ABSTRACT
Capsule: Great Black-backed Gulls Larus marinus breeding on Skokholm, UK, fed predominantly on seabirds, rabbits, refuse, and marine prey, with the majority of pairs being dietary generalists, but with some specialist pairs.
Aims: To understand the significance of Great Black-backed Gulls as top predators on a small offshore island with internationally important numbers of breeding seabirds (Skokholm, UK) by quantifying their diet and to determine how this varies within the breeding season, to test for pair-level dietary specialization and to examine the consequences of dietary differences for reproductive performance.
Methods: Regurgitated pellets were collected and analysed from 26 breeding pairs on Skokholm during 2017 and related to breeding success.
Results: Analysis of 1035 pellets revealed that, overall, Great Black-backed Gulls fed on seabirds (48% – mostly Manx Shearwaters Puffinus puffinus), mammals (38% – mostly European Rabbits Oryctolagus cuniculus), anthropogenic waste (7%), and marine prey (7%). Diet varied among pairs with 18 (73%) generalist pairs and 7 (27%) specialist pairs (of which, 5 were bird specialists and 2 were mammal specialists). Diet also varied seasonally, but pair-level dietary diversity was repeatable through the breeding season. Dietary diversity did not covary with breeding success.
Conclusion: Great Black-backed Gulls are top predators on Skokholm. Variation in diet among pairs emphasizes that not all individuals contribute equally in terms of predation. Understanding the incidence of this variation has important ecological implications, particularly where apex predators may exert a strong top-down influence.
Predator–prey dynamics often assume that individuals of the same species are identical in their resource use, despite marked between-individual variation in foraging and diet (Bolnick et al. Citation2003). Understanding the incidence and implications of such intra-population variation in foraging has important ecological and applied implications. For example, specialists that feed on a narrow range of food are expected to be more effective foragers than generalists that feed on a wide range of foods (Bolnick et al. Citation2003, Sargeant Citation2007, Woo et al. Citation2008). Moreover, where specialist predators exert a strong top-down influence, the most effective control measures could involve removal of specialized individuals (Votier et al. Citation2004a, Sanz-Aguilar et al. Citation2009, Swan et al. Citation2017).
Gulls (Larus species) are adaptable and successful generalist predators with a global distribution (Ramírez et al. Citation2012, Alonso et al. Citation2015, Navarro et al. Citation2017). This flexibility has, in tandem with the availability of anthropogenic food subsidies, been used to explain why many species have experienced population increases and range expansions in recent decades (Oro & Martinez-Abrain Citation2007, Duhem et al. Citation2008, Alonso et al. Citation2015). However, increasing gull numbers may also have deleterious impacts on their prey. For example, seabird populations have been negatively impacted by gulls because of the strong lethal effects of predation (Donehower et al. Citation2007, Sanz-Aguilar et al. Citation2009), as well as non-lethal effects due to behavioural compensation (Cresswell Citation2008). Mitigating this impact requires insight into gull foraging ecology, and particularly the role played by specialists, which may exert a disproportionate amount of top-down pressure (Swan et al. Citation2017). For example, on Benidorm Island, Spain, one pair of Yellow-Legged Gulls Larus michahellis nesting within a major European Storm Petrel Hydrobates pelagicus colony was responsible for the majority of petrel predation, and removal of this pair (plus 14 others) resulted in a 65% reduction in petrels killed, and led to an increase in petrel survival by 16% and breeding success by 23% (Sanz-Aguilar et al. Citation2009). In addition, specialists often have higher foraging efficiency and reproductive success than non-specialists leading to increased fitness (Annett & Pierotti Citation1999, Golet et al. Citation2000), with studies on skuas and gulls demonstrating a generally positive relationship between dietary choice and fitness correlates (Votier et al. Citation2004b, O’Hanlon et al. Citation2017). Such relationships may be particularly important as individual differences in behaviour may be heritable (van Oers et al. Citation2005, Araujo et al. Citation2011), potentially leading to greater future predation impacts on seabird populations.
The Great Black-backed Gull Larus marinus is an opportunistic scavenger and apex predator that breeds in coastal regions across the north Atlantic, feeding on seabirds, mammals, fish, refuse, and marine invertebrates (Buckley Citation1990, Good Citation1998, Gilliland et al. Citation2004, Reid Citation2004, Rome & Ellis Citation2004, Veitch et al. Citation2016). The relative importance of different foods varies greatly between areas; populations living sympatrically with other seabirds inflict significant levels of predation pressure (Veitch et al. Citation2016). Great Black-backed Gulls breeding on the island of Skokholm, Wales, UK have increased in population size from single figures in the 1970s to 93 pairs in 2017 (Brown & Eagle Citation2018). Here the species feeds on a range of prey, including many seabirds. For instance, they depredate the large colony of 65,000 pairs of Manx Shearwaters Puffinus puffinus, which represents approximately 20% of the global population. Predation rates have not been quantified, but anecdotal evidence suggests that numbers are high, with concerns raised for this internationally important seabird population. Additionally, there is speculation as to whether the decline in European Rabbit Oryctolagus cuniculus numbers on the island, another prey source for the gulls, may further increase predation on Manx Shearwaters if prey switching occurs.
The aims of this study are as follows. First, we quantify the population-level diet of breeding Great Black-backed Gulls on Skokholm using regurgitated pellets of indigestible material (Votier et al. Citation2003). Second, we test how diet, in terms of prey types and diversity, varies across the breeding season. Dietary diversity provides a quantitative indicator of specialization that can be used to test for seasonal variation. This is potentially important since gulls may exhibit seasonal fluctuations in diet (Steenweg et al. Citation2011), often in response to variations in food availability and energy requirements (Annett & Pierotti Citation1989). Third, we tested whether there were any consistent dietary differences among pairs. Narrower within-pair variation compared with among-pair variation, would suggest dietary specialization (Bolnick et al. Citation2003). Understanding the extent to which seabird predation, especially of Manx Shearwaters, is distributed across the population will not only shine a light on the foraging ecology of Great Black-backed Gulls but also help inform conservation decisions in the event of any potential management intervention (Swan et al. Citation2017). Finally, we investigated the relationship between dietary preference and breeding success.
Methods
Study site
The study was conducted on Skokholm Island National Nature Reserve, Pembrokeshire, Wales (51°41′N, 5°16′W) from 7 May to 9 July 2017. We focussed on 26 breeding pairs of Great Black-backed Gulls, representing 28% of the island population of 93 pairs. In addition to the 65,000 pairs of Manx Shearwaters, Skokholm also supports 1900 pairs of European Storm Petrel, 6500 individual Atlantic Puffins Fratercula arctica, 200 pairs of Northern Fulmar Fulmarus glacialis, 4000 individual Common Guillemots Uria aalge, 2,500 individual Razorbills Alca torda, 1400 pairs of Lesser Black-backed Gulls Larus fuscus and 300 pairs of European Herring Gulls Larus argentatus (Brown & Eagle Citation2018). These nesting seabirds use the surrounding marine environment, a Marine Nature Reserve, as well as more distant areas to forage.
Diet composition
Great Black-backed Gulls regurgitate pellets of indigestible prey items, enabling diet reconstruction. Before sampling began, all nests were cleared of old pellets before being sampled once every 4–10 days from 7 May until 9 July 2017. This period covered incubation, chick-rearing and finished when chicks began to fledge. Pellets were collected from within a 5 m radius of each nest to ensure pellets could be confidently assigned to a focal nest and removed to prevent repeat counting. Pellets were assigned to a pair since they aggressively defend their breeding territories.
Indigestible material in pellets was assigned to one of four broad categories: bird, mammal, refuse, and ‘other’ (Votier et al. Citation2003). Each pellet was assigned to a single category based on the predominant prey item and within these categories prey items were classified to the lowest taxonomic level possible. Pellets containing bird remains were identified on the basis of feathers (colour, size, and smell), skulls, feet, and wings. Mammal remains were classified using fur, wool, skulls, and other bones. Refuse pellets contained anthropogenic materials including plastic, cardboard, and tissue, likely from landfill. Pellets were classified as ‘other’ if they contained prey remains that did not fall into any of the previous three categories.
Diet specialization
Pairs were assigned as generalists or specialists following Votier et al. (Citation2004b): pairs with more than 70% of one of the four main dietary categories were classified as specialists, and the remaining pairs classified as generalists. In addition, to determine the extent to which diet varied seasonally (and thus the consistency of prey choice), we calculated Levin’s index of diet breadth for each two-week period (Levins Citation1968, Krebs Citation1989, Katzner et al. Citation2005). A high index value corresponds to high diversity in the diet (a generalist diet), while a low index value corresponds to low diversity in the diet (a specialist diet).
Data analysis
All nest sites were included in the analysis. We tested for seasonal variation in the four main dietary categories, grouped into two-week sampling periods using contingency tables. Levin’s index was calculated based upon all 16 prey categories (see Results). The relationship between dietary diversity and stage of the breeding season was tested using Spearman’s rank correlation.
To investigate among pair variation in diet, contingency table analysis with Pearson’s chi-squared test for count data was used to evaluate whether changes in the proportion of each prey category differed among pairs. We also calculate repeatability, the intra-class correlation coefficient (Lessells & Boag Citation1987), using variance components derived from a one-way analysis of variance of diet diversity over five two-week sampling intervals.
To determine whether diet affected breeding success, we analysed the relationship between each pairs’ diet diversity of their breeding success using Spearman’s rank correlation.
All analysis was carried out using R version 3.3.1 (R Core Team Citation2016).
Results
Population-level diet
In total, we collected and identified 1035 pellets from 26 pairs, with 11–73 pellets per nest (mean ± standard error = 39.8 ± 3.7) during 7 May to 9 July 2017 (). Birds were the most frequent prey type (n = 498, 48.1%), with the majority being Manx Shearwater (n = 416, 83.5%), followed by unidentified auk species (Atlantic Puffin, Razorbill, or Common Guillemot: n = 51, 10.2%), Common Pheasant (Phasianus colchicus: n = 6, 1.2%), unidentified passerine (n = 5, 1.0%), European Storm Petrel (n = 2, 0.4%), unidentified gull (n = 1, 0.2%), and unidentified bird (n = 17, 3.4%). Mammals were the second most common prey type (n = 393, 37.9%), consisting of rabbit (n = 384, 97.7%) and sheep (n = 9, 2.3%). Refuse was the next most frequent prey type (n = 77, 7.4%). The final category, ‘other’ (n = 67, 6.5%) consisted of whitefish (n = 38, 56.7%), unidentified molluscs (n = 14, 20.9%), vegetation (mostly grasses Poaceae: n = 8, 11.9%), unidentified bird eggshells (n = 5, 7.5%), and unidentified crustaceans (n = 2, 3.0%).
Table 1. Diet composition at a population level for 26 pairs of Great Black-backed Gulls breeding on Skokholm, UK in 2017, based on analysis of regurgitated pellets.
Seasonal variation in population-level diet
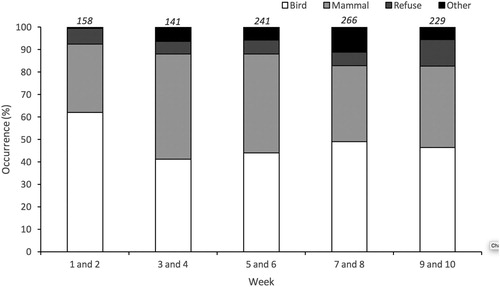
Stage of the breeding season had a significant effect upon prey type (Pearson’s chi-squared test, χ2 = 43.544, n = 12, P < 0.01). Bird pellets were most frequent during the first two-week period but decreased during the second and remained fairly similar thereafter ().
Figure 1. Seasonal change in the population-level diet for 26 pairs of Great Black-backed Gull breeding on Skokholm, UK in 2017, based on regurgitated pellets. Data are grouped into five time periods, each representing two weeks of sampling. Diet composition is divided into four broad categories; Bird, Mammal, Refuse, and ‘Other’.
Intra-population variation in diet
The proportion of different prey items varied significantly among pairs (Pearson’s chi-squared test, χ2 = 475.53, n = 75, P < 0.01; ). Five pairs were bird specialists (19%), feeding mostly on Manx Shearwater (). Two pairs were mammal specialists (8%), primarily eating rabbits. The remaining 18 pairs were classified as generalists (73%, ).
Figure 2. Intra-population variation in the diet of Great Black-backed Gull nest sites on Skokholm, UK in 2017, based on regurgitated pellets. Data are grouped according to nest site. ‘Other’ includes whitefish, mollusc, crustacean and vegetation.
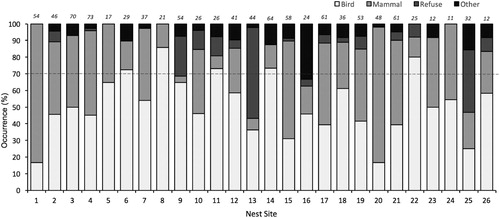
Figure 3. Intra-population variation in the diet of Great Black-backed Gull pairs breeding on Skokholm, UK in 2017, identified through pellet analysis. Pairs with more than 70% bird remains in pellets (marked with a dashed line) were classified as specialist bird predators. Pairs with more than 70% mammal remains in pellets were classified as specialist mammal predators.
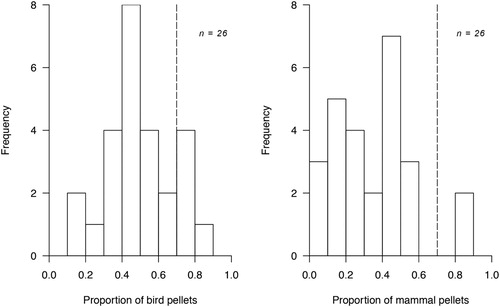
Table 2. Diet composition (%) of pellets categorized as ‘Bird’ for the five Great Black-backed Gull pairs on Skokholm, UK classified as bird specialists, determined by pellet analysis during the breeding season of 2017.
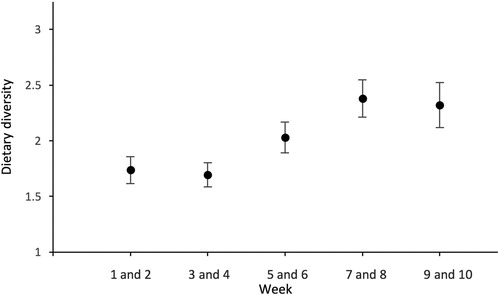
Overall Levin’s index of diet breadth was 2.03, becoming weakly but significantly higher as the breeding season progressed (Spearman’s rank correlation, rs = 0.308, n = 122, P < 0.01; ). Diet diversity was highly repeatability within-pairs (intra-class correlation coefficient: r = 0.749, n = 130, F = 14.0, P < 0.01).
Figure 4. Mean dietary diversity (Levin’s index) ± SE at the population level for 26 pairs of Great Black-backed Gull breeding on Skokholm, UK in 2017, determined by pellet analysis during the breeding season. Data are grouped into five time periods, each representing two weeks of sampling; Week 1 and 2 (1.68 ± 0.12), Week 3 and 4 (1.70 ± 0.11), Week 5 and 6 (2.03 ± 0.14), Week 7 and 8 (2.38 ± 0.17), and Week 9 and 10 (2.19 ± 0.20).
Breeding success and dietary specialization
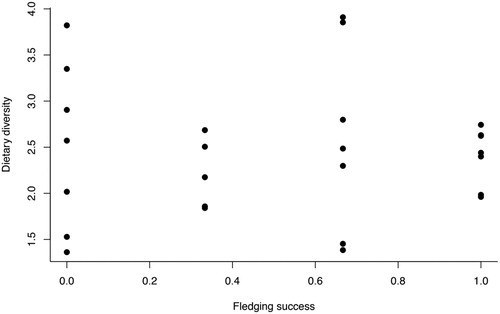
Of the 26 nests that were sampled during the breeding season, 7 failed (27%) and from the remaining 19 successful nests (73%), 40 chicks fledged. Therefore, the mean fledging success ± SE was 0.51 ± 0.08. Fledging success was not correlated with the dietary diversity of each pair (Spearman’s rank correlation, rs = −0.09, n = 26, P = 0.68; ), although statistical power was very low (0.0666).
Discussion
Our diet analysis based on pellets revealed a principally generalist population of Great Black-backed Gulls on Skokholm that fed extensively on seabirds (mostly Manx Shearwaters) and mammals (mostly rabbits). However, there were also a small number of pairs that specialized in feeding upon birds (19%: n = 5) and mammals (8%: n = 2).
Limitations and strengths of diet analysis based on pellets
Pellets of indigestible material may underestimate soft-bodied prey (Votier et al. Citation2001, Citation2003), requiring caution when interpreting the results. Nevertheless, Great Black-backed Gulls appear to only rarely feed on soft-bodied prey (Steenweg et al. Citation2011), and anecdotal observations of prey capture on Skokholm also indicate a diet dominated by prey with hard body parts (i.e. seabirds and rabbits). Despite some of the limitations, there are also clear strengths of using pellets over other dietary assessment techniques. First, sampling is non-invasive. Second, we were able to repeat sample across the breeding season to test for seasonal differences and consistency. Third, we were able to identify prey to a high taxonomic resolution, often to species level ().
Population-level diet
Overall, Great Black-backed Gulls fed mostly on other birds (48%) and terrestrial mammals (38%). Observations on Skokholm suggest that most of this prey is taken live, rather than scavenged, revealing Great Black-backed Gulls are apex predators. Refuse (7.3%) and ‘other’ (6.5%), comprised the rest of the diet items. Refuse may have come from landfill, with the nearest located 20 km (St. Davids Landfill – 51°88′N, 5°23′W) and 27 km (Withyhedge Landfill – 51°85′N, 4°16′W) from the island (Natural Resources Wales Citation2018). ‘Other’ diet items were comprised of fish and molluscs which may have come via scavenging from fishing vessels (Brown & Eagle Citation2018), intertidal foraging or kelptoparasitising other gulls (Veitch et al. Citation2016). Such a pattern of predatory and opportunistic behaviour is consistent with other studies of this species (Buckley Citation1990, Gilliland et al. Citation2004, Rome & Ellis Citation2004, Veitch et al. Citation2016).
Seasonal variation in population-level diet
Diet varied across the breeding season (). Bird pellet production was highest at the start of the season – a similar result for other studies of this species (Veitch et al. Citation2016). Overall, there was an increase in dietary diversity as the season progressed and this was attributable to an increase in auk species, marine dietary items and refuse (). Studies from other larids show similar seasonal dietary change. For example, Herring Gulls in the Netherlands increased dietary diversity during chick-rearing primarily due to an increase in the occurrence of crustaceans, fishery discards, and domestic refuse (van Donk et al. Citation2017). The increase in niche breadth may be due to changes in energy requirements during chick-rearing, altering food availabilities through an increase in the availability of alternative prey such as young seabirds or a combination of both.
Intra-population variation in diet
The majority of pairs (73%) showed no clear signs of specialization (or at least had <70% of any one prey type), feeding instead on a wide range of different prey (). However, not all diet items were evenly spread across territories, with some pairs specializing on birds (19%) and others on mammals (8%). Moreover, within-pair dietary diversity was highly repeatability (intra-class coefficient = 0.749) indicated that pairs’ foraging behaviours were maintained over time.
Even with our conservative method of assigning specialists, we found a higher proportion of specialists (∼27%) than in other large gull studies. For instance, Spear (Citation1993) and Sanz-Aguilar et al. (Citation2009) found that specialists only represented around 1% of the population of Western and Yellow-legged gulls respectively. This may be due to very high numbers of Manx Shearwaters and rabbits on Skokholm since the number of specialists is positively correlated with prey availability (Spear Citation1993, Votier et al. Citation2007). However, we were only able to study 28% of Skokholm’s breeding population, so it is unclear whether this represents the true number of specialist pairs.
Dietary specialization and breeding success
We found no relationship between dietary diversity and fledging success (). Earlier research suggests inconsistent patterns between specialization and fitness correlates (Pierotti & Annett Citation1991, Annett & Pierotti Citation1999, Votier et al. Citation2004b, van Donk et al. Citation2017). However, given our low statistical power (0.0666) of our study, it seems quite likely that this could be a Type II error.
Conservation implications
The population of Great Black-backed Gulls on Skokholm is increasing, following population control substantially reducing numbers between 1949 and 1985 (Brown & Eagle Citation2018). This is in contrast to the trend in the rest of UK, which showed an 11% decline between 1970 and 2002 (Reid Citation2004). Increasing gull populations are often linked to concerns over their predation impacts on populations of other seabirds (Votier et al. Citation2004b, Donehower et al. Citation2007, Sanz-Aguilar et al. Citation2009, Church et al. Citation2018) and there is potential for negative impacts of gull predation on Skokholm, particularly on Manx Shearwaters.
Reducing the impact of Great Black-backed Gull predation on Manx Shearwaters requires a more complete understanding of the numbers taken and the population-level consequences of this predation pressure. The present study hints that the removal of a relatively small number of specialist individuals may be an effective control measure to reduce impacts. However, large gulls specializing on birds often defend a feeding territory (Spear Citation1993, Votier et al. Citation2004b). Therefore, the presence of a small number of Great Black-backed Gulls specializing on Manx Shearwaters may, in fact, lead to lower levels of predation if they exclude other opportunistic predators. Further work is required to establish whether this is the case. In addition, increases in gull populations do not always negatively impact the bird populations being predated. Growth rates of Yellow-legged Gulls and sympatric waterbirds are positively correlated despite high predation rates (Oro & Martinez-Abrain Citation2007).
New environmental policies involving the closure of landfill sites and discard bans could lead to increased predation rates on synoptic species (Votier et al. Citation2004c, Oro et al. Citation2006, Bicknell et al. Citation2013), such as the Manx Shearwater. However, it is unclear the extent to which prey switching may occur, with foraging behaviours often learnt over several years (Bicknell et al. Citation2013).
To conclude, this study reveals the generalist diet of an island population of Great Black-backed Gulls, which consists of both generalists and specialist pairs. The gulls feed a range of prey – with some seasonal variation – but a preponderance of birds (mostly Manx Shearwaters) and rabbits. Seabird colonies involve complex predator-prey dynamics and this study emphasizes the importance of understanding that not all individuals equally contribute predation pressure, with management implications. The continued monitoring of the Great Black-backed Gull population, in tandem with details of their foraging ecology over multiple years, will be necessary to determine the effects of a growing population and a potential reduction in anthropogenic and marine resources on seabird predation rates.
Acknowledgements
The authors would like to thank Victoria Hope, Holly Pickett, Jacob Peterken and Maddy Rawlings for assisting with data collection.
References
- Alonso, H., Almeida, A., Granadeiro, J.P. & Catry, P. 2015. Temporal and age-related dietary variations in a large population of yellow-legged gulls Larus michahellis: implications for management and conservation. Eur. J. Wildlife Res. 61: 819–829. doi: 10.1007/s10344-015-0958-9
- Annett, C.A. & Pierotti, R. 1989. Chick hatching as a trigger for dietary switching in the Western gull. Colon. Waterbirds 12: 4–11. doi: 10.2307/1521306
- Annett, C.A. & Pierotti, R. 1999. Long-term reproductive output in Western gulls, consequences of alternate tactics in diet choice. Ecology 80: 288–297. doi: 10.1890/0012-9658(1999)080[0288:LTROIW]2.0.CO;2
- Araujo, M.S., Bolnick, D.I. & Layman, C.A. 2011. The ecological causes of individual specialisation. Ecol. Lett. 14: 948–958. doi: 10.1111/j.1461-0248.2011.01662.x
- Bicknell, A.W.J., Oro, D., Camphuysen, K.C.J. & Votier, S.C. 2013. Potential consequences of discard reform for seabird communities. J. Appl. Ecol. 50: 649–658. doi: 10.1111/1365-2664.12072
- Bolnick, D.I., Svanback, R., Fordyce, J.M., Yang, L.H., Davis, J.M., Hulsey, C.D. & Forister, M.L. 2003. The ecology of individuals: incidence and implications of individual specialisation. Am. Nat. 161: 1–28. doi: 10.1086/343878
- Buckley, N.J. 1990. Diet and feeding ecology of Great Black-backed Gulls (Larus marinus) at a southern Irish breeding colony. J. Zool.(Lond.) 222: 363–373. doi: 10.1111/j.1469-7998.1990.tb04038.x
- Brown, R. & Eagle, G. 2018. Skokholm Seabird Report 2017. [online] The Wildlife Trust of South and West Wales. https://www.welshwildlife.org/about-us/skokholm-reports/.
- Church, G.E., Furness, R.W., Tyler, G., Gilbert, L. & Votier, S.C. 2018. Change in the north Sea ecosystem from the 1970s to the 2010s: great skua diets reflect changing forage fish, seabirds, and fisheries. ICES J. Mar. Sci. fsy165, doi:10.1093/icesjms/fsy165.
- Cresswell, W. 2008. Non-lethal effects of predation in birds. Ibis 150: 3–17. doi: 10.1111/j.1474-919X.2007.00793.x
- Donehower, C.E., Bird, D.M., Hall, C.S. & Kress, S.W. 2007. Effects of gull predation and predator control on tern nesting success at Eastern Egg Rock, Maine. Waterbirds 30: 29–39. doi: 10.1675/1524-4695(2007)030[0029:EOGPAP]2.0.CO;2
- van Donk, S., Camphuysen, K.C.J., Shamoun-Baranes, J. & van der Meer, J. 2017. The most common diet results in low reproduction in a generalist seabird. Ecol. Evol. 7: 4620–4629. doi: 10.1002/ece3.3018
- Duhem, C., Roche, P., Vidal, E. & Tatoni, T. 2008. Effects of anthropogenic food resources on yellow-legged gull colony size on Mediterranean islands. Popul. Ecol. 50: 91–100. doi: 10.1007/s10144-007-0059-z
- Gilliland, S.G., Ankney, C.D. & Hicklin, P.W. 2004. Foraging ecology of great black-backed gulls during brood-rearing in the Bay of Fundy, New Brunswick. Can. J. Zool. 82: 1416–1426. doi: 10.1139/z04-124
- Golet, G.H., Kuletz, K.J., Roby, D.D. & Irons, D.B. 2000. Adult prey choice affects chick growth and reproductive success in pigeon guillemots. Auk 117: 82–91. doi: 10.1093/auk/117.1.82
- Good, T.P. 1998. Great black-backed gull (Larus marinus). In A. Poole & F. Gill. (eds) The Birds of North America, No. 30: 1–32. American Ornithologists’ Union, Washington, DC.
- Katzner, T.E., Bragin, E.A., Knick, S.T. & Smith, A.T. 2005. Relationship between demographics and diet specificity of Imperial Eagles Aquila heliacal in Kazakhstan. Ibis 147: 576–586. doi: 10.1111/j.1474-919X.2005.00443.x
- Krebs, C.J. 1989. Ecological Methodology. Harper Collins, New York.
- Lessells, C.M. & Boag, P.T. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104: 116–121. doi: 10.2307/4087240
- Levins, R. 1968. Ecology in Changing Environments: Some Theoretical Explorations. Princeton University Press, Princeton, NJ.
- Natural Resources Wales. 2018. Waste Permitting Map. [online] Natural Resources Wales. https://naturalresources.wales/evidence-and-data/maps/find-details-of-permitted-waste-sites/.
- Navarro, J., Gremillet, D., Ramírez, F.J., Afan, I., Bouten, W. & Forero, M.G. 2017. Shifting individual habitat specialisation of a successful predator living in anthropogenic landscapes. Mar. Ecol. Prog. Ser. 578: 243–251. doi: 10.3354/meps12124
- van Oers, K., de Jong, G., van Noordwijk, A.J., Kempenaers, B. & Drent, P.J. 2005. Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142: 1185–1206. doi: 10.1163/156853905774539364
- O’Hanlon, N.J., McGill, R.A.R. & Nager, R.G. 2017. Increased use of intertidal resources benefits breeding success in a generalist gull species. Mar. Ecol. Prog. Ser. 574: 193–210. doi: 10.3354/meps12189
- Oro, D., Martinez-Abrain, A., Paracuellos, M., Nevado, J.C. & Genovart, M. 2006. Infuence of density dependence on predator-prey seabird interactions at a large spatio-temporal scale. P. ROY. SOC. B-BIOL. SCI. 273: 379–383. doi: 10.1098/rspb.2005.3287
- Oro, D. & Martinez-Abrain, A. 2007. Deconstructing myths on large gulls and their impact threatened sympatric waterbirds. Anim. Conserv. 10: 117–126. doi: 10.1111/j.1469-1795.2006.00082.x
- Pierotti, R. & Annett, C. 1991. Diet and reproductive output in seabirds. Biosciences 40: 568–575. doi: 10.2307/1311297
- R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Ramírez, F., Navarro, J., Afan, I., Hobson, K.A., Delgado, A. & Forero, M.G. 2012. Adapting to a changing world: unravelling the role of man-made habitats as alternative feeding areas for slender-billed gull (Chroicocephalus genei). PLoS One 7: e47551. doi: 10.1371/journal.pone.0047551
- Reid, J.B. 2004. Great black-backed gull Larus marinus. In P. I. Mitchell, S. F. Newton, N. Ratcliffe & T. E. Dunn. (eds) Seabird Populations of Britain and Ireland, 263–276. T. & A.D. Poyser, London.
- Rome, M.S. & Ellis, J.C. 2004. Foraging ecology and interaction between herring gulls and great black-backed gulls in New England. Waterbirds 27: 200–210. doi: 10.1675/1524-4695(2004)027[0200:FEAIBH]2.0.CO;2
- Sargeant, B.L. 2007. Individual foraging specialisation: niche width versus niche overlap. Oikos 116: 1431–1437. doi: 10.1111/j.0030-1299.2007.15833.x
- Sanz-Aguilar, A., Martinez-Abrain, A., Tavecchia, G., Minguez, E. & Oro, D. 2009. Evidence-based culling of a facultative predator: Efficacy and efficiency components. Biol. Conserv. 142: 424–431. doi: 10.1016/j.biocon.2008.11.004
- Spear, L.B. 1993. Dynamics and effects of western gulls feeding in a colony of guillemots and Brandt’s cormorants. J. Anim. Ecol. 62: 399–414. doi: 10.2307/5190
- Steenweg, R.J., Ronconi, R.A. & Leonard, M.L. 2011. Seasonal and age-dependent dietary partitioning between the great black-backed and herring gulls. Condor 113: 795–805. doi: 10.1525/cond.2011.110004
- Swan, J.F.G., Redpath, S.M., Bearhop, S. & McDonald, R.A. 2017. Ecology of problem individuals and the efficacy of selective wildlife management. Trends Ecol. Evol. 32: 518–530. doi: 10.1016/j.tree.2017.03.011
- Veitch, B.G., Robertson, G.J., Jones, I.I. & Bond, A.L. 2016. Great black-backed gull (Larus marinus) predation on seabird populations at two colonies in eastern Canada. Waterbird 39: 235–245. doi: 10.1675/063.039.sp121
- Votier, S.C., Bearhop, S., Ratcliffe, N. & Furness, R.W. 2001. Pellets as indicators of diet in great skuas. Bird Study 48: 373–376. doi: 10.1080/00063650109461237
- Votier, S.C., Bearhop, S., MacCormick, A., Ratcliffe, N. & Furness, R.W. 2003. Assessing the diet of great skuas, Catharacta skua, using five different techniques. Polar Biol. 26: 20–26.
- Votier, S.C., Bearhop, S., Ratcliffe, N., Phillips, R.A. & Furness, R.W. 2004a. Predation by great skuas at a large Shetland seabird colony. J. Appl. Ecol. 41: 1117–1128. doi: 10.1111/j.0021-8901.2004.00974.x
- Votier, S.C., Bearhop, S., Ratcliffe, N. & Furness, R.W. 2004b. Reproductive consequences for great skuas specializing as seabird predators. Condor 106: 275–287. doi: 10.1093/condor/106.2.275
- Votier, S.C., Furness, R.W., Bearhop, S., Crane, J.E., Caldow, R.W.G., Catry, P., Ensor, K., Hamer, K.C., Hudson, A.V., Kalmbach, E., Klomp, N.I., Pfeiffer, S., Phillips, R.A., Prieto, I. & Thompson, D.R. 2004c. Changes in fisheries discard rates and seabird communities. Nature 427: 727–730. doi: 10.1038/nature02315
- Votier, S.C., Bearhop, S., Crane, J.E., Arcos, J.M. & Furness, R.W. 2007. Seabird predation by great skuas Stercorarius skua – intra-specific competition for food? J. Avian Biol. 38: 234–246. doi: 10.1111/j.0908-8857.2007.03893.x
- Woo, K.J., Elliott, K.H., Davidson, M., Gaston, A.J. & Davoren, G.K. 2008. Individual specialisation in diet by a generalist marine predator reflects specialisation in foraging behaviour. J. Anim. Ecol. 77(6): 1082–1091. doi: 10.1111/j.1365-2656.2008.01429.x