotland. Bird Study 45: 51–61. doi: 10.1080/00063659809461077
- Redpath, S.M., Arroyo, B.E., Etheridge, B., Leckie, F., Bouwman, K. & Thirgood, S.J. 2002. Temperature and hen harrier productivity: From local mechanisms to geographical patterns. Ecography 25: 533–540. doi: 10.1034/j.1600-0587.2002.250503.x
- Renou-Wilson, F., Bolger, T., Bullock, C., Convery, F., Curry, J., Ward, S. & Müller, C. 2011. BOGLAND: sustainable management of Peatlands in Ireland – final report. Environmental Protection Agency, Johnstown Castle, Co Wexford, Dublin, Ireland.
- Roche, J.R., Perrin, P.M., Barron, S.J. & Daly, O.H. 2014. National Survey of Upland Habitats (Phase 2, 2011–2012). National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland.
- Ruddock, M., Dunlop, B., O’Toole, L., Mee, A. & Nagle, T. 2012. Republic of Ireland National Hen Harrier Survey 2010. National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland.
- Ruddock, M., Mee, A., Lusby, J., Nagle, T., O’Neill, S. & O’Toole, L. 2016. The 2015 National Survey of Breeding Hen Harrier in Ireland. National Parks and Wildlife Service, Department of the Arts, Heritage and the Gaeltacht, Ireland.
- Sachslehner, L., Watzl, B., Schmalzer, A. & Trauttmansdorff, J. 2016. Die Kornweihe (Circus cyaneus) als Brutvogel in Niederösterreich – eine besonders schwierige Art. Ornithol News East Austria 27: 1–4.
- Schipper, W.J.A. 1977. Hunting in three European harriers (Circus) during the breeding season. Ardea 65: 53–72.
- Schipper, W.J.A. 1979. A comparison of breeding ecology in three European harriers (Circus). Ardea 66: 77–101.
- Small, M.F. & Hunter, M.L. 1988. Forest fragmentation and avian nest predation in forested landscapes. Oecologia 76: 62–64. doi: 10.1007/BF00379601
- Staneva, A. & Burfield, I. 2017. European Birds of Conservation Concern: populations, trends and national responsibilities. BirdLife International and Central Asian Partnership, Cambridge, UK.
- Tapia, I., Dominguez, J. & Rodriguez, L. 2004. Modeling habitat use and distribution of Hen Harriers (Circus cyaneus) and Montagu’s Harrier (Circus pygargus) in a mountainous area in Galicia, northwestern Spain. J. Raptor Res. 38: 133–140.
- Thompson, D.B.A., Stroud, A. & Pienkowski, M.W. 1988. Afforestation and upland birds: consequences for population ecology. In Usher, M.B. & Thompson, D.B.A. (eds) Ecological Change in the Uplands, 237–260. Blackwell Scientific Publications, Oxford.
- Thompson, D.B.A., MacDonald, A.J., Marsden, J.H. & Galbraith, C.A. 1995. Upland heather moorland in Great Britain: A review of international importance, vegetation change and some objectives for nature conservation. Biol. Cons. 71: 163–178. doi: 10.1016/0006-3207(94)00043-P
- Watson, D. 2017. The Hen Harrier. Bloomsbury Natural History, London, UK.
- Weathers, W.W. 1979. Climatic adaptation in Svian standard metabolic rate. Oecologia 42: 81–89. doi: 10.1007/BF00347620
- Whilde, T. 1993. Threatened Mammals, Birds, Amphibians and Fish in Ireland. Irish Red Data Book 2: Vertebrates. HMSO, Belfast.
- Wilson, M.W., Irwin, S., Norriss, D.W., Newton, S.F., Collins, K., Kelly, T.C. & O’Halloran, J. 2009. The importance of pre-thicket conifer plantations for nesting Hen Harriers Circus cyaneus in Ireland. Ibis 151: 332–343. doi: 10.1111/j.1474-919X.2009.00918.x
- Wilson, M.W., O’Donoghue, B., O’Mahony, B., Cullen, C., O’Donoghue, T., Oliver, G., Ryan, B., Troake, P., Irwin, S., Kelly, T.C., Rotella, J. & O’Haloran, J. 2012. Mismatches between breeding success and habitat preferences in Hen Harriers Circus cyaneus breeding in forested landscapes. Ibis 154: 578–589. doi: 10.1111/j.1474-919X.2012.01236.x
- Wilson, J.D., Anderson, R., Bailey, S., Chetcuti, J., Cowie, N.R., Hancock, M.H., Quine, C.P., Russell, N., Stephen, L. & Thompson, D.B.A. 2014. Modelling edge effects of mature forest plantations on peatland waders informs landscape-scale conservation. J. Appl. Ecol. 51: 204–213. doi: 10.1111/1365-2664.12173
- Wilson, M.W., Fernandez-Bellon, D., Irwin, S. & O'Halloran, J. 2017. Hen Harrier Circus cyaneus population trends in relation to wind farms. Bird Study 64: 20–29. doi: 10.1080/00063657.2016.1262815
- Wingfield, J.C. 1984. Influence of weather on reproduction. J. Exp. Zool. 232: 589–594. doi: 10.1002/jez.1402320327
- Wong, J. 2013. pdist: Partitioned Distance Function. R package version 1.2. Accessed 14th February 2018. Available at: https://CRAN.R-project.org/package=pdist
