ABSTRACT
Capsule: In large and medium wetlands with extended reed beds Phragmites and cattail Typha dominated areas, Water Rails Rallus aquaticus and Little Crakes Zapornia parva show higher differences in nesting sites in contrast with other populations from small and fragmented wetlands, where the ranges of values for environmental variables at nesting sites overlap greatly.
Aims: To investigate the nesting sites and nesting habitats of both species to a fine scale, on medium and large wetlands, to evaluate the potential overlapping of nesting habitat and to compare data with those obtained in small wetlands.
Methods: In the study area, the Fizeş Basin, Romania, 17 wetlands, ponds and reed beds ranging from 11.78 to 252.68 ha were investigated. The species were present on 4 wetlands, which were subsequently surveyed for nests. Between April and August 2010–2012, a total of 83 Water Rail and 46 Little Crake nests were found, measured and analysed in terms of nesting habitat and nest features.
Results: Principal component analyses suggest no overlap in nest site selection and a partial overlap in the nest features. An analysis of similarity confirmed significant nest structural differences. A discriminant function analysis highlights the main factors dividing the nest features of both species being: water depth and distance between the nest and a water surface. Little Crakes build their nests in cattail Typha sloped clumps, while Water Rails build their nests near reed Phragmites strains.
Conclusion: In large and medium wetlands, Water Rails nest deep in the reed bed rather than in other types of habitats, contrary to what has been reported for small ponds areas. Little Crakes nest in cattail independently of the size of the wetland where they occur.
Habitat fragmentation, the division of large contiguous areas of habitat into smaller patches isolated from one another (Diamond & May Citation1976, Lougheed et al. Citation2008) is a major concern in conservation biology. This phenomenon is especially relevant in developed countries and is becoming a massive process in developing areas. Yet, developing countries still own large and high-quality habitats, often with high biodiversity. Worldwide, some of the most fragmented habitats are wetlands (Celada & Giuseppe Citation1993, Lougheed et al. Citation2008). The phenomenon affects wetland-dependent species not only by reducing space availability and the amount of resources, but also by increasing intra- and inter-specific competition (Douglas Citation2011, Dhondt Citation2012).
Among the most affected bird species due to habitat loss, wetland fragmentation and interspecific competition are rails (Rallidae), an elusive and consequently poorly studied bird group (Taylor Citation1998, Perrins Citation2004, Brambilla et al. Citation2012, Stermin et al. Citation2017). Globally, almost a quarter of all rail species are threatened (Taylor Citation1998) which emphasizes the need to document their distribution, breeding habitats and heterospecific interactions, to help guide conservation efforts (Darrah & Krementz Citation2011, Bolenbaugh et al. Citation2012).
Seven rail species inhabit European wetlands and share their biotopes in terms of microhabitat structure of foraging and nesting places (Taylor Citation1998). This study focuses on two marsh-nesting species, the Little Crake Zapornia parva and the Water Rail Rallus aquaticus, which both occupy a wide range of freshwater habitats with tall and dense vegetation (Cramp Citation1980, Stermin et al. Citation2012, Jedlikowski et al. Citation2014), most of them semi-natural and used as fishponds (Brambila et al. Citation2012, Stermin et al. Citation2012).
Several studies have revealed the general habitat preferences of Water Rails in their breeding areas (Taylor Citation1998, Jenkins & Ormerod Citation2002, Brambilla & Rubolini Citation2004, Brambilla et al. Citation2012). However, few have investigated the microhabitat preferences for nest site in terms of water depth, vegetation type and density on Water Rails (De Kroon Citation1999, De Kroon Citation2000, De Kroon & Mommers Citation2002, De Kroon Citation2004) or Little Crakes (Schiermann Citation1929, Kux Citation1959, Bauer Citation1960, Dittberner & Dittberner Citation1990).
Little is currently known about the differences or similarities in the way that these two species share the habitat within wetlands (Bauer Citation1960, Jedlikowski et al. Citation2014). All previous studies on Water Rails and Little Crakes have been conducted in fragmented wetlands where there was no availability of larger areas of habitat. Those studies developed in medium and small ponds of 0.1–70 ha indicated an overlap in nesting sites and microhabitat characteristics (Jedlikowski et al. Citation2014, Jedlikowski & Brambilla Citation2017).
Habitat availability and patch size may influence the birds’ choice of nesting sites, as well as the extent of intra- and inter-specific competition (Dhondt Citation2012, Stermin et al. Citation2013). As the previous studies were carried out in small and medium wetlands we proposed to conduct our investigation in one of the largest inland wetlands in Europe with high availability of medium and large wetland areas (over 100 ha) (Stermin et al. Citation2013) covered by large areas of homogenous and heterogeneous swamp vegetation (Stermin et al. Citation2012).
Accordingly, the aims of our study are: (1) to describe, on a fine scale, the nesting habitat and nest placement of Water Rails and Little Crakes in medium and large wetlands, (2) to evaluate the level of overlap of nest site preferences in the two species, and (3) to compare similar data with those obtained in other studies on small wetlands (Kux Citation1959, Dittberner & Dittberner Citation1990, De Kroon Citation1999, Jenkins & Ormerod Citation2002, Brambilla & Rubolini Citation2004, Jedlikowski et al. Citation2014). We aim to provide new information on the breeding biology of these elusive species, which could be useful in the management of those increasingly rare and endangered habitats.
Methods
Study area
The research was carried out in the Fizeş Basin, an area of 56,000 ha in the central part of the Transylvanian Plain, Romania (N 46°50’, E 24°10’) (David Citation2008). The landscape is characterized by hills (400–450 m above sea level) and large valleys, mostly deforested and now cultivated, but also hosting the highest density of wetlands in the Transylvanian Plains (). Seventeen wetlands, ponds, and reed beds are present here with an area ranging from 11.78 to 252.68 ha (mean 66.67 ha), 11 of them range from 10 to 50 ha, 2 from 50 to 100 ha and 4 from 100 to 260 ha.
Figure 1. Study area location, wetlands, and distribution of breeding populations of Water Rails and Little Crakes in the Fizeş Basin, Romania.
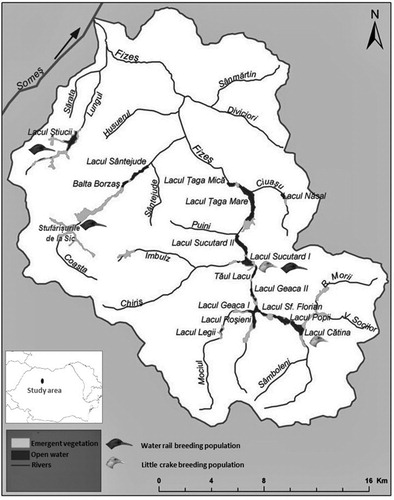
All wetlands have vegetation dominated by cattails Typha latifolia and T. angustifolia, Common Reed Phragmites australis and/or Carex spp. With the exception of two (Sic Reed Bed and Legi Valley), all wetlands are used as fishponds. A vast area, including parts of all the wetlands, is part of the Natura 2000 conservation network. Although we searched for breeding pairs in all 17 wetland areas, during our survey, nesting populations of Water Rails and Little Crakes were only located in four of them: Stufărişurile de la Sic (Sic Reed Bed), Lacul Ştiucii (Pike Lake), Sucutard and Cătina ().
The Sic Reed Bed is the largest reed bed area in the Transylvanian Plain (Stermin et al. Citation2011) and second largest in Romania after the Danube Delta. With an area of 252.68 ha, a length of 4.2 km and 550 m maximum width, it is 98% covered by compact reeds with small patches of cattail and reed mixture and an edge strip area of Carex and Juncus (Stermin et al. Citation2011). The vegetation density is around 400 plant stems per m2 and the water depth does not exceed 1.5 m, generally ranging between 0.2 and 0.5 m (Stermin et al. Citation2011). Pike Lake is a natural wetland with a maximum water depth of 5 m, but with a large marsh vegetation area surrounding it, where the water depth ranges from 0.2 to 1 m. The total area of this wetland is 170 ha, with around 40 ha of open water and 130 ha covered by emergent vegetation dominated by reeds (Stermin et al. Citation2011). Sucutard pond is an anthropogenic wetland with an area of 46 ha, covered 50% by open water, 15% by reed and 35% by cattails (15% dominated by T. latifolia and 20% by T. angustifolia) and a maximum water depth of 2 m. The spatial distribution and the structure of different plant communities make this pond a heterogeneous wetland (Stermin et al. Citation2012). Cătina pond is also an anthropogenic wetland, the water depth does not exceed 2 m, with a total surface area of 125 ha, it is 60% covered by emergent vegetation dominated by cattail.
Data collection
Data were collected in 2010–2012, from early April to end of August each year. During April and June playback surveys were used to locate breeding birds, and between May and August nests were located and monitored.
In the first part (April–June) of the breeding season (Cramp Citation1980, Taylor Citation1998), on the shore line around each of the 17 wetlands from the Fizeş Basin, points were selected every 30–50 m for broadcast surveys in areas with emergent vegetation (Dombrowski et al. Citation1993, Brambilla & Jenkins Citation2009, Johnson et al. Citation2009). The birds were stimulated using playback calls of Water Rail, Little Crake, Spotted Crake Porzana porzana and Baillon’s Crake Zapornia pusilla. We used playbacks of the four species with the intention of locating nesting populations of other rail species as well, and also because it is known that in different periods of the breeding season rails can react more aggressively to playbacks of heterospecific than conspecific calls (Stermin et al. Citation2017).
Playback stimulation, with territorial calls, was performed for 4 h around sunrise and 4 h around sunset and any call reaction from the focal species was noted (Polak Citation2005). Schematically, in each point, the method used was as follows: (a) broadcasting Water Rail calls (20 s); (b) waiting for response (30 s); (c) broadcasting Spotted Crake calls (25 s); (d) waiting for response (30 s); (e) broadcasting Little Crake calls (25 s); (f) waiting for response (30 s); (g) broadcasting Baillon’s Crake calls (25 s); and (h) waiting for response (30 s). The order of the calls was selected randomly at the beginning of the study. The monitoring was conducted at least three times for each wetland during April–June. The areas where birds reacted to the playbacks at least twice during the monitoring period were considered to be breeding sites.
At each identified breeding site, the vegetation was recorded and the position of all identified nests was marked using a global positioning system (GPS) handset (3 m average accuracy). Information on nest position, size and nesting habitat was collected using a tape line in the first part of the nesting period. Data recorded on the nest included: nest diameter (Ndiam), distance from the water surface to the centre of the nest cup bottom (Wndist) and, because in almost all the cases the nest was under slope vegetation clumps, we measured the distance from inside the nest cup to the nearest upright vegetation (NvegDis). In terms of nesting habitat, in a circular plot of 2 m radius around the nest, the following habitat variables were quantified: water depth (average value for three random measurements around the nest cup)(Wdepth), vegetation density (average counts of vegetation stems on two areas of 25 cm² random chosen in a circle plot of 2 m radius around the nest)(VegD), cattail percentage in that 25 cm² (Typha) and also reed percentage (Phr), distance to the nearest open water area (DistOpW) and distance to the shore (Shore)(calculated in GIS).
In our study area there was mainly one clutch per breeding season for both species (Cramp Citation1980, Taylor Citation1998, David et al. Citation2018), so new nests with small clutches, lower than the mean (David et al. Citation2018), located after 1st July, were considered to be replacement clutches.
Statistical analysis
Analyses were carried out using SPSS (SPSS, Inc. Citation2008) and R.3.3.1 (R Core Team Citation2018). For an exploratory evaluation of overlap in nest site selection and nest features between both species, principal component analysis (PCA) was applied. An analysis of similarity (ANOSIM) was performed to statistically evaluate the similarity of nest features between the two species. If the ANOSIM statistic R (which can range from −1 to 1) is positive it suggests dissimilarity between groups, values close to zero suggest a random grouping, and negative values suggest that dissimilarities are greater within groups than between groups (Clarke & Gorley Citation2001).
To compare habitat characteristics among species we used parametric (ANOVA) and nonparametric (Kruskal–Wallis) one-way models. Wdepth, NvegDis, DistOpW, and VegD values were transformed by log10 to meet the normality assumption.
A discriminant function analysis (DFA) was used to explore the multivariate differences between the two species and evaluate the hierarchy of variables involved in discrimination of the two groups. Prior to analysis, all continuous variables were z-transformed (standardized to an average of zero and a standard deviation of one) in order to increase comparability of the variable effects (Ćirovic et al. Citation2008). In this case, variables related to the nest site (Wdepth, VegD, Typha, Phr, Shore, DistOpW) and nest features (Ndiam, Wndist, NvegDis) were taken into account.
Results
During three breeding seasons, 83 nests of Water Rails and 46 nests of Little Crakes were found. Water Rail nests were located in three wetlands (Sic Reedbeds, n = 79; Pike Lake, n = 1; Sucutard Pond, n = 3) and Little Crake nests in two wetlands (Sucutard pond, n = 44; Cătina pond, n = 2). The earliest Water Rail nest was found on April 14th and the latest one on June 25th, most of the nests were found at the beginning of May, during the main nesting period. For Little Crakes the earliest nest was located on May 19th and the latest on July 15th, with the main nesting period for Little Crakes extending over six weeks from mid of May to the end of June. Because previous studies on this population of Little Crakes estimated a mean of 6.22 eggs per clutch (with a maximum of 8 eggs per clutch), we assumed that the nest found on July 15th with a complete clutch of only 4 eggs was a replacement nest.
The Water Rail nest density in Sic Reedbeds was estimated 1 pair/ha in each study year and the Little Crakes nest density in Sucutard pond was estimated at 5 pairs/ha in 2010, 1 pair/ha in 2011 and 2 pairs/ha in 2012.
Most (73.4%) of the Water Rail nests were located in reeds, 19.2% in reed beds, but in patches dominated by cattail, and 3.8% were located in habitats dominated only by cattail. For Little Crakes almost all nests (95.8%) were located in cattail dominated habitats and only 4.5% were located in reeds but close to the cattail dominated area. A summary of nesting sites and nest features for Water Rails and Little Crakes is given in and .
Figure 2. Comparison of nest site characteristics of Water Rails (1) and Little Crakes (0) (Boxplots: horizontal bar = median, whiskers = last observation within 1.5 times the interquartile range). Features are: water depth (Wdepth, cm), vegetation density (VegD, stems/m2), cattail percentage cover (Typha, %), reed percentage cover (Phr, %), distance to open water (DistOpW, m), distance to the shore (Shore, m), nest diameter (Ndiam, cm), distance from the water surface to the centre of the nest cup bottom (Wndist, cm) and distance from inside the nest cup to the nearest upright vegetation (NvegDis, cm).
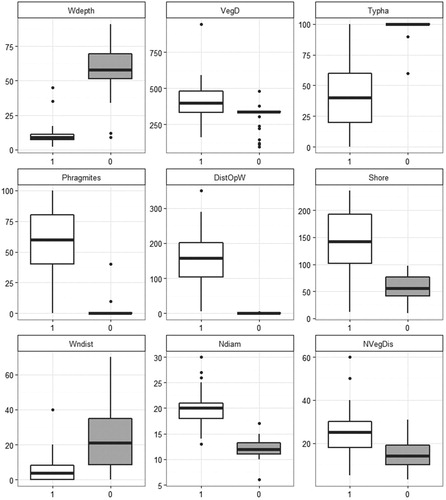
Table 1. Summary of nesting site characteristics and nest habitat features of Water Rails and Little Crakes. Parametric (ANOVA; F statistic) and nonparametric (Kruskal–Wallis; H statistic) one-way models compare differences between the two species. Features are: water depth (Wdepth, cm), vegetation density (VegD, stems/m2), cattail percentage cover (Typha, %), reed percentage cover (Phr, %), distance to open water (DistOpW, m), distance to the shore (Shore, m), nest diameter (Ndiam, cm), distance from the water surface to the centre of the nest cup bottom (Wndist, cm) and distance from inside the nest cup to the nearest upright vegetation (NvegDis, cm).
Even though the ranges of the recorded environmental variables used by both species were overlapping in some cases, the means were always significantly different (, ). The water depth ranged from 4 to 45 cm for Water Rail nest and from 9 to 91 cm for Little Crake nests, overlapping in the interval between 9 and 45 cm. The PCA suggested no overlaps in nest site selection (cumulative proportion of the first three principal components = 0.98019; ) and a partial overlap in the nest features (cumulative proportion of principal components = 2.7079; ). The ANOSIM statistic (R = 0.8209, P = 0.001) confirms the separation of nest features for both species. Parametric (ANOVA) and nonparametric (Kruskal–Wallis) one-way models revealed statistically significant differences between the two species in all measured variables (P < 0.001, ).
Figure 3. Two-dimensional ordination of nest site habitat characteristics of Water Rails (Rallus) and Little Crakes (Zapornia).
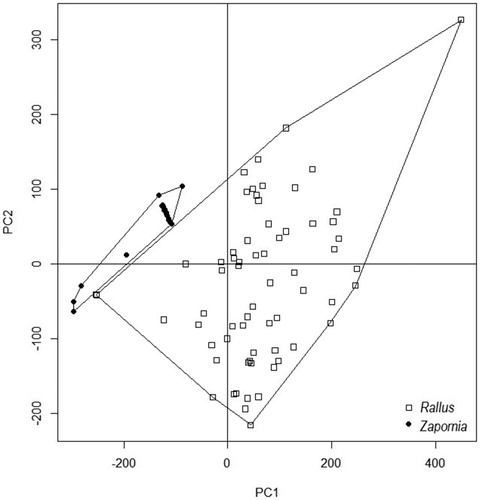
Figure 4. Two-dimensional ordination of nest features of Water Rails (Rallus) and Little Crakes (Zapornia).
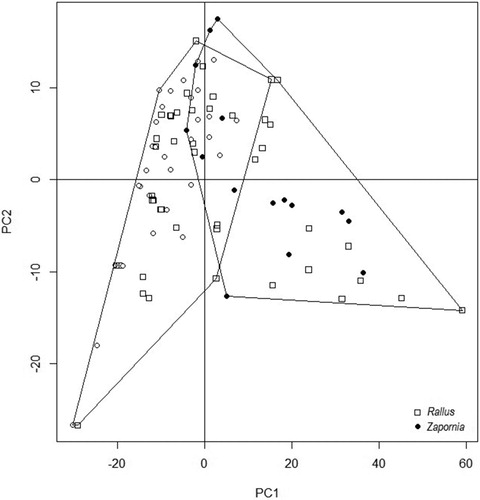
DFA generated one function that accounted for 100% of the variance between the two species (). The function was most highly correlated with distance from the water surface to the middle of the nest cup (r = 1) and water depth (r = 0.588).
Table 2. Summary statistics for the discriminant function analysis: correlations between original variables and discriminant function, and discriminant scores for each group (Eigenvalue = 2.284; Group centroids for Water Rail – 0.438; and Little Crake 0.780). Features are: water depth (Wdepth, cm), vegetation density (VegD, stems/m2), cattail percentage cover (Typha, %), reed percentage cover (Phr, %), distance to open water (DistOpW, m), distance to the shore (Shore, m), nest diameter (Ndiam, cm), distance from the water surface to the centre of the nest cup bottom (Wndist, cm) and distance from inside the nest cup to the nearest upright vegetation (NvegDis, cm).
Discussion
The results of this study indicate that the nesting habitats of Water Rails and Little Crakes differ and overlap only rarely. In terms of habitat structure, we found that the key factors in differentiating nest sites of the two species was water depth, followed by vegetation density. Water Rails nested in shallow waters and Little Crakes in deeper water, as has been found in previous studies (Kux Citation1959, Dittberner & Dittberner Citation1990, De Kroon Citation1999, Jedlikowski et al. Citation2014). The water depth has a wider range at Little Crake nests than at those of Water Rails because of the structure of the habitat, being dominated by cattail and reed respectively. Nesting places can overlap on the border of those two types of habitat, as was the case with Little Crake nests located in reeds but in deeper water. Jedlikowski et al. (Citation2014) also found water depth to be the most important factor differentiating the nest sites of the two species in areas with small ponds.
Nesting sites have a particular importance in breeding success (Regehr et al. Citation1998) for water birds, since deeper water and denser vegetation can decrease predation risk. In our two species, the main nest predator seems to be Marsh Harriers Circus aeruginosus and some mammal species such as the Raccoon Dog Nyctereutes procyonoides and the European Water Wole Arvicola amphibius (Stermin Citation2012, Jedlikowski et al. Citation2015). Deeper water offers better protection from terrestrial predators and dense vegetation offers high protection against aerial hunters.
Usually, in ponds, when water depth increases the density of the vegetation decreases. Close to the shore, the water is shallow and the vegetation density, dominated by reeds, is high. As the water gets deeper, the vegetation becomes dominated by cattail which has a lower stem density than reeds. In large deep wetlands, water depth influences the risk of predation: nests located in places where the water is deeper are better protected than the ones in shallow waters (Polak Citation2007, Austin & Buhl Citation2011) except in small ponds with shallow waters, where there is no correlation between water depth and nest survival (Jedlikowski et al. Citation2015).
Water Rails have large clutches (mean of 8.36 eggs, David et al. Citation2018), of white eggs with dark individual dots, in wide nests which are more visible and harder to hide. Thus, we suggest that eggs are more exposed to aerial predators and as such they prefer dense vegetation. In contrast, Little Crakes have smaller clutches (mean of 6.22 eggs, David et al. Citation2018), of darker eggs in narrow nests which can be hidden more easily in less dense vegetation. So we suggest that they prefer deeper water areas where they are more protected from terrestrial predators.
To compensate for the lack of the high vegetation density and to maintain protection, in Little Crakes, the distance between the middle of the nest cup and the nearest vertical vegetation stems was lower than it was for Water Rails (). Water Rails, do not need to hide their nests in a particular way because the vegetation density of reed where they nest is higher than that of cattail. Eight Little Crake nests were recorded in straight stem cattail areas. These nests looked like domed nests, having a roof above the nest cup built from the same materials as the nest.
According to the DFA results, the distance from the water to the nest cup is an important characteristic that distinguishes the nest features of the two species. Little Crakes build their nests up in the cattail sloped heaps and probably achieve more camouflage, while Water Rails build their nests close to the reed base.
In terms of vegetation, in small ponds Water Rails and Little Crakes nest almost in the same habitat type. Little Crakes have a tendency to nest in the cattail dominated habitat, while Water Rails tend to nest also in cattail, but in Carex (Glutz von Blotzheim et al. Citation1973, De Kroon Citation1999, Jedlikowski et al. Citation2014) and Juncus too (De Kroon Citation2004, Jedlikowski et al. Citation2014). De Kroon (Citation2004) suggests that nests located in Juncus are less visible than those located in reeds mixed with Carex. In our study, almost all Water Rail nests were located in habitats dominated by reed or reed and cattail, and only occasionally in habitats dominated only by cattail. This suggests that in the large wetland area of our study site, where there was a high availability of habitats and territories dominated by different vegetation types (approximately 400 ha dominated by reeds, 200 ha by cattail and 150 ha by Carex and Juncus), Water Rails mostly selected dense reed beds before cattail or Carex dominated habitats.
Even though all the other cattail dominated ponds were carefully investigated, most of the Water Rail nests were located in Sic Reedbeds and Pike Lake, wetlands dominated by dense reeds. In large scale studies, where the focus was the presence of birds during the breeding season but not nest location, the habitat used by Water Rails was also dominated by reed and cattail (Jenkins & Ormerod, Citation2002, Brambilla & Rubolini Citation2004).
Regarding the nest location related to the shore, in our study, in large reed beds, Water Rails had the possibility to nest deep inside the wetland, far away from the shore (, ) compared to smaller ponds where the distance from the nest to the shore was on average 9.6 ± 1.6 m (Jedlikowski et al. Citation2014).
The availability of large areas with dense reed can be a reason why there were no Water Rails nests in areas with Carex or Juncus, on the edge of the reed beds. In those cases, nests can be more exposed to predation risk than those deep in the reed beds (Batáry et al. Citation2004, Báldi & Batary Citation2005). In other studies on small ponds, a higher density of the vegetation close to the shore can offer a higher protection against aerial predators than a lower density of the vegetation from inside the wetland (Jedlikowski et al. Citation2015). In other cases, the distance from the nest to the shore can have no effect on predation risk (Picman et al. Citation1993).
On the nesting habitat of Little Crakes, in terms of dominant plant species, our results are similar to previous studies from small ponds (Jedlikowski et al. Citation2014) where almost all the nests were located in Typha habitats (around 80%) and Carex (around 20%) and only one nest was located in reeds. Although all the other large reed beds were carefully investigated, all Little Crake nests were located on Sucutard Pond and Cătina Pond wetlands, dominated by reed on the shore and by cattail near open water. In this context, we can say that in areas with small ponds and also in areas with large wetlands, with a high diversity and availability of habitats, Little Crakes nest in habitats dominated by cattail.
We conclude that in large wetlands with different available habitats, extensive reed beds and cattail dominated areas, Water Rails and Little Crakes showed greater differences in nesting habitat and nest features than they did in areas with only small ponds, where the ranges of values for all environmental variables overlapped a great deal (Jedlikowski et al. Citation2014). The most important factor distinguishing their nesting habitats, either in small or large wetlands, was the water level. In areas with large reed beds Water Rails nest deep into reed beds compared to other types of habitats, unlike in small ponds areas, while Little Crakes nest in cattail, in both large and small wetlands.
Finally, our results may have important implications for the conservation and management of wetlands where Water Rails and Little Crakes breed, by presenting new and specific information about how these two species are using their habitats in large wetlands, compared with the other studies conducted on small ponds.
Additional information
Funding
References
- Austin, J.E. & Buhl, D.A. 2011. Nest survival of American Coots relative to grazing, burning, and water depths. Avian Conserv. Ecol. 6: 1.
- Báldi, A. & Batáry, P. 2005. Nest predation in European reedbeds: Different losses in edges but similar losses in interiors. Folia Zool. 54: 285–292.
- Batáry, P., Winkler, H. & Baldi, A. 2004. Experiments with artificial nests on predation in reed habitats. J. Ornithol. 145: 59–63. doi: 10.1007/s10336-003-0010-9
- Bauer, K. 1960. Studies of less familiar birds. Little Crake. Br. Birds 53: 518–524.
- Bolenbaugh, J.R., Cooper, T., Brady, R.S., Willard, L.K. & Krementz, D.G. 2012. Population status and habitat associations of the King Rail in the Midwestern United States. Waterbirds 35: 535–545. doi: 10.1675/063.035.0404
- Brambilla, M. & Jenkins, R.K.B. 2009. Cost-effective estimates of Water Rail Rallus aquaticus breeding population size. Ardeola 56: 95–102.
- Brambilla, M. & Rubolini, D. 2004. Water Rail Rallus aquaticus breeding density and habitat preferences in Northern Italy. Ardea 92: 11–17.
- Brambilla, M., Rizzolli, F. & Pedrini, P. 2012. The effects of habitat and spatial features of wetland fragments on the abundance of two rallid species with different degrees of habitat specialization. Bird Study 59: 279–285. doi: 10.1080/00063657.2012.666226
- Celada, C. & Giuseppe, G. 1993. Breeding bird communities in fragmented wetlands. Bolletino di zoologia 60: 73–80. doi: 10.1080/11250009309355794
- Ćirovic, R., Radovic, D. & Vukov, T.D. 2008. Breeding site traits of European newts (Triturus macedonicus, Lissotriton vulgaris, and Mesotriton alpestris: Salamandridae) in the Montenegrin Karst Region. Arch. Biol. Sci. Belgrade 60: 459–468. doi: 10.2298/ABS0803459C
- Clarke, K.R. & Gorley, R.N. 2001. Primer v5: user manual/tutorial. Primer-E Ltd, Plymouth.
- Cramp, S. 1980. Handbook of the Birds of Europe, the Middle East and North Africa, Vol. 2. Oxford University Press, Oxford.
- Darrah, A.J. & Krementz, D.G. 2011. Habitat use of nesting and brood-rearing King Rails in the Illinois and Upper Mississippi River Valleys. Waterbirds 34: 160–167. doi: 10.1675/063.034.0204
- David, A. 2008. Ecologia populaţiilor de păsări din Câmpia Fizeşului. Presa Universitară Clujeană, Cluj-Napoca [in Romanian].
- David, A., Stermin, A.N. & Sevianu, E. 2018. Clutch size and egg repeatability in three elusive bird species: Little Bittern (Ixobrychus minutus), Little Crake (Zapornia parva) and Water Rail (Rallus aquaticus) from north-west Romanian populations. Studia Universitatis Babeş – Bolyao Biologia 63: 81–88. doi: 10.24193/subbbiol.2018.1.07
- De Kroon, G.H.J. 1999. Hoe diep is het oppervlaktewater in de broedhabitat van de Waterral Rallus aquaticus? Het Vogeljaar 47: 168–172.
- De Kroon, G.H.J. 2000. Over nesthabitat en nest van Waterral Rallus aquaticus in actief laagveen. Het Vogeljaar 48: 145–151.
- De Kroon, G.H.J. 2004. A comparison of two European breeding habitats of the Water Rail Rallus aquaticus. Acta Ornithol. 39: 21–27. doi: 10.3161/068.039.0107
- De Kroon, G.H.J. & Mommers, M.H.J. 2002. Breeding of the Water Rail Rallus aquaticus in Cladium mariscus vegetation. Ornis Svecica 12: 69–74.
- Dhondt, A.A. 2012. Interspecific Competition in Birds. Oxford University Press, Oxford.
- Diamond, J.M. & May, R.M. 1976. Island biogeography and the design of natural reserves. In May, R. M. (ed) Theoretical Ecology: principles and applications, 163–186. W.B. Saunders, Philadelphia, PA.
- Dittberner, H. & Dittberner, W. 1990. Öko-ethologische Beobachtungen am Nest der Kleinralle (Porzana parva). Bonn. Zool. Beitr 41: 27–58.
- Dombrowski, A., Mirosław, R. & Tabor, A. 1993. Use of the playback in estimating numbers of the Little grebe (Tachybaptus ruficollis), Water Rail (Rallus aquaticus), Little Crake (Porzana parva) and Moorhen (Gallinula chloropus). Notatki Ornitologiczene 34: 359–369.
- Douglas, J. 2011. Habitat fragmentation effects on birds in grasslands and wetlands: A critique of our knowledge. Gt. Plains Res. 11: 211–231.
- Glutz von Blotzheim, U.N., Bauer, K.M. & Bezzel, E. 1973. Handbuch der Vogel Mitteleuropas, Band 5. Akademische Verlagsgesellschaft, Frankfurt.
- Jedlikowski, J. & Brambilla, M. 2017. Effect of individual incubation effort on home range size in two rallid species (Aves: Rallidae). J. Ornithol. 158: 327–332. doi: 10.1007/s10336-016-1373-z
- Jedlikowski, J., Brambilla, M. & Suska-Malawska, M. 2014. Fine-scale selection of nesting habitat in Little Crake Porzana parva and Water Rail Rallus aquaticus in small ponds. Bird Study 61: 171–181. doi: 10.1080/00063657.2014.904271
- Jedlikowski, J., Brzeziński, M. & Chibowski, P. 2015. Habitat variables affecting nest predation rates at small ponds: A case study of the Little Crake Porzana parva and Water Rail Rallus aquaticus. Bird Study 62: 190–201. doi: 10.1080/00063657.2015.1031080
- Jenkins, R. & Ormerod, S. 2002. Habitat preferences of breeding Water Rail Rallus aquaticus. Bird Study 49: 2–10. doi: 10.1080/00063650209461238
- Johnson, D.H., Gibbs, J.P., Herzog, M., Lor, S., Niemuth, N.D., Ribic, C.A., Seamans, M., Shaffer, T.L., Shriver, W.G., Stehman, S.V. & Thompson, W.L. 2009. A sampling design framework for monitoring secretive marshbirds. Waterbirds 32: 203–215. doi: 10.1675/063.032.0201
- Kux, Z. 1959. Ein beitrag zur bionomie der Bartmeise (Panurus biarmicus russicus Brehm) und des Kleinen Sumpfhuhns (Porzana parva Scop.) an südmährischen Teichen. Acta Mus. Morav 44: 139–170.
- Lougheed, V., McIntosh, M., Christian, P. & Stevenson, J. 2008. Wetland degradation leads to homogenization of the biota at local and landscape scales. Freshw. Biol. 53: 2402–2431. doi: 10.1111/j.1365-2427.2008.02064.x
- Perrins, C. 2004. The New Encyclopedia of Birds. Oxford University Press, Oxford.
- Picman, J., Milks, M.L. & Leptich, M. 1993. Patterns of predation on passerine nests in marshes: Effects of water depth and distance from edge. Auk 110: 89–94. doi: 10.2307/4088408
- Polak, M. 2005. Temporal pattern of vocal activity of Water Rail Rallus aquaticus and the Little Crake Porzana parva in the breeding season. Acta Ornithol. 40: 21–26. doi: 10.3161/068.040.0107
- Polak, M. 2007. Nest-site selection and nest predation in the Great Bittern Botaurus stellaris population in eastern Poland. Ardea 95: 31–38. doi: 10.5253/078.095.0104
- R Core Team. 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Regehr, H.M., Rodway, M.S. & Montevecchi, W.A. 1998. Antipredator benefits of nest-site selection in black-legged kittiwakes. Can. J. Zool. 76: 910–915. doi: 10.1139/z98-016
- Schiermann, G. 1929. Zur Brutbiologie des Kleinen Sumpfhuhnes, Porzana parva. J. Ornithol. 77: 221–228. doi: 10.1007/BF01917253
- SPSS, Inc. 2008. Advanced Statistics v. 17.0. SPSS, Chicago, IL.
- Stermin, A.N. 2012. Biology and Ecology of Some Problematic Species: Water Rail (Rallus aquaticus) and Little Crake (Porzana parva) – studies on the Fizeş Basin’s populations. PhD Thesis, Babes-Bolyai University.
- Stermin, A.N., Pripon, L.R., David, A. & Coroiu, I. 2011. Wetlands management for Little Crake (Porzana parva) conservation in a “Natura 2000” site. Int. Conf. Environ. Sci. Develop., IPCBEE 4: 91–94.
- Stermin, A.N., Pripon, L.R. & David, A. 2012. The importance of homogenous vs. heterogenous wetlands in rallid (Rallidae) phenological seasons. Brukenthal Acta Musei 7: 549–554.
- Stermin, A.N., David, A. & Sevianu, E. 2013. An evaluation of acoustic monitoring methods for a Water Rail (Rallus aquaticus) population in a large reed bed. Waterbirds 36: 463–469. doi: 10.1675/063.036.0403
- Stermin, A.N., David, A. & Sevianu, E. 2017. The response of Water Rails (Rallus aquaticus) to the playback of conspecific and heterospecific calls. Wilson J. Ornithol. 129: 481–491. doi: 10.1676/16-078.1
- Taylor, B. 1998. Rails. A Guide to the Rails, Crakes, Gallinules and Coots of the World. Yale University Press, New Haven, CT and London.
