ABSTRACT
Capsule: Nest site selection of Black-bellied Sandgrouse Pterocles orientalis in an Algerian arid environment is dictated by a combination of topography, human presence, landscape and space.
Aims: To disentangle the drivers affecting Black-bellied Sandgrouse nest habitat use in an Algerian arid environment.
Methods: We used data on a series of topographic, anthropogenic, landscape and space variables, to identify the predictors of the occurrence probability of Black-bellied Sandgrouse nests. These variables were measured at nests (n = 33) and random points (n = 33) within landscape plots of 250 m radius.
Results: The probability of a site being selected for nesting by Black-bellied Sandgrouse was negatively related to distance to the nearest cereal crops, but positively associated with the cover of natural water, cover of rocks, and distance to the nearest road. This probability was also high at elevations between 251 and 289 m.
Conclusion: From a practical perspective, it would be interesting to reproduce the same investigation in other Mediterranean arid environments to find out if this species follows a similar pattern of nesting habitat use. This would provide guidance for future conservation actions and inform appropriate habitat management for this species.
In order to develop management actions appropriate for the conservation of a species, it is important to understand all of its ecological requirements, including nesting habitats (Hanane Citation2018). Nest site choice in birds is often the result of trade-offs that allow them to maximize their reproductive success. Overall, habitat selection takes into account a range of factors, including availability of food (Wiehn & Korpimäki Citation1997, Setchfield & Peach Citation2016), protection from weather (Sadoti Citation2008), predators (Hatchwell et al. Citation1999), human disturbance (Rogers Citation1996, Hanane & Baâmal Citation2011, Hanane Citation2012, Citation2014) and the presence of competitors (Illera & Diaz Citation2008). This is particularly the case in harsh conditions, such as North African arid environments, where birds are often constrained by abiotic factors (Palomino et al. Citation2008, Traba et al. Citation2013). Substrate type and slope are known to be important factors in habitat selection by Cream-coloured Coursers Cursorius cursor (Palomino et al. Citation2008), Houbara Bustards Chlamydotis undulate (Carrascal et al. Citation2008) and Dupont’s Larks Chersophilus duponti (Garza et al. Citation2005, Seoane et al. Citation2006), whereas altitude and slope determine nest site selection of the Canary Islands Stonechat Saxicola dacotiae (Illera et al. Citation2010). This study investigates nesting habitat selection of a ground-nesting (Benítez-López et al. Citation2014) granivorous species known to be well adapted to extreme conditions of arid regions: the Black-bellied Sandgrouse Pterocles orientalis.
Black-bellied Sandgrouse are a characteristic species of open arid, semi-desert, steppes and pseudo-steppes of southwest Europe, North Africa and the Middle East (De Juana Citation1997, Isenmann & Moali Citation2000, BirdLife International Citation2004, Seoane et al. Citation2010, Traba et al. Citation2013). From a biological point of view, rather little attention has been given to the study of most sandgrouse species (Morales & Traba Citation2016), likely due to their secretive nature and cryptic behaviour. To date, the majority of studies undertaken on Black-bellied Sandgrouse have been conducted in southwest Europe, especially Spain (Herranz & Suárez Citation1999, Benítez-López et al. Citation2014, Martín et al. Citation2014, Benítez-López et al. Citation2017) and Portugal (Cardoso et al. Citation2007), and in North Africa, studies have been limited to Morocco (Aourir et al. Citation2008, Znari et al. Citation2008, Traba et al. Citation2013) and Algeria (Farhi Citation2015, Farhi & Belhamra Citation2015). None of these studies have specifically focused on the factors influencing nesting habitat selection of Black-bellied Sandgrouse in the entire Mediterranean basin, but instead have concentrated on general habitat use (Benítez-López et al. Citation2014, Martín et al. Citation2014, Benítez-López et al. Citation2017). These works have, however, documented how Black-bellied Sandgrouse interacted with their environments in arid southwest European areas. It has, therefore, been shown that, in the breeding period, Black-bellied Sandgrouse: (1) are sensitive to human disturbance (Cardoso et al. Citation2007, Martín et al. Citation2014, Benítez-López et al. Citation2017) and (2) generally use rocky areas (Znari et al. Citation2008, Martín et al. Citation2014, Benítez-López et al. Citation2017) in close proximity to water-holes and cereal crops (Cardoso et al. Citation2007, Martín et al. Citation2014). This pattern remains nonetheless specific to the birds’ presence and not of their nests. This has resulted in gaps, which need to be filled if we are to (1) build more knowledge on the species, (2) identify the characteristics of areas where nesting will occur, (3) highlight priority areas for conservation management, (4) develop a long-term conservation programme and (5) suitably manage the species.
Here we investigate the effects of a series of variables, measured at occupied nest sites and an equivalent number of random unoccupied sites, on the probability of presence of Black-bellied Sandgrouse nests in an Algerian arid environment. Specifically, we aim to disentangle the roles of (i) topography; (ii) human disturbance, (iii) landscape and (iv) space. We have used Variation Partitioning, as the technique of choice for this with an unbiased statistical estimator to ensure the correct interpretation of the results (Legendre Citation2008). Such an approach will identify which of these four potential predictors is the most relevant in the determining Black-bellied Sandgrouse nest presence. A marked sensitivity towards an individual predictor will determine the nature and the scope of management measures to be undertaken.
Overall, the results of this investigation will help inform the management and conservation of this species. Given the abovementioned Black-bellied Sandgrouse habitat associations for adults birds, we hypothesized that the selected nest sites would be: (1) more distant from human settlements, (2) closer to water-holes and cereal crops for foraging and (3) more rocky. Considering the relative importance of these factors, we expected that the selection of nesting habitat would be a trade-off between them.
Methods
Study area
Observations were made in Algeria, in the arid region of Biskra (34°50.222N 5°45.063"W). The area (35.1 km2) has a Saharan climate, with annual average rainfall of 149.7 mm, most of which falls during the winter season (December–January) (ONM Citation2016). Temperatures vary widely, being more moderate during the winter, and hot in summer (especially in July) with peaks that can often reach 44°C. Mean relative humidity is 35%, with a minimum of 25.6% during July and a maximum of 59.1% during December. Altitude ranges from 125 to 300 m above sea level.
The predominant landscape is mainly composed of: (i) sparse vegetation dominated by of short perennial grasses and undershrubs (chamaephytes) such as Arthrophytum scoparium, Anvillea radiata and Salsola vermiculata, (ii) a fairly flat rock area (376 ha), (iii) cereal crops (61.4 ha) consisted mainly of Barley Hordeum vulgare and secondarily of Wheat Triticum turgidum, and (iv) a salt dump (19.4 ha). This latter, quite weakly attended, constitutes the place where waste, coming from salt extraction from the neighbouring quarry, are accumulated. The area is also a permanent pasture area for camels and sheep (DSA Citation2017). The study area is crossed by the Bougatou’s wadi in which vegetation is dominated by Astragalus armatus and Retama raetam. No human habitations exist in the studied arid environment (Farhi Citation2015).
Data collection
Prior to starting field work, a survey was carried out among the various researchers, foresters, shepherds and land owners connected with Black-bellied Sandgrouse, to define the areas that they perceived as being preferred by the species within the region of Biskra. Once completed, monitoring took place during the 2013 and 2014 breeding seasons (mid-March to end of August). To maximize the chances of finding the nests of this extremely secretive species, systematic and non-systematic approaches were combined. During each weekly visit, the study area was been arbitrarily divided into four equal sub-areas. Each sub-area was assigned to two people and a systematic search for nests was undertaken. The systematic approach used was to walk on a zig-zag pattern from one end of each sub-area to the other, with one surveyor moving along the right side and the other on the left side so as not overlap. This method has the advantage of allowing almost complete coverage of the study area. During these back-and-forth movements, all signs of Black-bellied Sandgrouse presence (e.g. droppings, footprints, flying birds) were recorded. Once a bird that was suspected to be nesting was located, a furthermore intense search was undertaken to locate the site of the nest. Surveys began one hour after sunrise and lasted until one hour before sunset under favourable weather conditions (no rain and low wind). This information was further supplemented by a non-systematic approach which consisted of nests found by people using the study area (land owners, shepherds, etc.) who were distributed across almost the entire study area almost all day.
To compare habitat characteristics, an equal number of non-nest points (n = 14 in 2013 and 19 in 2014) were randomly selected. Random points were selected, using the QGIS random selection tool (Quantum GIS Development Team Citation2017) after excluding all points at which Black-bellied Sandgrouse were recorded.
Selection of scales
To assess habitat at the landscape scale, we used the spatial analyst tool in QGIS to calculate land cover around each nest and random point within four radii (50, 100, 250, 500 m) surrounding each survey point. To select the radius values for the landscape variables, we treated the sets of variables belonging to the four different radii as competing sets of variables and selected the radius leading to the most parsimonious models. The lowest value of Akaike information criterion corrected for small sample sizes (AICc) was obtained for the 250 m radius (26.7; AICc null model = 93.6), followed by those of 500 m (28.8), 100 m (52.0), and 50 m (64.9). Based on these statistical analyses, the 250 m radius was selected for the landscape-scale analyses.
Selection of variables and GIS procedures
We selected nine environmental variables related to topography, human influence and land use. These variables were used because they could potentially influence the presence of nests of Black-bellied Sandgrouse (). All variables were measured using Quantum QGIS 2.18.14. Elevation and slope were derived from the Shuttle Radar Topography Mission Global 1 Digital Elevation Model, with a 30 × 30 m resolution.
Table 1. Description of variables used in models to explain the probability of the presence of Black-bellied sandgrouse nests in a North African arid environment.
Because of the lack of geospatial data for the study area, we produced a land use map to calculate distances and landscape variables from its vector layers. This map was produced following a supervised object-oriented classification of satellite images from the Landsat 8 Operational Land Imager (OLI) and Thermal Infrared Sensor (TIRS) Level 2 collection (30 m spatial resolution) acquired from the Earth Explorer website of the U.S. Geological Survey (USGS, earthexplorer.usgs.gov). This platform allowed us to obtain pre-processed Landsat data, with the transformation of the spectral values into surface reflectance, which offers the possibility of exploiting them without making prior corrections. The analysed period corresponds to the year 2015. The validation of this map was made from base-map images from Bing (Aerial Bing) and the field data. We choose Universal Transverse Mercator (UTM) zone 31 for our study area basing on UTM grid zones on a projected map of the world.
Spatial variables were created by means of Moran’s eigenvector maps (MEMs) (Dray et al. Citation2006), a method that produces flexible spatial predictors through principal coordinate analysis of a truncated geographic distance matrix among different points, while capturing spatial effects at multiple spatial scales. These orthogonal spatial variables (MEMs) can be used in regression (independent variables) to account for spatial autocorrelation in the distribution of probability of the presence of Black-bellied Sandgrouse nests (Borcard et al. Citation2011, Assandri et al. Citation2019).
Statistical analyses
Possible correlations among variables were checked using variance inflation factors (VIFs). To prevent multicolinearity, the variables were tested with VIF Analysis (Quinn & Keough Citation2002). Variables with a VIF > 5 were removed as recommended by Zuur et al. (Citation2009).
To test the effect of the covariates on Black-bellied Sandgrouse nest presence, we used Generalized Linear Models (GLM) with binomial errors (logistic regression). We separately tested the effect of each group of topographical, anthropogenic, landscape and space predictors on the probability of nest presence. For each set of predictors, we developed an all-inclusive design (all possible combination models) by using multimodel inference (Burnham & Anderson Citation2002). Models were then ordered by increasing AICc (Burnham & Anderson Citation2002). We considered all models with ΔAICc lower than 2 as equally good (Burnham & Anderson Citation2002). Model weights were used to define the relative importance of each explanatory variable across the full set of models evaluated by summing weight values of all models that included the explanatory variable of interest. We tested the model performance by the Hosmer–Lemeshow Goodness of Fit Test (Hosmer & Lemeshow Citation2000). For subsequent variation partitioning (VP) analyses, we retained only the variables with confidence intervals of parameter estimates not encompassing zero. VP was applied to evaluate the specific contribution of each of the four sets of predictors and their joint fractions in the nest habitat selection by Black-bellied Sandgrouse. We applied VP to the final and parsimonious models (simplified models resulting from model selection, Peres-Neto & Legendre Citation2010, Assandri et al. Citation2019).
To ensure that observations were independent of each other and to allow us to subsequently address spatial autocorrelation in data prior to data analysis, we implemented Moran’s I correlogram with 10 lags of 500 m each to assess the pattern of spatial autocorrelation of the probability of nest presence. We assessed the significance of the values for each lag with a Monte-Carlo test of 999 permutations. A correlogram was significant if at least one lag resulted in P < 0.005 (adjusted Bonferroni correction).
All statistical analyses were performed in R-3.0.2 software (R Development Core Team Citation2013). We used the package ‘car’ (Fox & Weisberg Citation2011) to calculate Variance Inflation Factor (VIF), the package ‘MuMIn’ to calculate AICc (Bartoń Citation2015), the package ‘ResourceSelection’ to test the best model performance, the package ‘vegan’ (Oksanen et al. Citation2013) to carry out the variation partitioning analysis. Package ‘sp’, ‘spdep’, ‘adespatial’, ‘ade4’, ‘maptools’, and ‘adegraphics’ were used generate spatial predictors (Dray et al. Citation2006). Means are quoted ± standard errors.
Results
A total of 33 Black-bellied Sandgrouse nests (n = 14 for 2013, n = 19 for 2014) were assessed by this study. The average characteristics of those nests, as well as the random points, are summarized in . Systematic and non-systematic searches resulted in the finding of 12 and 21 nests, respectively.
Table 2. Descriptive statistics for variables (mean ± se) measured at Black-bellied sandgrouse nests and random points in a Mediterranean arid environment. Comparison between nest points and random points are shown by the t tests.
The most supported models on the effect of topographic, landscape, human and spatial predictors on the nest presence probability are summarized in and . The probability of nest presence was negatively influenced by distance to the nearest dry cereal crops, positively associated with the cover of natural water, cover of rocks, and distance to the nearest road. This probability was also high at elevation between 251 and 289 m.
Table 3. Most supported models on the effect of topographic, landscape, anthropogenic, spatial predictors on probability of Black-bellied sandgrouse nest presence. Models are ranked according to Akaike’s information criterion corrected for small sample size (AICc) and only models within an interval of ΔAICc < 2 are shown. The difference in AICc from the best supported model (ΔAICc), Akaike’s weights (wi), −2 log-likelihood values (logLik), and Hosmer–Lemeshow Goodness of Fit Test (GOF) are also given. Negative (−) or positive (+) relationships between predictors and probability of the presence of Black-bellied sandgrouse nests are shown.
Table 4. Models parameters (estimate; based on models with ΔAICc < 2), standard error (SE) from the most supported topographic, landscape, anthropogenic and spatial models for probability of Black-bellied sandgrouse nest presence. 95% confidence intervals (C.I.) for the parameter estimates are given; in bold, the parameters in which confidence intervals do not include zero.
Moran correlogram showed that both the residuals of topographical (best model (ELV + ELV2): Moran’s I = 0.275, P = 0.001, (a)) and landscape (CWT + CRO: Moran’s I = 0.253, P = 0.004, (b)) variables exhibited significant positive spatial autocorrelation in the first 500 m, whereas residuals of anthropogenic (DCE + DRT: Moran’s I = 0.214-0.747, P < 0.002, (c)) variables exhibited a significant positive spatial autocorrelation in the first 1500 m. Space was unimportant at further distances, respectively.
Figure 1. Correlograms of predicted Black-bellied Sandgrouse nest presence. Black symbols represent significant Moran's I values at the 0.05 level. Asterisk-marked symbols represent significant Moran's I values at the corrected Bonferroni level (0.05/10 = 0.005).
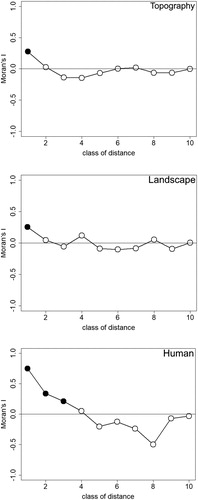
Because of the presence of spatial autocorrelation for each set of variables, we created spatial variables by means of Moran’s eigenvector maps (three eigenvectors: MEM1, MEM8 and MEM5). Only one of them was retained after model selection [MEM1 represents a positive spatial correlation at 500 m (Moran's I = 0.266, P = 0.001)] which has confidence intervals for the estimate not encompassing zero. The Hosmer–Lemeshow Goodness of Fit test indicated very good fit of these models ().
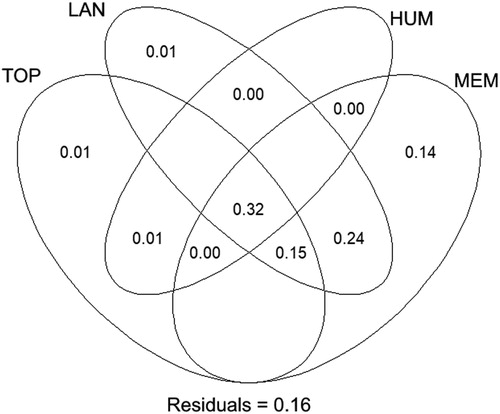
Variation partitioning highlighted a clear contribution of the four sets of predictors in explaining variation in the probability of nest presence, indicating that multiple factors co-explain the observed pattern. The probability of nest presence was mainly jointly explained (32%) by space (MEM1), landscape (covers of rock and natural water area), human presence (distance to the nearest dry cereal field and to nearest road), and topography (elevation), followed by the joint effect of space and landscape (24%), then by that of space, landscape and topography (15%) (). The results also suggest important spatial patterns. Indeed, taken alone, the space has the greatest effect in explaining the Black-bellied Sandgrouse nest presence (14%), while the pure effects of topography, landscape and human disturbance are very negligible ().
Figure 2. Venn diagrams for variation partitioning showing the percentage contribution of topography, landscape, human presence, and spatial components in explaining the presence of Black-bellied Sandgrouse nests. Each number shows the fractions of variation explained by variables included in each different scale or each combination of scales. Values less than zero are not shown. TOP = topography, HUM = human influence, LAN = landscape, MEM = Moran’s eigenvector maps.
Discussion
In the present study, we investigated the effects of topographic, anthropogenic, landscape, and space variables on the presence of Black-bellied Sandgrouse nests in a Mediterranean arid environment. Our results revealed that the probability of a site being selected for nesting was negatively related to distance to the nearest dry cereal farmland, positively associated with the cover of natural water, cover of rocks, and distance to the nearest road. This probability was also high at elevation between 251 and 289 m. To our knowledge, this is the first study to investigate nest site selection of Black-bellied Sandgrouse in the Mediterranean basin. This work would help to complete the picture of the habitat use by this species, providing new information on nest habitat selection.
The distance to nearest dry cereal farmland was negatively correlated with the presence of Black-bellied Sandgrouse nests. This result provides information on the importance of the presence of cereals around nests (less than 600 m). In the study area, the breeding period of Black-bellied Sandgrouse mainly coincides with the post-sowing ploughing stage of cereals. This ensures the availability of food resources in close proximity to nests (birds can rapidly find seeds). Seeds are known to be the most important dietary component of sandgrouse (Herranz & Suarez Citation1999, Martín et al. Citation2014, Benítez-Lopez et al. Citation2017). This result also informs on the aptitude of the species to nest in close proximity to habitats intensely managed by humans, as found by Benítez-Lopez et al. (Citation2013) while explaining adult sandgrouse presence. It seems that Black-bellied Sandgrouse can withstand a certain level of human disturbance to meet their food needs. Martín et al. (Citation2014), in studying habitat preference for breeding sympatric sandgrouse, have reported a high tolerance to changes in environmental conditions for Black-bellied Sandgrouse.
In this arid environment, Black-bellied Sandgrouse place their nests further from roads than random placement would suggest. Such choice could be interpreted as an anti-disturbance strategy. Noises generated by vehicles and their presence are likely to disturb sandgrouse in this area as demonstrated in Morocco for the Barbary Partridge Alectoris barbara (Hanane Citation2018). The negative effects of roads depend undeniably on the level of traffic as previously stated by Reijnen et al. (Citation1996) and Martín et al. (Citation2014). Sandgrouse species are known to be sensitive to human activity because they strongly avoided trails and human settlements (Martín et al. Citation2014, Benítez-Lopez et al. Citation2017). Other studies have also reported an avoidance of human-disturbance factors such as roads and villages (Cardoso et al. Citation2007, Seoane et al. Citation2010). It is now known that urbanization and associated infrastructure development are important drivers of decline of sandgrouse (Santos & Suárez Citation2005, Cardoso et al. Citation2007, Benítez-Lopez et al. Citation2013). In the study area, urbanization infrastructures are widespread on elevations below 245 m, and the relatively high level of anthropogenic disturbance could be the major cause of the absence of Black-bellied Sandgrouse nests at these altitudes. Our results also suggest the absence of nests on elevations above 295 m. Above this height, the topographic conditions (presence of cliffs) are not really appropriate (see Znari et al. Citation2008, Yosef & Zduniak Citation2011 and Traba et al. Citation2013) to accommodate the nests of this species. The choice of the altitude range between 245 and 295 m for nesting would be a strategy trade-off between topographic (shallow slope and flat areas), anthropogenic factors (low human presence), and landscape factors (habitat cover). This is not surprising since that the distribution of the species is, in itself, intimately linked to both topographic and anthropogenic factors (Whittaker et al. Citation2007, Benítez-Lopez et al. Citation2013).
At the landscape scale (approximately 20 ha), natural water cover and that of rocky area (flat stoney ground) were the best predictors of the probability of nest presence. To nest, Black-bellied Sandgrouse selected high rock cover areas as previously found by Cardoso et al. (Citation2007), Znari et al. (Citation2008) and Traba et al. (Citation2013). The use of this habitat type is undoubtedly dictated by an antipredatory strategy (Znari et al. Citation2008). Rock areas can help to reduce predation risk by concealing nests and eggs from predators (Lloyd et al. Citation2001, Martín et al. Citation2014), but also by providing a suitable camouflage background for adults and chicks (Cardoso et al. Citation2007). Furthermore, at this scale, the probability of Black-bellied Sandgrouse nest presence is also associated with the cover of natural water. This is in agreement with Yosef & Zduniak (Citation2011) who, in studying the drinking schedule of four sandgrouse species, have suggested that Black-bellied Sandgrouse breed and forage in proximity to water holes. According to Hinsley et al. (Citation1993), this choice is probably a prerequisite for survival because the species is known to be a ‘less able thermoregulator under hot conditions’ than other species. In a hot and arid environment, it is also possible that water availability in proximity to nests would be vital for chicks. Indeed, time spent searching for water by adults would be markedly reduced, thereby providing better breeding opportunities through increased nest attendance as well as reduced risk of dehydration.
In this arid environment, Black-bellied Sandgrouse suitable nesting sites are space dependent and not randomly distributed. Topographic, anthropogenic, landscape and space factors jointly explained most of the variation in the probability of presence of Black-bellied Sandgrouse nests (32% of total variation). The importance of this joint scale probably results from the fact that Black-bellied Sandgrouse search for particular characteristics (suitable areas) for more successful reproduction (69.7% of nests have hatched, n = 33). To reach this objective, Black-bellied Sandgrouse jointly take into account: (i) sensitivity to human disturbance (anti-disturbance strategy), (ii) the need to have food resources in close proximity (food strategy), and (iii) the need to have appropriate landscape composition (anti-predation and water strategy). As for most animals (Cowlishaw Citation1997), food acquisition and human/predator avoidance are principal components of Black-bellied Sandgrouse nest habitat selection strategies.
Conclusions and implications
As predicted, the observed nest distribution pattern was explained by multiple factors. Some of our results are similar to those found for sandgrouse in southwest Europe (Seoane et al. Citation2010, Benítez-Lopez et al. Citation2013, Martín et al. Citation2014). Further studies could usefully focus on identifying the overlap between habitats used for nesting by Black-bellied Sandgrouse and habitat use for other purposes, and at different times of the year. Conservation guidelines for this species during the breeding season should consider the spatial configuration of their nesting habitats. Protecting this breeding zone in its entirety, while performing awareness campaigns with the local farmers would be the most effective way to guarantee nest survival. Brotons et al. (Citation2004) have also highlighted the importance of protected areas for maintaining viable populations of steppe birds. Agricultural land management should also take into account the requirements of Black-bellied Sandgrouse. When a species adopts a strategy based on trade-offs, especially in harsh conditions, the balance between the different components constituting this trade-off are often sensitive to even minor changes. Each of the four components should be monitored both spatially and temporally. For instance, the assessment of the cover of cereals (certainly at the expense of natural habitats) and location of nest sites should inform on the tolerance of this species to agricultural activities. Overall, research on the sandgrouse should enable land managers to prevent significant land cover changes (i.e. agricultural intensification) near areas with the highest probabilities of the occurrence of nesting Black-bellied Sandgrouse.
The combined model has explained 84% of the variance. Although high, this suggests that there are yet a number of other non-evaluated variables that may also influence selection of nest site location. These may include hunting and predation pressure in the area. It would, therefore, be useful to assess further variables affecting nest site selection processes in this species, to enhance conservation actions. Finally, it is important to extend the same analytical approach to other arid Mediterranean environments to find out if this species follows a similar nesting habitat selection pattern. This would provide guidance for future conservation actions and appropriate habitat management for the species.
Acknowledgements
We thank Borhane-eddine Farhi, Oussama Ben nacer, Djamel eddine Boubidi, Messaoud, and Dehina for their help during the field work. We are grateful to Stephen Browne for very helpful comments on an earlier draft of this paper. We thank the two anonymous reviewers and the Editor of Bird Study for their comments and advice.
ORCID
Saâd Hanane http://orcid.org/0000-0003-2008-1728
References
- Aourir, M., Znari, M., El Abbassi, A., Radi, M. & Melin, J.M. 2008. Reproductive parameters in captive hand-reared Black-bellied Sandgrouse. Zoo Biol. 27: 269–281. doi: 10.1002/zoo.20185
- Assandri, G., Bogliani, G., Pedrini, P. & Brambilla, M. 2019. Toward the next Common Agricultural Policy reform: determinants of avian communities in hay meadows reveal current policy's inadequacy for biodiversity conservation in grassland ecosystems. J. Appl. Ecol. 56: 604–617. doi: 10.1111/1365-2664.13332
- Bartoń, K. 2015. MuMIn: multi-Model Inference. R package. Version 1.15.1.
- Benítez-López, A., Viñuela, J., Suárez, F., Hervás, I. & García, J.T. 2013. Modelling sandgrouse (Pterocles spp.) distributions and large-scale habitat requirements in Spain: implications for conservation. Environ. Conserv. 41: 132–143. doi: 10.1017/S0376892913000192
- Benítez-López, A., Viñuela, J., Suárez, F., Hervás, I. & García, J.T. 2014. Niche-habitat mechanisms and biotic interactions explain the coexistence and abundance of congeneric sandgrouse species. Oecologia 176: 193–206. doi: 10.1007/s00442-014-3010-y
- Benítez-López, A., Viñuela, J., Mougeot, F. & García, J.T. 2017. A multi-scale approach for identifying conservation needs of two threatened sympatric steppe birds. Biodiver. Conserv. 26: 63–83. doi: 10.1007/s10531-016-1222-7
- Birdlife International. 2004. Black-bellied Sandgrouse Pterocles orientalis. Data Zone.
- Borcard, D., Gillet, F. & Legendre, P. 2011. Numerical Ecology with R. Springer-Verlag, New York: 306.
- Brotons, L., MaÑosa, S. & Estrada, J. 2004. Modelling the effects of irrigation schemes on the distribution of steppe birds in Mediterranean farmland. Biodiv. and Conserv. 13: 1039–1058. doi: 10.1023/B:BIOC.0000014468.71368.35
- Burnham, K.P. & Anderson, D.R. 2002. Model Selection and Inference: A Practical Information-Theoretic Approach. 2nd ed. Springer-Verlag, New York: 1–488.
- Cardoso, A.C., Poeiras, A.S. & Carrapato, C. 2007. Factors responsible for the presence and distribution of black bellied sandgrouse Pterocles orientalis in the Nature Park “Vale do Guadiana”. Ardeola 54: 205–215.
- Carrascal, L.M., Palomino, D., Seoane, J. & Alonso, C.L. 2008. Habitat use and population density of the houbara bustard Chlamydotis undulata in Fuerte ventura (CanaryIslands). Afr. J. Ecol. 46: 291–302. doi: 10.1111/j.1365-2028.2008.00971.x
- Cowlishaw, G. 1997. Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Anim. Behav. 53: 667–686. doi: 10.1006/anbe.1996.0298
- De Juana, E. 1997. Family Pteroclididae (Sandgrouse). In J. del Hoyo, A. Elliott & J. Sargatal. (ed) Handbook of the Birds of the World, Vol. 4: 679. Lynx Edicions, Barcelona.
- Dray, S., Legendre, P. & Peres-Neto, P.R. 2006. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Modell. 196: 483–493. doi: 10.1016/j.ecolmodel.2006.02.015
- DSA. 2018. Bilan annuel sur l'agriculture dans la région de Biskra. Algérie. (Compagne 2017/2018). Ed. Direction des services agricole: 13.
- Farhi, K. 2015. Bio-ecologie des populations du Ganga unibande (Pterocles orientalis) dans les Ziban Thèse Doct. Univ. Mohamed khider. Biskra. 111.
- Farhi, K. & Belhamra, M. 2015. Nidification et ponte du Ganga unibande Pterocles orientalis (Linneaus, 1758) dans la région de Biskra, Algérie. Courier du savoir 19: 74–84.
- Ferns, P.N. & Hinsley, S.A. 1995. Importance of topography in the selection of drinking sites by Sandgrouse. Funct. Ecol. 9: 371–375. doi: 10.2307/2389999
- Fox, J. & Weisberg, S. 2011. An R Companion to Applied Regression. 2nd ed. Sage, Thousand Oaks.
- Garza, V., Suárez, F., Herranz, J., Traba, J., García de la morena, E.L., Morales, M., González, R. & Castañeda, M. 2005. Space use and habitat selection of dupont’s lark (Chersophilus duponti) during the breeding and post breeding periods. Ardeola 52: 133–146.
- Hanane, S. 2012. Do age and type of plantings affect turtle dove Streptopelia turtur nest placement in olive agroecosystems? Ethol. Ecol. Evol. 24: 284–293. doi: 10.1080/03949370.2011.634439
- Hanane, S. 2014. Plasticity in nest placement of the Turtle Dove (Streptopelia turtur): experimental evidence from Moroccan agro-ecosystems. Avian Biol. Res. 7: 65–73. doi: 10.3184/175815514X13947297062869
- Hanane, S. 2018. Local versus landscape-scale determinants of nest-site selection in the Barbary Partridge Alectoris barbara. Bird Study 65: 495–504. doi: 10.1080/00063657.2018.1559797
- Hanane, S. & Baâmal, L. 2011. Are Moroccan fruit orchards suitable breeding habitats for Turtle Doves Streptopelia turtur? Bird Study 58: 57–67. doi: 10.1080/00063657.2010.518230
- Hatchwell, B.J., Russell, A.F., Fowlie, M.K. & Ross, D.J. 1999. Reproductive success and nest-site selection in a cooperative breeder: effect of experienceand a direct benefit of helping. The Auk. 116: 355–363. doi: 10.2307/4089370
- Herranz, J. & Suarez, F. 1999. La Ganga Ibérica (Pterocles alchata) y la Ganga Ortega (Pterocles orientalis) en España: Distribucion, abundancia, bioloíga y conservación. Colección Técnica, Ministerio de Medio Ambiente, Organismo Autónomo Parques Nacionales, Madrid: 327.
- Hinsley, S.A., Ferns, P.N., Thomas, D.H. & Pinshow, B. 1993. Black-Bellied Sandgrouse (Pterocles orientalis) and Pin-Tailed Sandgrouse (Pterocles alchata): Closely related species with Differing Bioenergetic Adaptations to arid zones. Physiol. Zool. 66: 20–42. doi: 10.1086/physzool.66.1.30158285
- Hosmer, D.W. & Lemeshow, S. 2000. Applied logistic regression. Second edition: 369.
- Illera, J.C. & Diaz, M. 2008. Site fidelity in the Canary Islands stonechat Saxicola dacotiae in relation to spatial and temporal patterns of habitat suitability. Acta Oecol. 34: 1–8. doi: 10.1016/j.actao.2008.01.003
- Illera, J.C., Wehrden, H.V. & Wehner, J. 2010. Nest site selection and the effects of land use in a multi-scale approach on the distribution of a passerine in an island arid environment. J. Arid Environ. 74: 1408–1412. doi: 10.1016/j.jaridenv.2010.04.012
- Isenmann, P. & Moali, A. 2000. Oiseaux d’Algérie – Birds of Algeria. Ed. Société d’études ornithologiques de France, Mus. nati. hist. natu, Paris: 30.
- Legendre, P. 2008. Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J Plant Ecol. 1: 3–8. doi: 10.1093/jpe/rtm001
- Lloyd, P., Little, R.M. & Crowe, T.M. 2001. The breeding biology of the Namaqua Sandgrouse, Pterocles namaqua. Ostrich 72: 169–178. doi: 10.2989/00306520109485313
- Morales, M.B. & Traba, J. 2016. Prioritising research in steppe bird conservation: a literature survey. Ardeola. 63: 137–150. doi: 10.13157/arla.63.1.2016.rp6
- Martín, B., Martín, C.A., Palacín, C., Sastre, P., Ponce, C. & Bravo, C. 2014. Habitat preferences of sympatric sandgrouse during the breeding season in Spain: a multi-scale approach. Eur. J. Wildlife Res. 60: 625–636. doi: 10.1007/s10344-014-0826-z
- Palomino, D., Seoane, J., Carrascal, L.M. & Alonso, C. 2008. Competing effects of topographic, lithological, vegetation structure and human impact in the habitat preferences of the Cream-coloured Courser. J. Arid Environ. 72: 401–410. doi: 10.1016/j.jaridenv.2007.07.007
- Peres-Neto, P.R. & Legendre, P. 2010. Estimating and controlling for spatial structure in the study of ecological communities. Glob. Ecol. Biogeogr. 19: 174–184. doi: 10.1111/j.1466-8238.2009.00506.x
- Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H. & Wagner, H.H. 2013. Vegan: Community Ecology R Package, v2. 0-10.
- ONM. 2016. Données météorologiques de la région Biskra (Algérie) de 1980 jusqu’a 2016 de. Ed. Office national de la météorologie.
- QGIS Development Team. 2017. QGIS Geographic Information System, Version 2.18.14-LasPalmas. Open Source Geospatial Foundation, Las-palmas. http://www.qgis.org/.
- Quinn, G. & Keough, M. 2002. Experimental Design and Data Analysis for Biologists. Cambridge University Press. 527.
- R Core Team. 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- Reijnen, R., Foppen, R.P.B. & Meeuwsen, H. 1996. The effect of traffic on the density of breeding birds in Dutch agricultural grasslands. Biol. Conserv. 75: 255–260. doi: 10.1016/0006-3207(95)00074-7
- Rogers, L. 1996. Behavioral, structural and neurochemical asymmetries in the Avian Brain: a model system for studying visual development and processing. Neurosci. Biobehav. Rev. 20: 487–503. doi: 10.1016/0149-7634(95)00024-0
- Sadoti, G. 2008. Nest-site selection by Common Black Hawks in south western New Mexico. J. Field Ornithol. 79: 11–19. doi: 10.1111/j.1557-9263.2008.00140.x
- Santos, T. & Suárez, F. 2005. Biogeography and population trends of Iberian steppe birds. In G. Bota, M. B. Morales, S. Mañosa & J. Camprodon. (ed) Ecology and Conservation of Steppe-Land Birds, 69–102. Lynx Edicions & Centre Tecnològic Forestal de Catalunya, Barcelona.
- Seoane, J., Carrascal, L.M., Palomino, D. & Alonso, C. 2010. Population size and habitat relationships of Black-bellied Sandgrouse Pterocles orientalis in the Canary Islands, Spain. Bird Conserv. Int. 20: 161–175. doi: 10.1017/S0959270909990207
- Seoane, J., Justribo, J.H., Garcia, F., Retamar, J., Rabadan, C. & Atienza, J.C. 2006. Habitat-suitability modelling to assess the effects of land-use changes on Dupont’s lark Chersophilus duponti: a case study in the Layna. Important Bird Area. Biol. Conserv. 128: 241–252. doi: 10.1016/j.biocon.2005.09.032
- Setchfield, R.P. & Peach, W.J. 2016. The influence of crop tiller density on the breeding performance of a cereal-nesting specialist. J. Appl. Ecol. 53: 1430–1439. doi: 10.1111/1365-2664.12704
- Traba, J., Acebes, P., Malo, J.E., García, J.T., Carriles, E., Radi, M. & Znari, M. 2013. Habitat selection and partitioning of the Black-bellied Sandgrouse (Pterocles orientalis), the Stone Curlew (Burhinus oedicnemus) and the Cream-coloured Courser (Cursorius cursor) in arid areas of North Africa. J. Arid Environ. 94: 10–17. doi: 10.1016/j.jaridenv.2013.02.007
- Whittaker, R.J., Ladle, R.J., Araújo, M.B., Fernández-Palacios, J.M., Delgado, J.D. & Arévalo, J.R. 2007. The island immaturity – Speciation pulse model of island evolution: An alternative to the “diversity begets diversity” model. Ecography 30: 321–327. doi: 10.1111/j.0906-7590.2007.04761.x
- Wiehn, J. & Korpimäki, E. 1997. Food limitation on brood size: experimental evidence in the Eurasian kestrel. Ecology 78: 2043–2050. doi: 10.1890/0012-9658(1997)078[2043:FLOBSE]2.0.CO;2
- Yosef, R. & Zduniak, P. 2011. Drinking schedule of four sandgrouse species (Pterocles spp.) in relation to sunrise and season. Acta Ethol. 14: 35–41. doi: 10.1007/s10211-010-0088-z
- Znari, M., Aourir, M., Radi, M. & Melin, J.M. 2008. Breeding biology of the Black-bellied Sandgrouse Pterocles orientalis in west-central Morocco. Ostrich 79: 53–60. doi: 10.2989/OSTRICH.2008.79.1.6.363
- Zuur, A.F., Ieno, E.N. & Elphick, C.S. 2009. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1: 3–14. doi: 10.1111/j.2041-210X.2009.00001.x
