ABSTRACT
Capsule Different methods of comparing bill morphology between woodpecker species show different, but not contradictory results.
Aims Differences among similar, closely related species which co-occur are still highly debated. In such a scenario, species should diverge morphologically to reduce competition. We studied this phenomenon, in three closely related woodpecker species that co-occur in eastern Slovakia: Great Spotted Woodpecker Dendrocopos major, Syrian Woodpecker Dendrocopos syriacus and White-backed Woodpecker Dendrocopos leucotos. The Great Spotted Woodpecker has the widest niche and lives sympatrically with both Syrian and White-backed Woodpeckers, while the distributions of the latter two species do not overlap because of their use of different habitats. We predicted that Great Spotted Woodpeckers should differ morphologically from both sympatric species: Syrian and White-backed Woodpeckers.
Methods Comparisons were made between species, based on dorsal and lateral views of maxilla using both geometric morphometric techniques and traditional measurements.
Results We confirmed our hypothesis, and found significant differences in bill shape between Great Spotted Woodpecker and both congeners. This is in contrast to the observed higher similarity of Great Spotted Woodpecker and Syrian Woodpecker in traditional body measurements. However, surprisingly, Syrian Woodpecker and White-backed Woodpecker do not differ significantly in bill shape.
Conclusions This work indicates that geometric morphometry appears to be a promising tool for the investigation of how interspecific competition influences the shape of the bill between co-occurring species.
The study of community assembly emphasizes competition-mediated ecological interactions (Meiri et al. Citation2014). Competition for resources is regarded as one of the major causes of phenotypic differentiation in closely related species (Schluter Citation2000). Such divergence in both phenotype and available resources in sympatric congeneric species is caused and/or maintained by ecological character displacement. This phenomenon has recently been considered to be one of the key drivers generating biodiversity (Pfennig & Pfennig Citation2012, Stuart & Losos Citation2013). However, the status of ecological character displacement is still the subject of debate, and not all examples of this phenomenon are considered to be fully compelling (Schluter Citation2000, Dayan & Simberloff Citation2005, Stuart & Losos Citation2013, Dufour et al. Citation2015). This is because, for most cases, interspecific competition has not been indubitably documented and confounding mechanisms have not been unequivocally ruled out. Therefore, most documented cases of ecological character displacement are also consistent with other evolutionary and ecological processes (Stuart & Losos Citation2013). Ecological competition takes place not only between species, but mainly within them (Doebeli Citation2011). Sexual size dimorphism can enable males and females of the same species to specialize on different sized food items and therefore minimize intraspecific competition. However, interspecific competition is thought to limit sexual dimorphism, since larger competitors in the community will prevent the larger sex from evolving to a larger size, and smaller species may prevent the smaller sex from becoming even smaller (Meiri et al. Citation2014). Strong intraspecific competition can select for character divergence between individuals within a population (Bolnick et al. Citation2003, Scott et al. Citation2003).
Food is usually considered to be a major factor limiting population sizes (MacArthur & Wilson Citation1967). When the sexes compete over food, theory suggests that sexual dimorphism can evolve via frequency-dependent natural selection, enabling males and females to specialize on different types of food (Slatkin Citation1984, Bolnick & Doebeli Citation2003). Therefore, strong intersexual competition can lead to selection for accentuated sexual differences. Sexual size dimorphism is often assumed to be more likely to evolve where intraspecific competition is fierce, but where interspecific competition, which can prevent the sexes from diverging, is weak (Greenberg & Olsen Citation2010, Cooper et al. Citation2011, Luther & Greenberg Citation2011). In the case of birds, bill characteristics are crucial for effective foraging in a given niche (Grant Citation1999, Benkman Citation2003, Grant & Grant Citation2006, Berns & Adams Citation2010). Changes in bill traits together with other features, such as different food preferences and foraging behaviour, can give a competitive advantage over species that have overlapping food and habitat preferences. European woodpeckers (Picidae) are a good example of a group of species living in sympatry (Angelstam & Mikusiński Citation1994, Kosiński & Kempa Citation2007) that form an ideal system for the study of species and sexual shape dimorphism. Three closely related species of woodpeckers: Great Spotted Woodpecker Dendrocopos major, Syrian Woodpecker Dendrocopos syriacus, and White-backed Woodpecker Dendrocopos leucotos, co-exist in large parts of their ranges in central and southeastern Europe (Winkler & Christie Citation2002). The Great Spotted Woodpecker is the most numerous woodpecker in the Palearctic and Europe (Michalek & Miettinen Citation2003), with a range that covers almost the whole of Europe and much of Asia. The Great Spotted Woodpecker is the most opportunistic of the European woodpeckers because it is able to occupy many different habitats, from pure coniferous forests to deciduous forests (Michalek & Miettinen Citation2003, Mazgajski & Rejt Citation2006), and has a large tolerance to fragmentation of habitats (Myczko et al. Citation2014, Michalczuk & Michalczuk Citation2016a). The Syrian Woodpecker is an expansive species, which began to extend its distribution through the Balkans toward northwest Europe around 1890 (Nowak Citation1971); it now also inhabits Central Europe and has been a regular breeder there since the 1950s (Ferianc Citation1950, Ciosek & Tomiałojć Citation1982, Danko et al. Citation2002, Michalczuk Citation2014). The Syrian Woodpecker occurs sympatrically with the Great Spotted Woodpecker in the majority of the former’s range and both species can use many different types of habitat (Michalczuk & Michalczuk Citation2016a, Kajtoch & Figarski Citation2017). However, the Syrian Woodpecker is more common in more open and synanthropic areas than the Great Spotted Woodpecker, especially in orchards and vineyards (Danko et al. Citation2002, Michalczuk & Michalczuk Citation2006, Citation2016a, Citation2016b, Ciach & Fröhlich Citation2013). Furthermore, the Syrian Woodpecker does not occur in boreal/hemiboreal areas or coniferous forests (Angelstam & Mikusiński Citation1994). In comparison to the two previous species, the White-backed Woodpecker is declining in northern Europe (Carlson Citation2000) but generally stable in the remainder of its range (Pasinelli Citation2006). Most of the range of this species overlaps with that of the Great Spotted Woodpecker, especially in Asia (Winkler & Christie Citation2002). It is the most specialized of the three studied species and is associated with old-growth deciduous or mixed forests (Wesołowski Citation1995, Carlson Citation2000). For this species, one of the most important factors is a large amount of dead wood in the habitat (Czeszczewik & Walankiewicz Citation2006). The Great Spotted and Syrian Woodpeckers are very closely related (Fuchs & Pons Citation2015) and hybridize relatively often (Cramp Citation1985, Michalek & Miettinen Citation2003, Michalczuk et al. Citation2014), but Great Spotted and White-backed Woodpecker hybrids are also known (Cramp Citation1985, Michalek & Miettinen Citation2003). Surprisingly, Syrian Woodpeckers is the least well known of the European woodpeckers (Pasinelli Citation2006) with a lack of morphological data from Central Europe (Cramp Citation1985).
Little is known about the differences in morphometry between these three species in co-occurring populations. Such information may be crucial, not only for understanding the niche separation between these very closely related species, but also to provide more general conclusions for causes of success under mutual competition. Earlier classical studies, for example, on Darwin finches, suggested that external measurements, especially bill size and shape, as well as body size, are important for understanding niche utilization by similar species (Boag & Grant Citation1981, Citation1984, Price Citation1987). Two other traits that should be related to foraging behaviour are wing length and tail length. Changes in size and/or shape of the wing should affect the capability of the animal to fly and manoeuvre (James Citation1982, Fitzpatrick Citation1999, Hromada et al. Citation2003a). Tail feathers are used to store kinetic energy during excavating behaviour (Winkler & Christie Citation2002).
An excellent, but often overlooked, source of ecological data is museum collections. Saris Museum in Bardejov, Slovakia holds one of the most prominent collections in the Carpathian region, including birds, accompanied with excellent secondary data (Hromada et al. Citation2003b, Kuczyński et al. Citation2003, Roselaar Citation2003, Hromada et al. Citation2015, Kaczmarski et al. Citation2017). Great Spotted, Syrian and White-backed Woodpeckers are well represented in this collection.
In this study, we predicted that Great Spotted Woodpeckers should differ morphologically from both sympatric species (Syrian and White-backed Woodpeckers) due to interspecific competition. However, bill measurements traditionally used for comparisons between species, such as bill length, do not sufficiently demonstrate differences in bill structure. It is possible to compare other traits only when the bill possesses specific and characteristic measurable points. But, like most bird beaks, woodpecker bills do not have such features. Therefore, we have here tested the suitability of geometric morphometrics which allows a comparison of bill shapes in the absence of the specific landmarks needed for comparison using traditional methods.
Methods
Research area
Woodpeckers from sympatric populations were collected in eastern Slovakia, mainly in its northern part, in the transition between the Eastern and Western Carpathians. The landscape in the region is hilly and diverse, with many valleys entering the mountains from the south. Elevation varies between 100 and 1100 m above sea level. Managed fir-beech forests with small relics of primeval forests dominate in the mountains, while the valleys are characterized by farmland, with a patchy mosaic of arable fields, pasture, orchards and gardens, interspersed with small woods, and scrub (Hromada et al. Citation2003b, Hromada et al. Citation2015). The Great Spotted Woodpecker lives here in sympatry with either the White-backed Woodpecker (in mixed and coniferous forests) or the Syrian Woodpecker (in valleys, lowlands, urbanized areas, and orchards). However, the White-backed and Syrian Woodpecker niches in this area do not overlap and can be described as allotopic (Rivas Citation1964). The Syrian Woodpecker expanded its range from the southeast into the studied area at the beginning of the 1950s (Danko et al. Citation2002, Michalczuk Citation2014).
Woodpecker specimens
The analysed material was based on the extensive collection of Great Spotted, Syrian and White-backed Woodpeckers collected from 1957 to 1974, and now held in the Saris museum in Bardejov, Slovakia (Hromada et al. Citation2003b, Roselaar Citation2003, Hromada et al. Citation2015) and additionally in the East Slovakian museum, Košice, Slovakia. Birds were collected throughout the year except for July, although in August and September only a few specimens were obtained. To assess bill shape morphology we used only sexually mature specimens of the three species. The samples used for geometric morphometric comparisons consisted of 34 specimens of White-backed Woodpeckers (20 females and 14 males), 89 specimens of Great Spotted Woodpeckers (43 females and 46 males), and 33 specimens of Syrian Woodpeckers (13 females and 20 males). For the data on traditional measurements we used only museum specimens from the Saris museum. These measurements had been made on freshly obtained birds before preparation and conservation, using the same methodology, by one person (Tibor Weisz), who was not aware of the future use of the data (Hromada et al. Citation2003b, Hromada et al. Citation2015). The six traditional measurements were as follows: body mass to an accuracy of 0.05 g, lengths of wing, body, and tail to an accuracy of 0.5 mm, and bill length (skull to bill tip) and tarsus lengths to an accuracy of 0.05 mm. Measurement methods followed Svensson (Citation1992): wing length was the flattened wing, tail length was taken on the underside using a rule, and tarsus length was taken as the distance between the sole side of the opened foot, abutted on a calliper at a right angle and measured to the proximal point of the tarsometatarsus. Some measurements were missing for some specimens because of damage during collection, and the numbers of specimens actually used are shown in the tables.
Data collection for geometric morphometry
Each bill was photographed in two different projections: dorsal (from above) and lateral (from the side) using a Sony Cyber-shot DSC-WX60 digital camera. The photographs were taken with a 4.5 mm focal length. The bill was photographed against a white background in a laboratory, using graph paper as a scale. The camera was mounted on a tripod and photographs were taken from the same distance and position for each bill. We used tpsUtil software (Rohlf Citation2012) to build a tps file for each bill from the image, separately for the two projections. Afterwards, the tps file was opened in tpsDig2 software (Rohlf Citation2010) where every image was scaled to the graph paper. Then, on every image in the same projection, three landmarks were distributed at local minima and maxima of the bill edge (). Next, two curves with five equally spaced locations (semi-landmarks) were digitized on bill edges between landmarks (). Semi-landmarks are landmarks digitized on the curve or surface with positions along the curvature (Mitteröecker & Gunz Citation2009). On the dorsal view, a landmark on the end of the bill and two landmarks on the left and right side of the bill near the head were digitized. The first curve was made on the right-hand side of the bill from the head to the end of the bill, and the second curve on the left-hand side. On the lateral view, landmarks were located at the end of the bill and at the most upper and lower location near the head. The first curve was then made on the superior (upper) edge of the maxilla ((b)), and the second on the inferior (lower) edge. Before taking the photographs, the feathers were arranged to expose the greatest extent of the bill. This ensured that the first landmarks were digitized near the skull. Both curves were made in the same direction, from the head to the end of the bill. On the lateral view of the bill, landmarks and semi-landmarks were distributed only on the maxilla, because the mandible was partially covered by the upper part of the bill.
Figure 1. Image of a woodpecker bill with semi-landmarks shown. A – Dorsal view and B – Lateral view. Red points are landmarks, white points are semi-landmarks digitized along the curves.
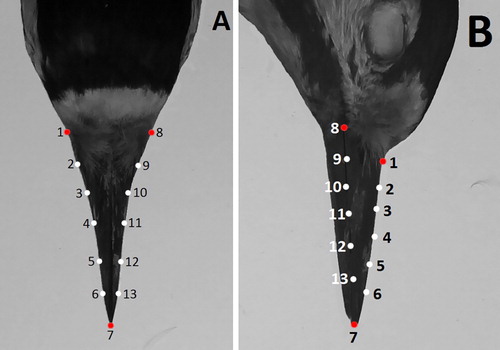
The bills of woodpeckers do not have many characteristic landmarks which could be useful in geometric morphometrics. Thus, in our study we also used semi-landmarks to analyse shape differences within and between species. A landmark superimposition was then undertaken using a Procrustes Generalized Analysis (GPA) with adjustment to each semi-landmark during each iteration of the GPA optimization. Slide of semi-landmarks along curves was carried out to reduce the bending energy between each specimen. This part of the analysis was calculated using TPSRelw (Rohlf Citation2017). Next, all semi-landmarks were converted to landmarks in order to carry out geometric morphometric analysis in MorphoJ. This procedure allowed a comparison of shapes through translation (averaging the position of landmarks), scaling to one size, and rotation (minimizing the summed squared distances between landmarks; Zelditch et al. Citation2012). Centroid size was used as a measure of geometric scale and calculated as the square root of the summed squared distances of each landmark from the centre of the landmark configuration (Zelditch et al. Citation2012). To estimate the technical error measurement, the landmarks and semi-landmarks were distributed by two independent observers (ZO and AMK) twice each on 30 randomly selected specimens.
Geometric morphometric analysis
A Procrustes analysis of variance (anova) test was carried out to analyse the level of the measurement error associated with semi-landmark digitization. The Procrustes anova used in geometric morphometric analysis is a variant of the anova used in traditional statistical analysis. This method compares variation in shape within groups to variation in shape between groups, and is applied to test the hypothesis that samples do not differ in their means (Zeldith et al. Citation2012). A multivariate regression was subsequently carried out for each view (anterior and lateral) separately to analyse how the size of the bill (centroid size) influenced its shape (Procrustes coordinates). This type of association is called allometry and can be a crucial component of shape variation (Outomuro & Johansson Citation2017). In the case of a significant multivariate regression, geometric morphometric analysis would be carried out taking the allometry component into account. We used principal components analysis (PCA) to describe major trends in shape variation within the entire sample of birds. To focus on the differences between sexes within the three species a canonical variate analysis (CVA) was then carried out. To test the significance of shape differences we computed tests based on 10,000 randomizations in CVA. The significance level of the CVA was Bonferroni-adjusted (Sokal & Rohlf Citation1995) to 0.05/15 = 0.0033 owing to the large number (15) of sex/species comparisons on each view. The semi-landmarks were established using tpsRelw while all geometric morphometric analyses were carried out using MorphoJ software (version 1.06d, Klingenberg Citation2011).
Linear measurements
We compared interspecific and intersexual morphometric differences using anova based on three species groups and two sex groups and their interaction followed by post hoc Tukey multiple comparisons. All six traditional variables passed homogeneity of variance tests but two failed Kolmogorov Smirnoff normality tests. Consequently, as a precaution, all multiple comparisons were derived from bootstrapping based on 1000 samples. Pearson correlations were calculated between morphometric measurements, separately for each species. All statistical tests were two-tailed and the significance threshold was 0.05.
Results
The suitability of geometric morphometry for comparison of bill shapes
In the Procrustes anova, the Procrustes coordinates of each sample were the dependent variable, in turn the observer who digitized landmarks and semi-landmarks on bills was the independent variable. Variation among samples (mean square = 3.8 × 10−4) was much higher than that between the double distribution of semi-landmarks in both observers (mean square = 8.3 × 10−7). Moreover, Procrustes anova did not show significant differences (P > 0.05) in semi-landmark distribution between the two observers; hence, high intra- and inter-observer agreement was achieved. The multivariate regression which analyses association between bill size (centroid size as the independent variable) and bill shape (coordinates as the dependent variable) was not significant for either analysed view (dorsal view: per cent predicted 0.853%, P = 0.3075; lateral view: per cent predicted 0.186%, P = 0.6984) thus, subsequent geometric morphometric analysis was carried out without taking into account the allometric component.
Bill shape differences
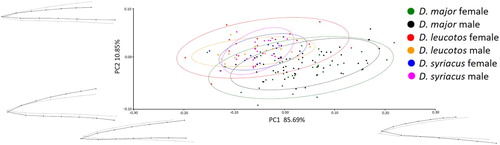
The PCA for bill shape showed that the female and male Great Spotted Woodpeckers were most variable in the two projections. A total of 88.06% of the variability in the dorsal view and 85.69% in the lateral view could be explained by the first component (PC1). PC1 in the dorsal view represented the width of the proximal part of the bill; in the lateral view it represented the extent of curve ( and ). PC2 explained 9.43% of the variability for the dorsal view and 10.85% for the lateral view. PC2 represented the width and height in the central part of the bill ( and ).
Figure 2. Results of PCA of bill shapes for the dorsal view. Grey lines represent bill shape for the principal component.
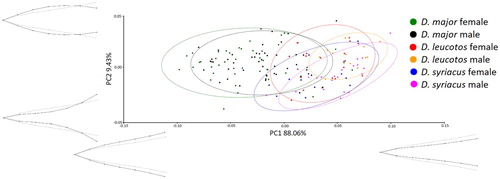
Figure 3. Results of PCA of bill shapes for the lateral view. Grey lines represent bill shape for the principal component.
The CVA showed that White-backed and Syrian Woodpecker bill shapes differed significantly from bills of Great Spotted Woodpeckers in both views (lateral and dorsal), the results are presented in and , and and . In general, White-backed and Syrian Woodpeckers were not distinct in bill shape in the two views. Only male White-backed Woodpeckers were characterized by significantly different bill shape from female Syrian Woodpeckers in the anterior view. CV1 explained 81.95% of the variation in the dorsal view and 79.67% in the lateral view. In both views, CV1 described bill width over its entire length and the extent of curve of the bill. In the analysed material, CV2 explained 9.51% of the variation in the dorsal view and 10.59% in the lateral view. CV2 represented the width and height in the central part of the bill. Both sexes of White-backed and Syrian Woodpecker were characterized by negative values of CV1 indicating a relatively narrower and less curved bill than female and male Great Spotted Woodpeckers which were characterized by positive values of CV1. In turn, positive values of CV2 characterized a relatively wider bill near the head. Significant differences between sexes were noted only for White-backed Woodpeckers in the anterior view (). Male White-backed Woodpeckers generally had a relatively narrower bill near the head.
Figure 4. Results of the CVA of bill shapes for the dorsal view. Grey lines represent bill shape for the canonical variate.
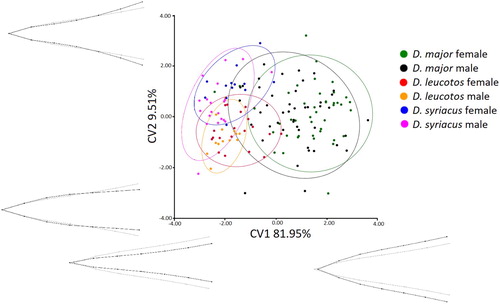
Figure 5. Results of the CVA of bill shapes for the lateral view. Grey lines represent bill shape for the canonical variate.
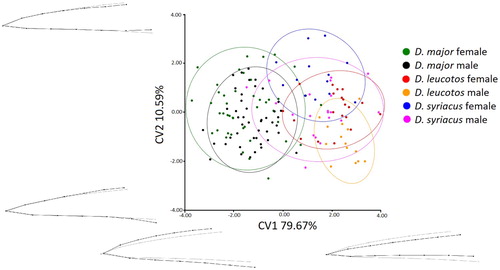
Table 1. P-values from permutation tests in CVA. Analysis carried out on the dorsal view of the bill.
Table 2. P-values from permutation tests in CVA. Analysis carried out on the lateral view of the bill.
Body trait differences
A summary of traditional morphometric measurements analysed by species, sex and species*sex interaction revealed significant differences between groups in all body traits ((a–f)). In each species, mean bill length was significantly longer in males than females ((a)). The longest bills in both males and females were recorded for White-backed Woodpeckers and were significantly longer than all other analysed groups. The shortest mean bill length was recorded for female Great Spotted Woodpeckers but was not significantly different from female Syrian Woodpeckers. Bills of male Syrian Woodpeckers were significantly longer than male Great Spotted Woodpeckers. Sex differences in body mass were significant only for the White-backed Woodpecker, where males were heavier than females ((b)). Both sexes of the White-backed Woodpecker were heavier than birds from all other groups. Male Great Spotted Woodpeckers were significantly heavier than male Syrian Woodpeckers. Statistically significant sex differences in wing length only occurred in the White-backed Woodpecker ((c)). Both sexes of the White-backed Woodpecker had significantly longer wings than all other groups of birds. Both sexes of the Syrian Woodpecker had significantly shorter wings than all other groups. We did not find any statistically significant sex differences in mean tail length of the analysed groups ((d)). Only the Syrian Woodpecker had significantly shorter tails than all other analysed groups. Tarsus length was significantly greater only in male White-backed Woodpeckers compared to other groups ((e)). We did not find any statistically significant sex differences between mean body length in analysed groups except for Great Spotted Woodpeckers ((f)). Only the Syrian Woodpecker had a significantly shorter mean body length than all other groups and male White-backed Woodpeckers had significantly longer mean body length than both sexes of Great Spotted Woodpecker.
Table 3. Summary of analyses of traditional body trait differences in White-backed, Syrian and Great Spotted Woodpeckers. Means that do not share the same superscript letter are significantly different (P < 0.05).
Discussion
The traditional body trait measurements showed a higher similarity between the Great Spotted and Syrian Woodpeckers than either did with White-backed Woodpecker. However, as predicted, the bill shape analysis indicated a specific shape of the Great Spotted Woodpecker bill which differed significantly not only from that of the White-backed Woodpecker but also from that of the closer related Syrian Woodpecker. Surprisingly, despite the significant difference in the bill length of Syrian and White-backed Woodpeckers, the variation of bill shapes in these two species was very similar. The lack of differences between the bill shapes of Syrian and White-backed Woodpeckers (if we compare the same sexes) may be a result of the lack of competition between the two, because of the spatial isolation in the environment of these two species which inhabit completely different habitats (Wesołowski Citation1995, Danko et al. Citation2002, Czeszczewik & Walankiewicz Citation2006, Michalczuk & Michalczuk Citation2016b). Moreover, it is surprising in these different habitats that the optimum shapes of the bills are similar ().
Because of a lack of direct data about the existing competition of the studied species in Slovakia in terms of dietary analysis, we cannot confirm unequivocally that differences in bill morphology in co-occurring populations are a direct effect of character displacement. However, our findings can at least suggest the existence of niche differentiation in the three studied woodpecker species. For investigation of co-evolutionary morphological response to interspecific competition, Dayan & Simberloff (Citation1998) suggested a careful consideration both of guild composition and proper morphological traits. Yet, both White-backed and Syrian Woodpeckers co-occur widely with Great Spotted Woodpecker, which is a generalist and uses habitats preferred by both other species (Cramp Citation1985, Wesołowski Citation1995, Michalczuk & Michalczuk Citation2016a, Citation2016b). The foraging behaviour of Great Spotted and Syrian Woodpeckers is similar. The most important differences are a higher frequency of gleaning and probing, and less excavating, in Syrian Woodpecker than in the Great Spotted Woodpecker (Winkler Citation1973). All three examined woodpecker species mainly forage on arthropods, especially insect larvae, and all species include plant material in their diet (Cramp Citation1985, Michalek & Miettinen Citation2003). Great Spotted Woodpeckers show a greater tendency than Syrian Woodpeckers to seek deep wood-boring insects, and White-backed Woodpeckers – in comparison to Great Spotted Woodpeckers – forage more frequently pecking deep into wood (Hogstad Citation1978, Cramp Citation1985). A major component of the White-backed Woodpecker diet is large wood-boring insect larvae, which occur much less frequently in the diet of Great Spotted and Syrian Woodpeckers (Cramp Citation1985, Michalek & Miettinen Citation2003). There are strong differences between winter foraging niches of the three species (Hogstad Citation1971, Cramp Citation1985). Great Spotted Woodpeckers forage on conifer seeds, mainly Scots Pine Pinus sylvestris (Hogstad Citation1971, Myczko & Benkman Citation2011). This can explain their specific bill shape which could be an adaptation for processing conifer cones.
Thus, it is clear that there are considerable dietary niche overlaps in the interspecific pairs, White-backed/Great Spotted Woodpeckers and Syrian/Great Spotted Woodpeckers, which can lead to competition and accentuation of trait divergence (Gray et al. Citation2005, Stuart & Losos Citation2013) in studied sympatric species. Moreover, all three species are closely related (Fuchs & Pons Citation2015) and more closely related species compete more fiercely for resources (Wiens & Rotenberry Citation1981). We did not confirm any significant shape differences between White-backed and Syrian Woodpeckers in comparisons of the same sexes in the dorsal view of the maxilla, and independent of sex in the lateral view. This means that their bill shapes are morphologically similar (, and ). Syrian Woodpeckers prefer more open areas greatly modified by humans (Mošanský & Mošanský Citation1999, Michalczuk & Michalczuk Citation2016b) in comparison to the semi-natural forests preferred by White-backed Woodpeckers (Czeszczewik & Walankiewicz Citation2006). This difference in habitat preferences results in strict spatial isolation between the two species and a lack of direct competition for resources. Despite a lack of any difference in bill shape between White-backed and Syrian Woodpeckers, both species differed significantly in bill length. The bills of White-backed Woodpeckers were significantly longer, which may simply reflect the difference in body size between the two species. For both sexes, mean dimensions of almost all analysed body traits were significantly larger in White-backed Woodpeckers than in the other analysed species ((a–f)). Only female White-backed Woodpecker wing length and body length were similar to those of female Great Spotted Woodpeckers. White-backed Woodpecker tail length was similar to tail length in the Great Spotted Woodpecker, and female White-backed Woodpecker tarsus length was similar to all other groups. On the other hand, longer bills in White-backed Woodpeckers may be a consequence of their foraging behaviour involving excavation of prey deeper within the tree (Cramp Citation1985), therefore, longer bill and greater body mass can be beneficial. The comparisons of body mass within the same sexes between sympatrically occurring Great Spotted and Syrian Woodpeckers showed a lack of significant differences, thus between these two species we should expect the strongest interspecific concurrence.
Sex differences in foraging niches are well known in woodpeckers. Most studies on foraging niches of sympatric European woodpeckers reveal many differences in their foraging behaviour (Jenni Citation1983, Török Citation1990). For example, Hogstad (Citation1978) reported differences between sexes in foraging niches for White-backed and Great Spotted Woodpeckers and suggested dominance by males in optimum feeding sites. Moreover, Osiejuk (Citation1998) reported many intersexual differences in foraging patterns in Great Spotted Woodpeckers, especially in winters of poor Scots Pine cone crops. However, in winters with a rich crop of Scots Pine cones, the foraging behaviour of both sexes became very similar (Osiejuk Citation1994, Citation1998). It is obvious to expect that such differences in foraging niche should also encourage sexual dimorphism; particularly in bill size and shape. We confirmed significant sex differences in bill length of all three woodpecker species ((a)), but following Bonferroni correction the only significant difference between the sexes in shape of bills was in the dorsal view of White-backed Woodpeckers ( and ).
We have demonstrated that different methods of comparing bill morphology between species show different, but not contradictory results. The traditional measurement of bill length to compare morphology shows different results than the landmark-based geometric morphometric analysis described here. This apparent discrepancy is a consequence of woodpecker bills, like those of most birds, not having characteristic points enabling repeatable measurements for describing bill shape. It follows that using the results of these two techniques together allows us to more thoroughly compare differences in bill morphology between groups of birds.
Acknowledgements
We thank T. Jászay, the curator of the Natural History collection of Saris Museum in Bardejov for help during investigations and P. Tryjanowski for useful comments on this paper and for statistical advice.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Angelstam, P. & Mikusiński, G. 1994. Woodpecker assemblages in natural and managed boreal and hemiboreal forests - a review. Ann. Zool. Fenn. 31: 157–172.
- Benkman, C.W. 2003. Divergent selection drives the adaptive radiation of crossbills. Evolution 57: 1176–1181. doi: 10.1111/j.0014-3820.2003.tb00326.x
- Berns, C.M. & Adams, D.C. 2010. Bill shape and sexual shape dimorphism between two species of temperate hummingbirds: Black-Chinned hummingbird (Archilochus alexandri) and Ruby-Throated hummingbird (A. colubris). Auk 127: 626–635. doi: 10.1525/auk.2010.09213
- Boag, P.T. & Grant, P.R. 1981. Intense natural selection in a population of Darwin's Finches (Geospizinae) in the Galapagos. Science 214: 82–85. doi: 10.1126/science.214.4516.82
- Boag, P.T. & Grant, P.R. 1984. Darwin's Finches (Geospiza) on Isla Daphne Major, Galapagos: Breeding and feeding ecology in a climatically variable environment. Ecol. Monogr. 54: 463–489. doi: 10.2307/1942596
- Bolnick, D.I. & Doebeli, M. 2003. Sexual dimorphism and adaptive speciation: Two sides of the same ecological coin. Evolution 57: 2433–2449. doi: 10.1111/j.0014-3820.2003.tb01489.x
- Bolnick, D.I., Swanbäck, R., Fordyce, J.A., Yang, L.H., Davis, J.M., Hulsey, C.D. & Forister, M.L. 2003. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 161: 1–28. doi: 10.1086/343878
- Carlson, A. 2000. The effect of habitat loss on a deciduous forest specialist species: The White-backed Woodpecker (Dendrocopos leucotos). Forest Ecol. Manag. 131: 215–221. doi: 10.1016/S0378-1127(99)00215-7
- Ciach, M. & Fröhlich, A. 2013. Habitat preferences of the Syrian Woodpecker Dendrocopos syriacus in urban environments: An ambiguous effect of pollution. Bird Study 60: 491–499. doi: 10.1080/00063657.2013.847899
- Ciosek, J. & Tomiałojć, L. 1982. Dzięcioł syryjski, Dendrocopos syriacus (Hempr. et Ehrenb.) ptakiem lęgowym w Polsce. [Syrian Woodpecker, Dendrocopos syriacus (Hempr. and Ehrenb.), breeding in Poland]. Prz. Zool. 26: 101–109. (in Polish).
- Cooper, I.A., Gilman, R.T. & Boughman, J.W. 2011. Sexual dimorphism and speciation on two ecological coins: Patterns from nature and theoretical predictions. Evolution 65: 2553–2571. doi: 10.1111/j.1558-5646.2011.01332.x
- Cramp, S. 1985. The Birds of the Western Palearctic, Vol. IV. Oxford University Press, Oxford.
- Czeszczewik, D. & Walankiewicz, W. 2006. Logging affects the white-backed woodpecker Dendrocopos leucotos distribution in the Białowieża Forest. Ann. Zool. Fenn. 24: 221–227.
- Danko, Š, Darolová, A. & Krištín, A. 2002. Birds Distribution in Slovakia. Veda, Bratislava.
- Dayan, T. & Simberloff, D. 1998. Size patterns among competitors: Ecological character displacement and character release in mammals, with special reference to island populations. Mammal Rev. 28: 99–124. doi: 10.1046/j.1365-2907.1998.00029.x
- Dayan, T. & Simberloff, D. 2005. Ecological and community-wide character displacement: The next generation. Ecol. Lett. 8: 875–894. doi: 10.1111/j.1461-0248.2005.00791.x
- Doebeli, M. 2011. Adaptive Diversification. Princeton University Press, Princeton, NJ.
- Dufour, C.M.S., Meynard, C., Watson, J., Rioux, C., Benhamou, S., Perez, J., du Plessis, J.J., Avenant, N., Pillay, N. & Ganem, G. 2015. Space use variation in co-occurring sister species: Response to environmental variation or competition? PLoS One 10: e0117750. doi: 10.1371/journal.pone.0117750
- Ferianc, O. 1950. Dryobates syriacus balcanicus (GENGL. et STRES.) na Slovensku. Sylvia 11-12: 51–56. (in Slovak).
- Fitzpatrick, S. 1999. Tail length in birds in relation to tail shape, general flight ecology and sexual selection. J. Evol. Biol. 12: 49–60. doi: 10.1046/j.1420-9101.1999.00009.x
- Fuchs, J. & Pons, J.M. 2015. A new classification of the Pied Woodpeckers assemblage (Dendropicini, Picidae) based on a comprehensive multi-locus phylogeny. Mol. Phylogenet. Evol. 88: 28–37. doi: 10.1016/j.ympev.2015.03.016
- Grant, P.R. 1999. Ecology and Evolution of Darwin's Finches. Princeton University Press, Princeton, NJ.
- Grant, P.R. & Grant, B.R. 2006. Evolution of character displacement in Darwin's finches. Science 313: 224–226. doi: 10.1126/science.1128374
- Gray, S.M., Robinson, B.W. & Parsons, K.J. 2005. Testing alternative explanations of character shifts against ecological character displacement in brook sticklebacks (Culaea inconstans) that coexist with ninespine sticklebacks (Pungitius pungitius). Oecologia 146: 25–35. doi: 10.1007/s00442-005-0184-3
- Greenberg, R. & Olsen, B.J. 2010. Bill size and dimorphism in tidal-marsh sparrows: Island-like processes in a continental habit. Ecology 91: 2428–2436. doi: 10.1890/09-1136.1
- Hogstad, O. 1971. Notes on the winter food of the Great Spotted Woodpecker, Dendrocopos major. Sterna 10: 233–241.
- Hogstad, O. 1978. Sexual dimorphism in relation to winter foraging and territorial behavior of the three-toed Woodpecker Picoides tridactylus and three Dendrocopos species. Ibis 120: 198–203. doi: 10.1111/j.1474-919X.1978.tb06775.x
- Hromada, M., Kuczynski, L., Krištín, A. & Tryjanowski, P. 2003a. Animals of different phenotype differentially utilise dietary niche - the case of the Great Grey Shrike Lanius excubitor. Ornis Fenn. 80: 71–78.
- Hromada, M., Kuczyński, L., Skoracki, M., Antczak, M. & Tryjanowski, P. 2003b. The value of the bird collections and associated data in regional museums: Lanius excubitor specimens in Šarišské Museum Bardejov, Slovakia. Bull. Br. Ornithol. Club 123A: 226–233.
- Hromada, M., Čanády, A., Mikula, P., Peterson, A. & Tryjanowski, P. 2015. Old natural history collections for new millennium - birds and mammals in the collection of PHMR. Tibor Weisz in Sarisske museum Bardejov, Slovakia. Folia Oecol. 7: 115–132.
- James, F.C. 1982. The ecological morphology of birds: A review. Ann. Zool. Fenn. 19: 265–275.
- Jenni, L. 1983. Habitatnutzung, Nahrungserwerb und Nahrung von Mittel- und Buntspecht (Dendrocopos medius, D. major) sowie Bemerkungen zur Verbreitungsgeschichte des Mittelspechts. Orn. Beob. 80: 29–57 (in German).
- Kaczmarski, M., Kubicka, A.M., Hromada, M. & Tryjanowski, P. 2017. Robustness of newt heads in condition of co-existence: A case of the Carpathian newt and the alpine newt. Zoomorphology 136: 511–521. doi: 10.1007/s00435-017-0366-7
- Kajtoch, Ł & Figarski, T. 2017. Comparative distribution of Syrian and great spotted woodpeckers in different landscapes of Poland. Folia Zool. 66: 29–36. doi: 10.25225/fozo.v66.i1.a5.2017
- Klingenberg, C.P. 2011. Morphoj: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11: 353–357. doi: 10.1111/j.1755-0998.2010.02924.x
- Kosiński, Z. & Kempa, M. 2007. Density, distribution and nest-sites of woodpeckers Picidae in a managed forest of Western Poland. Pol. J. Ecol. 55: 519–533.
- Kuczyński, L., Tryjanowski, P., Antczak, M., Skoracki, M. & Hromada, M. 2003. Repeatability of measurements and shrinkage after skinning: The case of the Great Grey Shrike Lanius excubitor. Bonn. Zool. Beitr. 51: 127–130.
- Luther, D. & Greenberg, R. 2011. The island syndrome in coastal wetland ecosystems: Convergent evolution of large bills in mangrove passerines. Auk 128: 201–204. doi: 10.1525/auk.2011.10262
- MacArthur, R.H. & Wilson, E.O. 1967. Theory of Island Biogeography. Princeton University Press, Princeton.
- Mazgajski, T.D. & Rejt, Ł. 2006. The effect of forest patch size on the breeding biology of the great spotted woodpecker Dendrocopos major. Ann. Zool. Fenn. 43: 211–220.
- Meiri, S., Kadison, A.E., Novosolov, M., Pafilis, P., Foufopoulos, J., Itescu, Y., Raia, P. & Pincheira-Donoso, D. 2014. The number of competitor species is unlinked to sexual dimorphism. J. Anim. Ecol. 83: 1302–1312. doi: 10.1111/1365-2656.12248
- Michalczuk, J. 2014. Expansion of the Syrian woodpecker Dendrocopos syriacus in Europe and Western Asia. Ornis Polonica 55: 149–161.
- Michalczuk, J. & Michalczuk, M. 2006. Reaction to playback and density estimations of Syrian Woodpeckers Dendrocopos syriacus in agricultural areas of south-eastern Poland. Acta Ornithol. 41: 33–39. doi: 10.3161/068.041.0109
- Michalczuk, J. & Michalczuk, M. 2016a. Coexistence of Syrian Woodpecker Dendrocopos syriacus and Great Spotted Woodpecker Dendrocopos major in nonforest tree stands of the agricultural landscape in SE Poland. Turk. J. Zool. 40: 743–748. doi: 10.3906/zoo-1601-13
- Michalczuk, J. & Michalczuk, M. 2016b. Habitat preferences of Picidae woodpeckers in the agricultural landscape of SE Poland: Is the Syrian Woodpecker Dendrocopos syriacus colonizing a vacant ecological niche? North-West. J. Zool. 12: 14–21.
- Michalczuk, J., McDevitt, A.D., Mazgajski, T.D., Figarski, T., Ilieva, M., Bujoczek, M., Malczyk, P. & Kajtoch, Ł. 2014. Tests of multiple molecular markers for the identification of Great Spotted and Syrian Woodpeckers and their hybrids. J. Ornithol. 155: 591–600. doi: 10.1007/s10336-014-1040-1
- Michalek, K.G. & Miettinen, J. 2003. Dendrocopos major Great Spotted Woodpecker. BWP Update 5: 101–184.
- Mitteröecker, P. & Gunz, P. 2009. Advances in geometric morphometrics. Evol. Biol. 36: 235–247. doi: 10.1007/s11692-009-9055-x
- Mošanský, L. & Mošanský, A. 1999. Development of Syrian Woodpecker (Dendrocopos syriacus) and Great Spotted Woodpecker (Dendrocopos major) population in Košice urban area. Tichodroma 12: 97–106.
- Myczko, Ł & Benkman, C.W. 2011. Great spotted woodpeckers Dendrocopos major exert multiple forms of phenotypic selection on Scots pine Pinus sylvestris. J. Avian Biol. 42: 429–433. doi: 10.1111/j.1600-048X.2011.05326.x
- Myczko, Ł, Rosin, Z.M., Skórka, P. & Tryjanowski, P. 2014. Urbanization level and woodland size are major drivers of woodpecker species richness and abundance. PLoS One 9: e94218. doi: 10.1371/journal.pone.0094218
- Nowak, E. 1971. The range expansion of animals and its causes. Zesz. Nauk. Inst. Ekol. PAN 3: 1–255. (in Polish).
- Osiejuk, T.S. 1994. Sexual dimorphism in foraging behavior of the Great Spotted Woodpecker Dendrocopos major during winters with rich crops of Scotch pine cones. Ornis Fenn. 71: 144–150.
- Osiejuk, T.S. 1998. Study on the intersexual differentiation of foraging niche in relation to abundance of winter food in Great Spotted Woodpecker Dendrocopos major. Acta Ornithol. 33: 137–141.
- Outomuro, D. & Johansson, F. 2017. A potential pitfall in studies of biological shape: Does size matter? J. Anim. Ecol. 86: 1447–1457. doi: 10.1111/1365-2656.12732
- Pasinelli, G. 2006. Population biology of European woodpecker species: A review. Ann. Zool. Fenn. 43: 96–111.
- Pfennig, D.W. & Pfennig, K.S. 2012. Evolution's Wedge: competition and the origins of diversity. University of California Press, Berkeley.
- Price, T.D. 1987. Diet variation in a population of Darwin's finches. Ecology 68: 1015–1028. doi: 10.2307/1938373
- Rivas, L.R. 1964. A reinterpretation of the concepts “Sympatric” and “Allopatric” with proposal of the additional terms “Syntopic” and “Allotopic”. Syst. Zool. 13: 42–43. doi: 10.2307/2411436
- Rohlf, F.J. 2010. TPSDig2, v. 2.12. State University, Stony Brook, NY.
- Rohlf, F.J. 2012. TPSUtil, v. 1.40. State University, Stony Brook, NY.
- Rohlf, F.J. 2017. Ecology and Evolution and Anthropology. Stony Brook University, New York.
- Roselaar, K. 2003. An inventory of major European bird collections. Bull. Br. Ornithol. Club 123: 253–337.
- Schluter, D. 2000. Ecological character displacement in adaptive radiation. Am. Nat. 156: 4–16. doi: 10.1086/303412
- Scott, S.N., Clegg, S.M., Blomberg, S.P., Kikkawa, J. & Owens, I.P.F. 2003. Morphological shifts in island-dwelling birds: The roles of generalist foraging and niche expansion. Evolution 57: 2147–2156. doi: 10.1111/j.0014-3820.2003.tb00392.x
- Slatkin, M. 1984. Ecological causes of sexual dimorphism. Evolution 38: 622–630. doi: 10.1111/j.1558-5646.1984.tb00327.x
- Sokal, R.R. & Rohlf, F.J. 1995. Biometry: the principles and practice of statistics in biological research. W.H. Freeman, New York.
- Stuart, Y.E. & Losos, J.B. 2013. Ecological character displacement: Glass half full or half empty? Trends Ecol. Evol. 28: 402–408. doi: 10.1016/j.tree.2013.02.014
- Svensson, L. 1992. Identification Guide to European Passerines. British Trust for Ornithology, Thetford.
- Török, J. 1990. Resource partitioning among three woodpecker species Dendrocopos spp. during the breeding season. Holarct. Ecol. 13: 257–264.
- Wesołowski, T. 1995. Ecology and behaviour of White-backed Woodpecker (Dendrocopos leucotos) in a primaeval temperate forest (Białowieża National Park, Poland). Vogelwarte 38: 61–75.
- Wiens, J.A. & Rotenberry, J.T. 1981. Morphological size ratios and competition in ecological communities. Am. Nat. 117: 592–599. doi: 10.1086/283744
- Winkler, H. 1973. Food acquisition and competition of the Syrian Woodpecker, Picoides syriacus. Oecologia 12: 193–208. doi: 10.1007/BF00345517
- Winkler, H. & Christie, D.A. 2002. Family Picidae (Woodpeckers). In J. Hoyo, A. Elliott & J. Sargatal. (eds) Handbook of the Birds of the World. Vol. 7. Jacamars to Woodpeckers, 296–558. Lynx Edicions, Barcelona.
- Zelditch, M.L., Swiderski, D.L. & Sheets, H.D. 2012. Geometric Morphometrics for Biologists. Elsevier Academic Press, London.
